Effect of Graphene Oxide and Silver Nanoparticle Hybrid Composite on Acinetobacter baumannii Strains, Regarding Antibiotic Resistance and Prevalence of AMP-C Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. A. baumannii Isolation and Antibiotic Susceptibility Testing
2.2. Detection of AMP-C ß-Lactamases
2.3. GO–Ag Hybrid Nanocomposite Effect on A. baumannii Strains
3. Results
3.1. Antibiotic Resistance and AMP-C β-lactamases Production
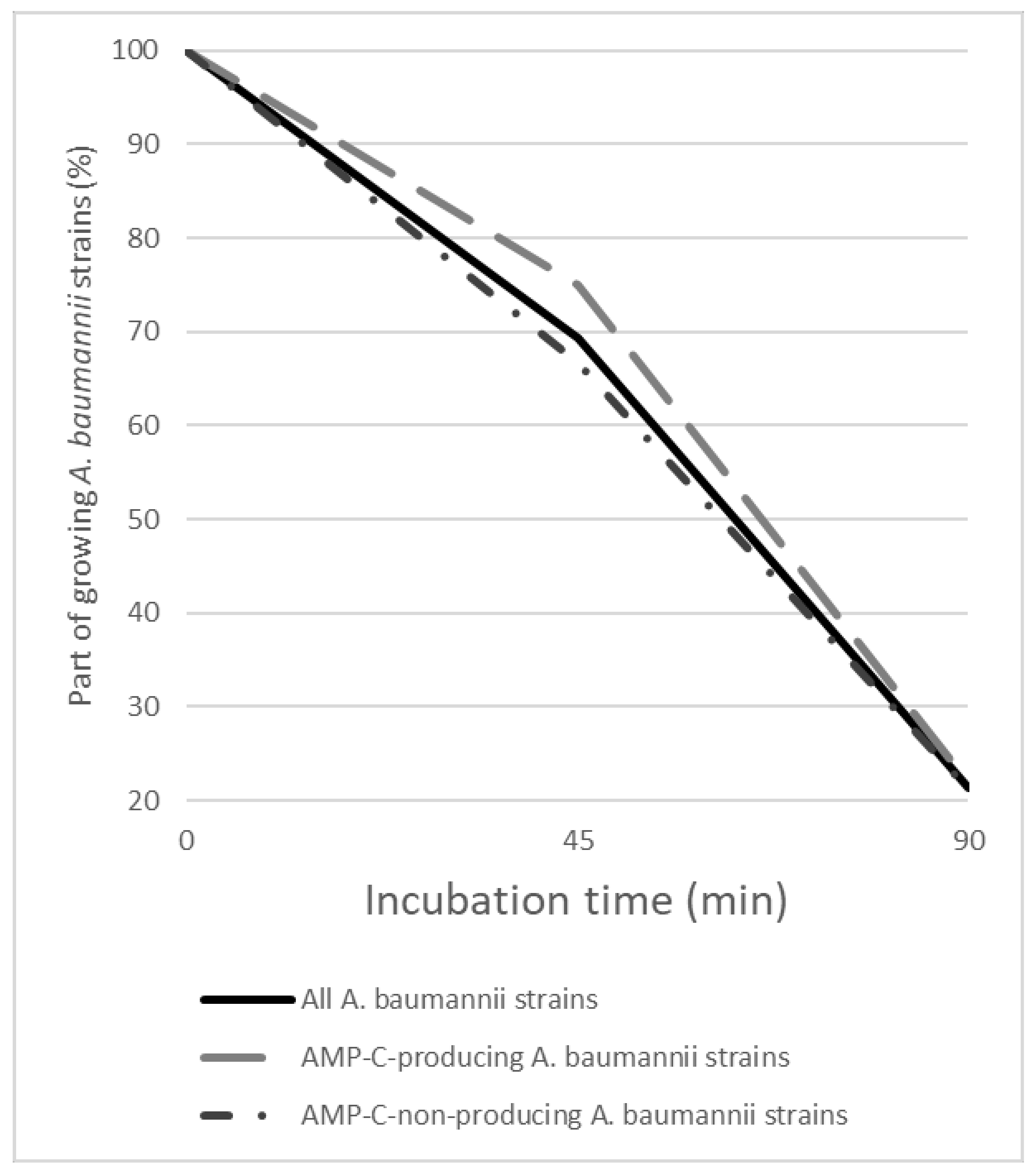
3.2. Effect of GO–Ag Hybrid Nanocomposite on AMP-C-Producing and AMP-C-Non-Producing A. baumannii Strains
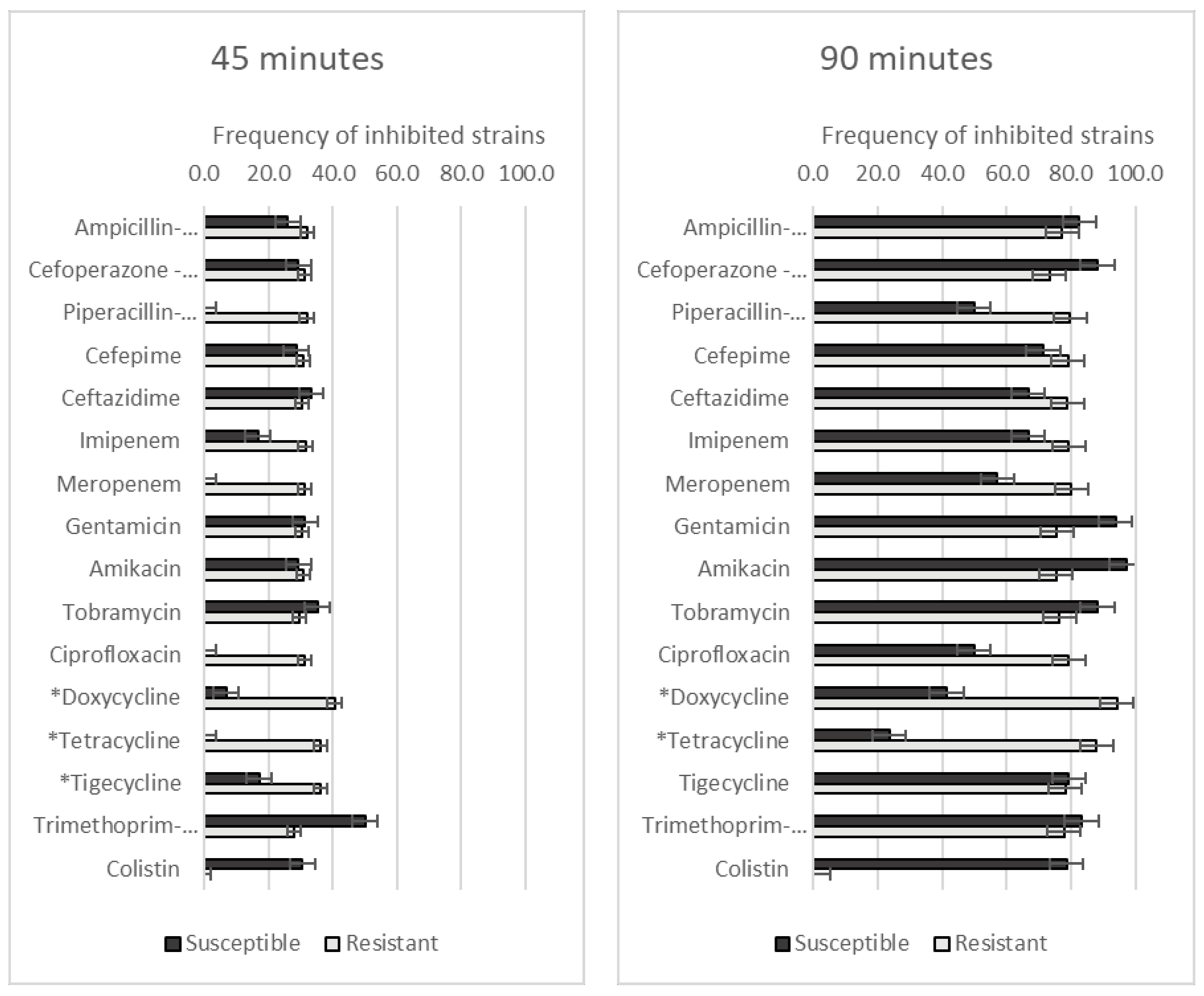
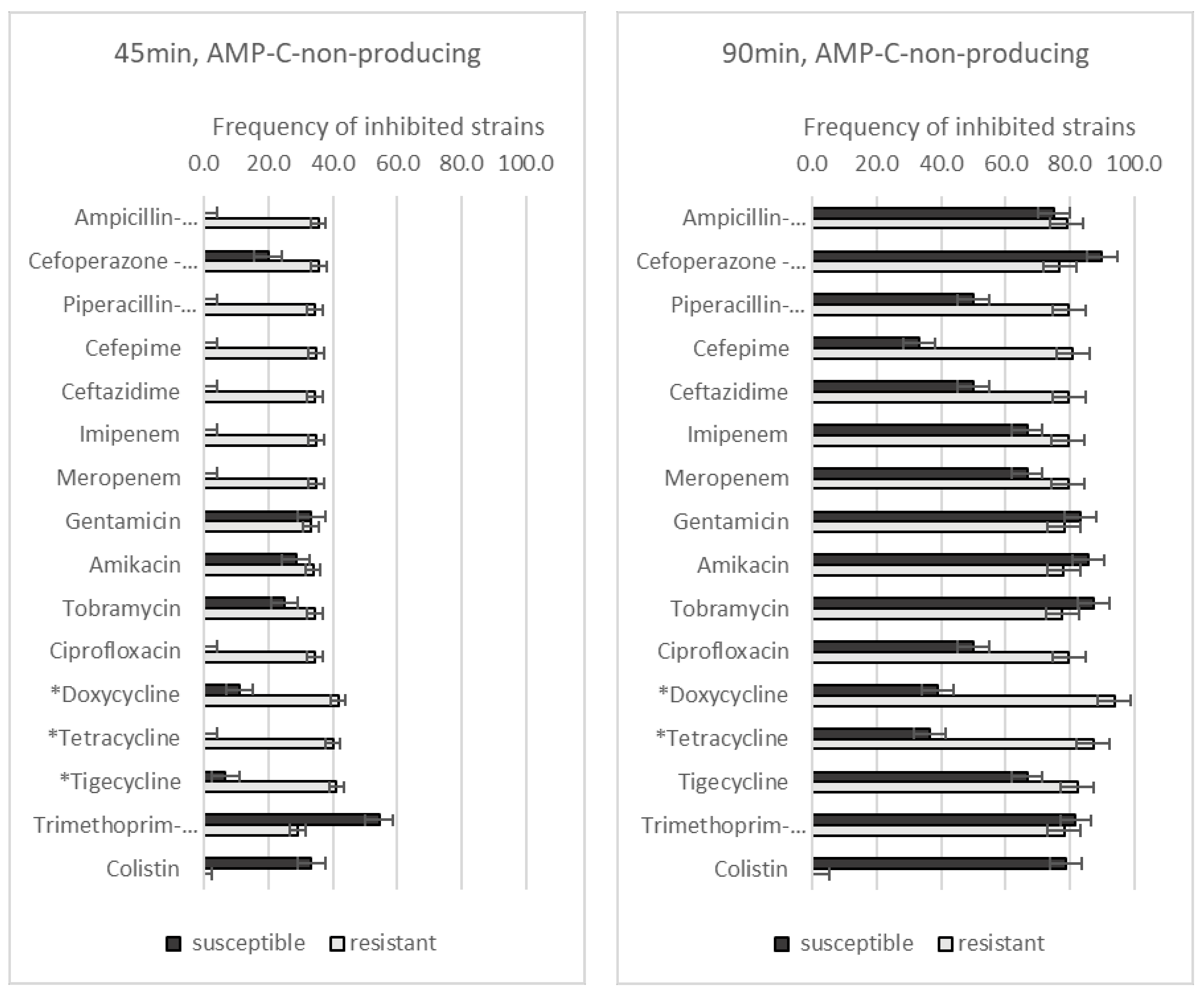
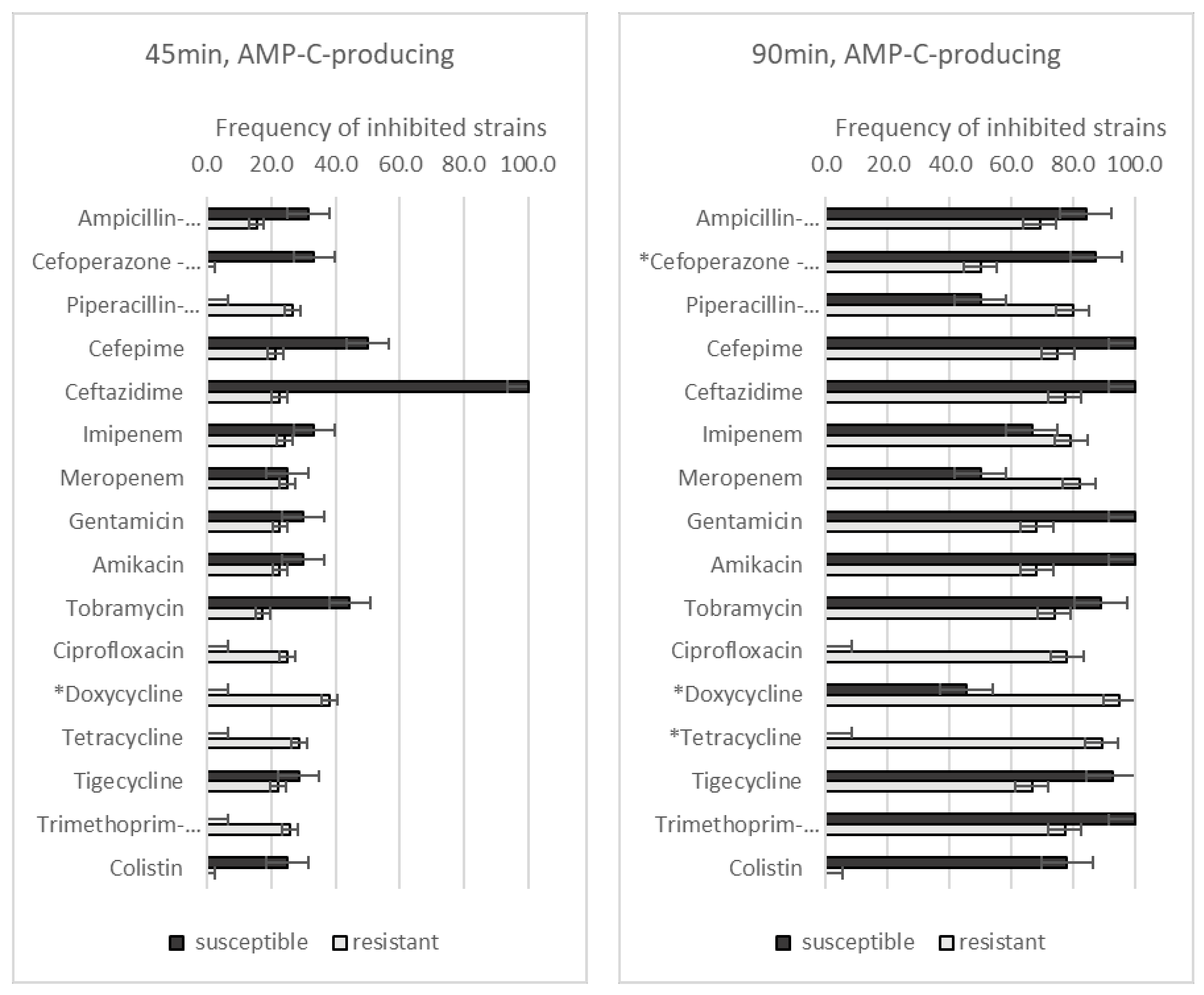
3.3. Effect of GO–Ag Hybrid Nanocomposite on Antibiotic-Resistant A. baumannii Strains
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saini, V.; Jain, C.; Singh, N.P.; Alsulimani, A.; Gupta, C.; Dar, S.A.; Haque, S.; Das, S. Paradigm Shift in Antimicrobial Resistance Pattern of Bacterial Isolates during the COVID-19 Pandemic. Antibiotics 2021, 10, 954. [Google Scholar] [CrossRef]
- Gould, I.M. A review of the role of antibiotic policies in the control of antibiotic resistance. J. Antimicrob. Chemother. 1999, 43, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, N.K.; Arora, N.K.; Chandy, S.J.; Fairoze, M.N.; Gill, J.P.; Gupta, U.; Hossain, S.; Joglekar, S.; Joshi, P.C.; Kakkar, M.; et al. Rationalizing antibiotic use to limit antibiotic resistance in India. Indian J. Med. Res. 2011, 134, 281–294. [Google Scholar] [PubMed]
