Chromatographic Analyses of Spirulina (Arthrospira platensis) and Mechanism of Its Protective Effects against Experimental Obesity and Hepatic Steatosis in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Prepartion of Spirulina (SP)
2.3. Characterization of Phenolic Compounds of Spirulina by HPLC-DAD-ESI MS/MS Analysis
2.4. Preparation of Cafeteria Diet
2.5. Animals and Treatment
2.6. Biochemical Assessment of Liver Tissue
2.7. Metabolic Parameters
2.8. Biochemical Analysis
2.9. Oxidative Stress Assessment
2.10. Antioxidant Enzyme Activity Assays
2.11. Determination of Reactive Oxygen Species
2.12. Histopathological Study
2.13. Assessment of Liver Cytokines
2.14. Data Analysis
3. Results
3.1. HPLC-DAD-ESI-MS/MS Analysis of Spirulina
3.2. Effect of SP and CD on Body, Liver, Abdominal Fat Weight, Weight Gain, and Food Intake
3.3. Effect of SP and a CD on Liver and Plasma Lipid Profiles
3.4. Effect of SP and a CD on Liver Function
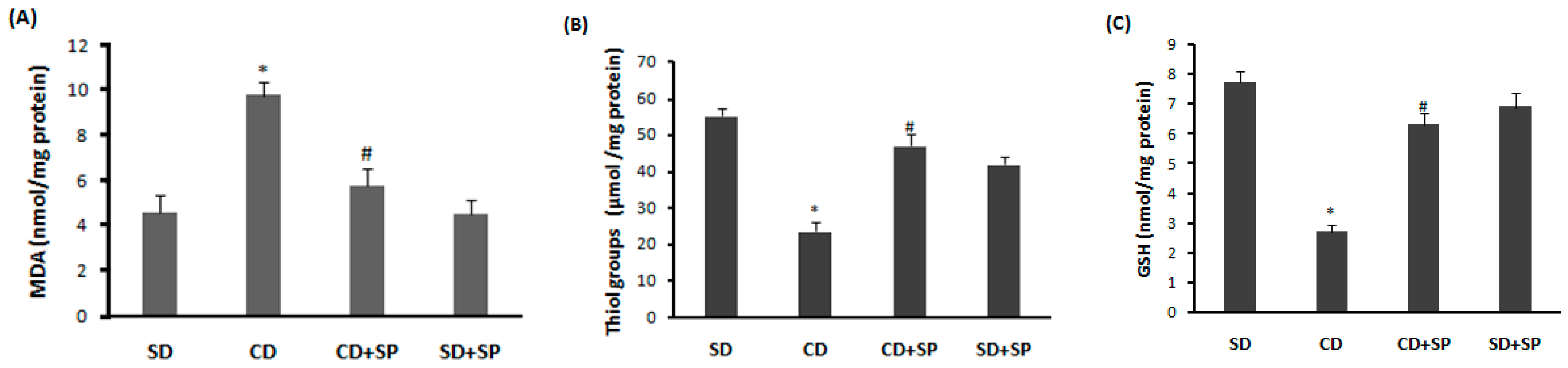
3.5. Effect of SP and a CD on Liver Oxidative Stress
3.6. Effect of SP and a CD on Liver Antioxidant Enzyme Activity
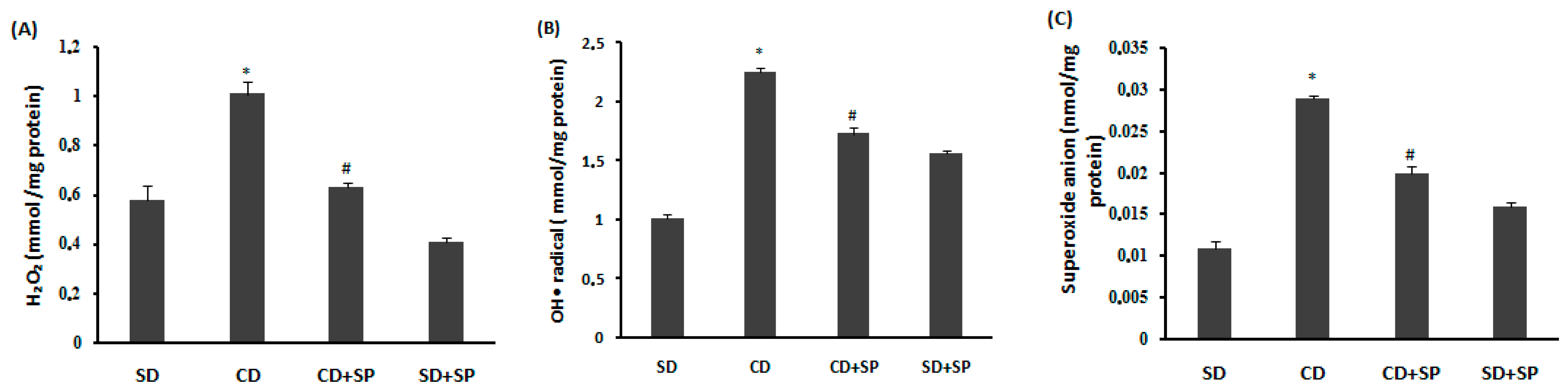
3.7. Effect of SP and a CD on Liver Reactive Oxygen Species (ROS)
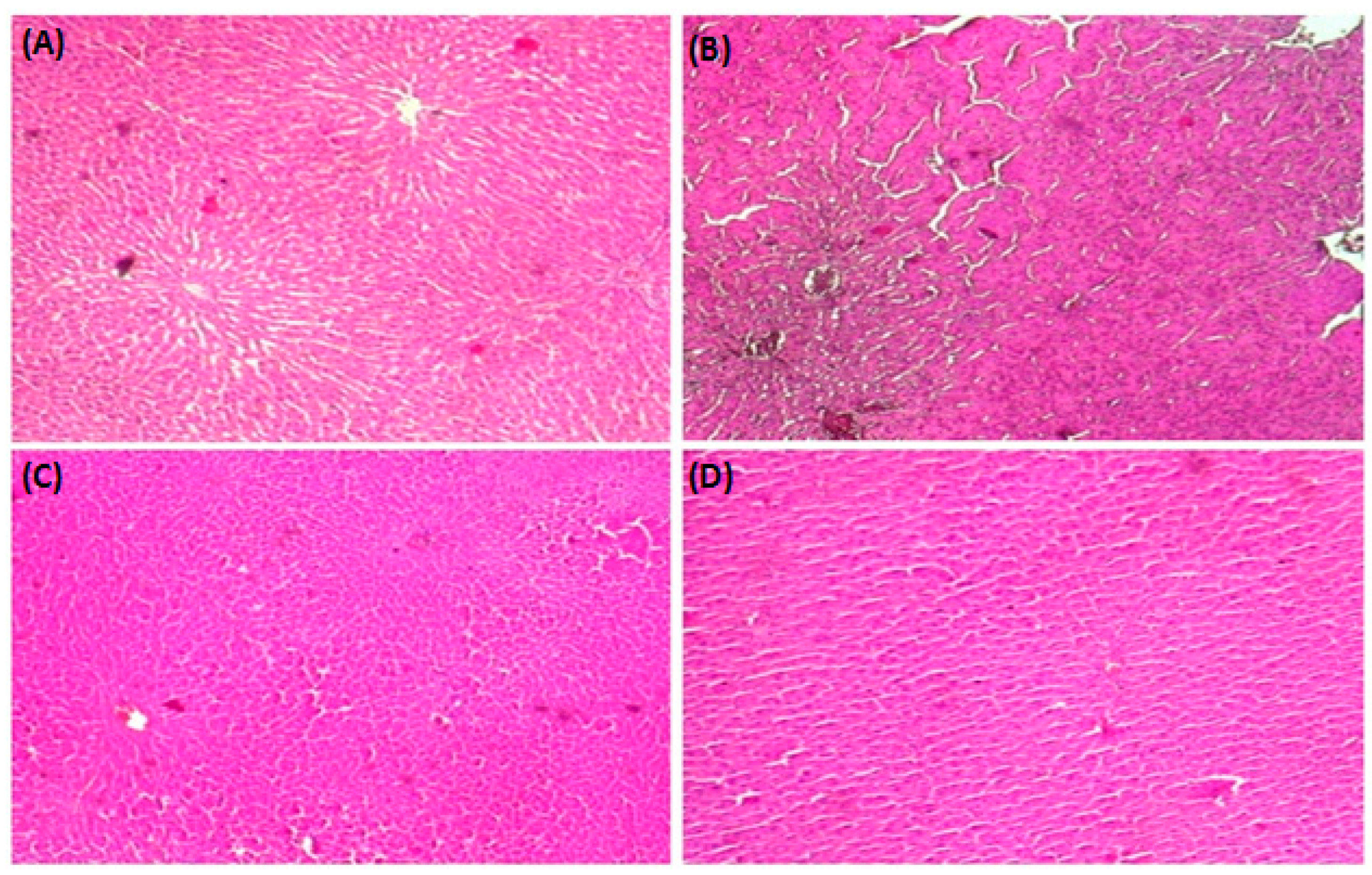
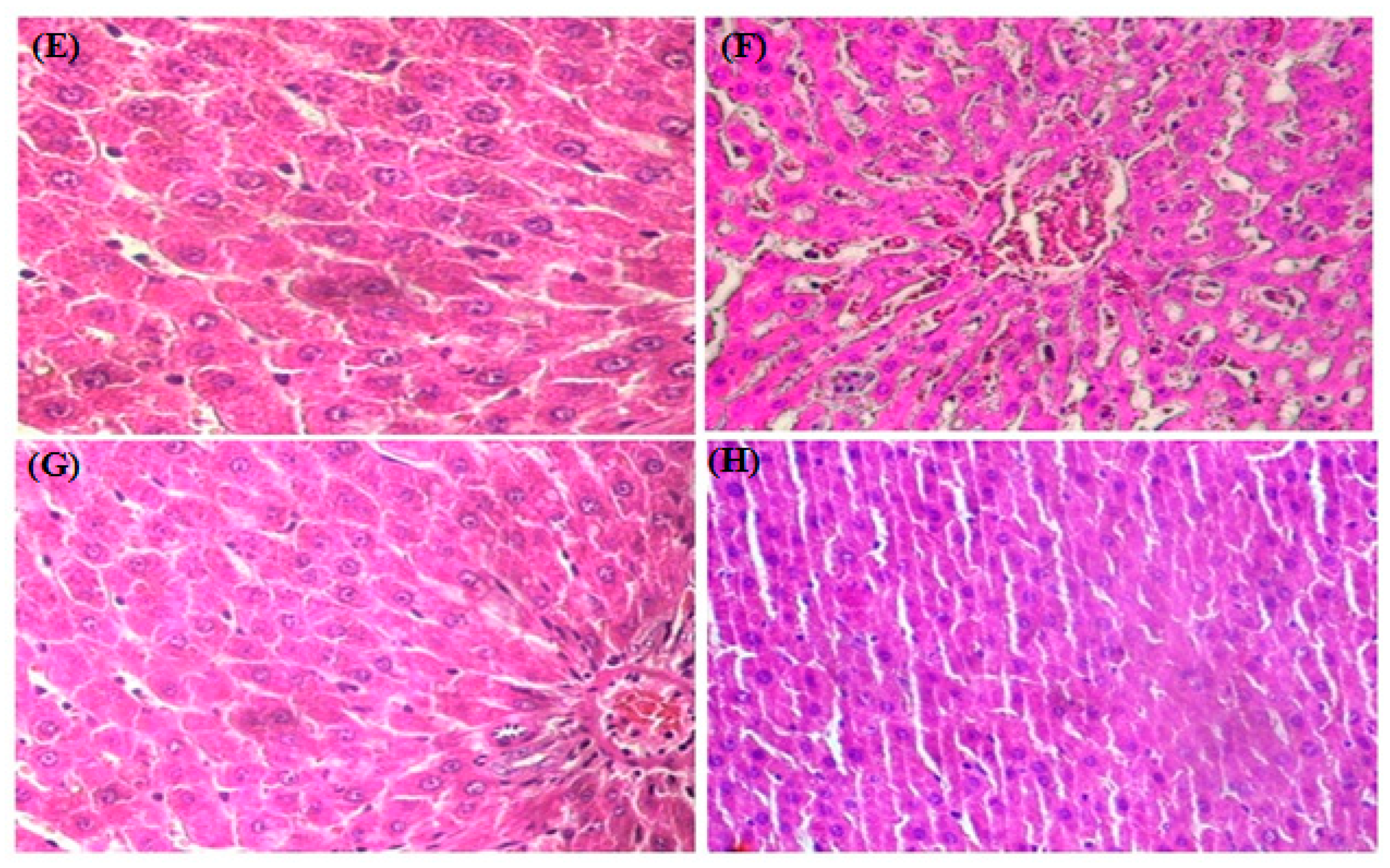
3.8. HistopathologicalStudy