- Browne, A.J.; Chipeta, M.G.; Haines-Woodhouse, G.; Kumaran, E.P.A.; Hamadani, B.H.K.; Zaraa, S.; Henry, N.J.; Deshpande, A.; Reiner, R.C., Jr.; Day, N.P.J.; et al. Global antibiotic consumption and usage in humans, 2000–2018: A spatial modelling study. Lancet Planet. Health 2021, 5, e893–e904. [Google Scholar] [CrossRef]
- Medic, D.; Bozic Cvijan, B.; Bajcetic, M. Impact of Antibiotic Consumption on Antimicrobial Resistance to Invasive Hospital Pathogens. Antibiotics 2023, 12, 259. [Google Scholar]
- Guo, J.; Liao, M.; He, B.; Liu, J.; Hu, X.; Yan, D.; Wang, J. Impact of the COVID-19 pandemic on household disinfectant consumption behaviors and related environmental concerns: A questionnaire-based survey in China. J. Environ. Chem. Eng. 2021, 9, 106168. [Google Scholar] [CrossRef]
- Aunkor, M.T.H.; Raihan, T.; Prodhan, S.H.; Metselaar, H.S.C.; Malik, S.U.F.; Azad, A.K. Antibacterial activity of graphene oxide nanosheet against multidrug resistant superbugs isolated from infected patients. R. Soc. Open Sci. 2020, 7, 200640. [Google Scholar] [CrossRef]
- Perreault, F.; de Faria, A.F.; Nejati, S.; Elimelech, M. Antimicrobial Properties of Graphene Oxide Nanosheets: Why Size Matters. ACS Nano 2015, 9, 7226–7236. [Google Scholar] [CrossRef]
- Azizi-Lalabadi, M.; Hashemi, H.; Feng, J.; Jafari, S.M. Carbon nanomaterials against pathogens; the antimicrobial activity of carbon nanotubes, graphene/graphene oxide, fullerenes, and their nanocomposites. Adv. Colloid Interface Sci. 2020, 284, 102250. [Google Scholar] [CrossRef]
- Cobos, M.; De-La-Pinta, I.; Quindós, G.; Fernández, M.J.; Fernández, M.D. Graphene Oxide–Silver Nanoparticle Nanohybrids: Synthesis, Characterization, and Antimicrobial Properties. Nanomaterials 2020, 10, 376. [Google Scholar]
- Zavascki, A.P.; Carvalhaes, C.G.; Picão, R.C.; Gales, A.C. Multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii: Resistance mechanisms and implications for therapy. Expert Rev. Anti-Infect. Ther. 2010, 8, 71–93. [Google Scholar] [CrossRef] [PubMed]
- Dijkshoorn, L.; Nemec, A.; Seifert, H. An increasing threat in hospitals: Multidrug-resistant Acinetobacter baumannii. Nat. Rev. Microbiol. 2007, 5, 939–951. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-K.; Kuo, S.-C.; Chang, K.-C.; Cheng, C.-C.; Yu, P.-Y.; Chang, C.-H.; Chen, T.-Y.; Tseng, C.-C. Clinical Antibiotic-resistant Acinetobacter baumannii Strains with Higher Susceptibility to Environmental Phages than Antibiotic-sensitive Strains. Sci. Rep. 2017, 7, 6319. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-R.; Lee, J.H.; Park, M.; Park, K.S.; Bae, I.K.; Kim, Y.B.; Cha, C.-J.; Jeong, B.C.; Lee, S.H. Biology of Acinetobacter baumannii: Pathogenesis, Antibiotic Resistance Mechanisms, and Prospective Treatment Options. Front. Cell. Infect. Microbiol. 2017, 7, 55. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X. Detection of AmpC β-lactamases in Acinetobacter baumannii in the Xuzhou region and analysis of drug resistance. Exp. Ther. Med. 2015, 10, 933–936. [Google Scholar] [CrossRef]
- Eliopoulos, G.M.; Maragakis, L.L.; Perl, T.M. Acinetobacter baumannii: Epidemiology, Antimicrobial Resistance, and Treatment Options. Clin. Infect. Dis. 2008, 46, 1254–1263. [Google Scholar] [CrossRef]
- Lozovskis, P.; Jankauskaitė, V.; Guobienė, A.; Kareivienė, V.; Vitkauskienė, A. Effect of Graphene Oxide and Silver Nanoparticles Hybrid Composite on P. aeruginosa Strains with Acquired Resistance Genes. Int. J. Nanomed. 2020, 15, 5147–5163. [Google Scholar] [CrossRef]
- Sabour, A. Global Risk Maps of Climate Change Impacts on the Distribution of Acinetobacter baumannii Using GIS. Microorganisms 2023, 11, 2174. [Google Scholar]
- Aldali, J.A. Acinetobacter baumannii . Saudi Med. J. 2023, 44, 732–744. [Google Scholar] [CrossRef]
- Hafiz, T.A.; Alghamdi, S.S.; Mubaraki, M.A.; Alghamdi, S.S.M.; Alothaybi, A.; Aldawood, E.; Alotaibi, F. A two-year retrospective study of multidrug-resistant Acinetobacter baumannii respiratory infections in critically Ill patients: Clinical and microbiological findings. J. Infect. Public Health 2023, 16, 313–319. [Google Scholar] [CrossRef]
- Jalali, Y.; Liptáková, A.; Jalali, M.; Payer, J. Moving toward Extensively Drug-Resistant: Four-Year Antimicrobial Resistance Trends of Acinetobacter baumannii from the Largest Department of Internal Medicine in Slovakia. Antibiotics 2023, 12, 1200. [Google Scholar]
- Vázquez-López, R.; Solano-Gálvez, S.G.; Juárez Vignon-Whaley, J.J.; Abello Vaamonde, J.A.; Padró Alonzo, L.A.; Rivera Reséndiz, A.; Muleiro Álvarez, M.; Vega López, E.N.; Franyuti-Kelly, G.; Álvarez-Hernández, D.A.; et al. Acinetobacter baumannii Resistance: A Real Challenge for Clinicians. Antibiotics 2020, 9, 205. [Google Scholar]
- Yadav, S.K.; Bhujel, R.; Hamal, P.; Mishra, S.K.; Sharma, S.; Sherchand, J.B. Burden of Multidrug-Resistant Acinetobacter baumannii Infection in Hospitalized Patients in a Tertiary Care Hospital of Nepal. Infect. Drug Resist. 2020, 13, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Malik, A.; Rizvi, M.; Ahmed, M. Presence of metallo-beta-lactamases (MBL), extended-spectrum beta-lactamase (ESBL) & AmpC positive non-fermenting Gram-negative bacilli among Intensive Care Unit patients with special reference to molecular detection of bla(CTX-M) & bla(AmpC) genes. Indian J. Med. Res. 2016, 144, 271–275. [Google Scholar] [CrossRef]
- Kumar, P.; Huo, P.; Zhang, R.; Liu, B. Antibacterial Properties of Graphene-Based Nanomaterials. Nanomaterials 2019, 9, 737. [Google Scholar]
- Estévez-Martínez, Y.; Vázquez Mora, R.; Méndez Ramírez, Y.I.; Chavira-Martínez, E.; Huirache-Acuña, R.; Díaz-de-León-Hernández, J.N.; Villarreal-Gómez, L.J. Antibacterial nanocomposite of chitosan/silver nanocrystals/graphene oxide (ChAgG) development for its potential use in bioactive wound dressings. Sci. Rep. 2023, 13, 10234. [Google Scholar] [CrossRef]
- Sarshar, M.; Behzadi, P.; Scribano, D.; Palamara, A.T.; Ambrosi, C. Acinetobacter baumannii: An Ancient Commensal with Weapons of a Pathogen. Pathogens 2021, 10, 387. [Google Scholar]
- Bengtsson-Palme, J.; Kristiansson, E.; Larsson, D.G.J. Environmental factors influencing the development and spread of antibiotic resistance. FEMS Microbiol. Rev. 2017, 42, fux053. [Google Scholar] [CrossRef]
- Oz, T.; Guvenek, A.; Yildiz, S.; Karaboga, E.; Tamer, Y.T.; Mumcuyan, N.; Ozan, V.B.; Senturk, G.H.; Cokol, M.; Yeh, P.; et al. Strength of Selection Pressure Is an Important Parameter Contributing to the Complexity of Antibiotic Resistance Evolution. Mol. Biol. Evol. 2014, 31, 2387–2401. [Google Scholar] [CrossRef]
| Antibiotic | All A. baumannii Strains (n = 98) (n (%)) | AMP-C -Producing (n = 32) (n (%)) | AMP-C -Non-Producing (n = 66) (n (%)) | p-Value |
|---|---|---|---|---|
| Ampicillin-sulbactam | 75 (76.5) | 13 (40.6) | 62 (93.9) | <0.001 |
| Cefoperazone-sulbactam | 64 (65.3) | 8 (25.0) | 56 (84.8) | <0.001 |
| Piperacillin-tazobactam | 94 (95.5) | 30 (93.8) | 64 (97.0) | 0.45 |
| Cefepime | 91 (92.9) | 28 (87.5) | 63 (95.5) | 0.152 |
| Ceftazidime | 95 (96.9) | 31 (96.9) | 64 (97.0) | 0.98 |
| Imipenem | 92 (93.9) | 29 (90.6) | 63 (95.5) | 0.35 |
| Meropenem | 91 (92.9) | 28 (87.5) | 63 (95.5) | 0.152 |
| Gentamicin | 82 (83.7) | 22 (68.8) | 60 (90.9) | 0.005 |
| Amikacin | 81 (82.7) | 22 (68.8) | 59 (89.4) | 0.011 |
| Tobramycin | 81 (82.7) | 23 (71.9) | 58 (87.9) | 0.049 |
| Ciprofloxacin | 96 (98.0) | 32 (100.0) | 64 (97.0) | 0.32 |
| Doxycycline | 69 (70.4) | 21 (65.6) | 48 (72.7) | 0.47 |
| Tetracycline | 83 (84.7) | 29 (90.6) | 55 (83.3) | 0.591 |
| Tigecycline | 69 (70.4) | 18 (56.3) | 51 (77.3) | 0.033 |
| Trimethoprim-sulfamethoxazole | 86 (87.8) | 31 (96.9) | 55 (83.3) | 0.055 |
| Colistin | 0 (0.0) | 0 (0.0) | 0 (0.0) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lozovskis, P.; Skrodenienė, E.; Jankauskaitė, V.; Vitkauskienė, A. Effect of Graphene Oxide and Silver Nanoparticle Hybrid Composite on Acinetobacter baumannii Strains, Regarding Antibiotic Resistance and Prevalence of AMP-C Production. Medicina 2023, 59, 1819. https://doi.org/10.3390/medicina59101819
Lozovskis P, Skrodenienė E, Jankauskaitė V, Vitkauskienė A. Effect of Graphene Oxide and Silver Nanoparticle Hybrid Composite on Acinetobacter baumannii Strains, Regarding Antibiotic Resistance and Prevalence of AMP-C Production. Medicina. 2023; 59(10):1819. https://doi.org/10.3390/medicina59101819
Chicago/Turabian StyleLozovskis, Povilas, Erika Skrodenienė, Virginija Jankauskaitė, and Astra Vitkauskienė. 2023. "Effect of Graphene Oxide and Silver Nanoparticle Hybrid Composite on Acinetobacter baumannii Strains, Regarding Antibiotic Resistance and Prevalence of AMP-C Production" Medicina 59, no. 10: 1819. https://doi.org/10.3390/medicina59101819





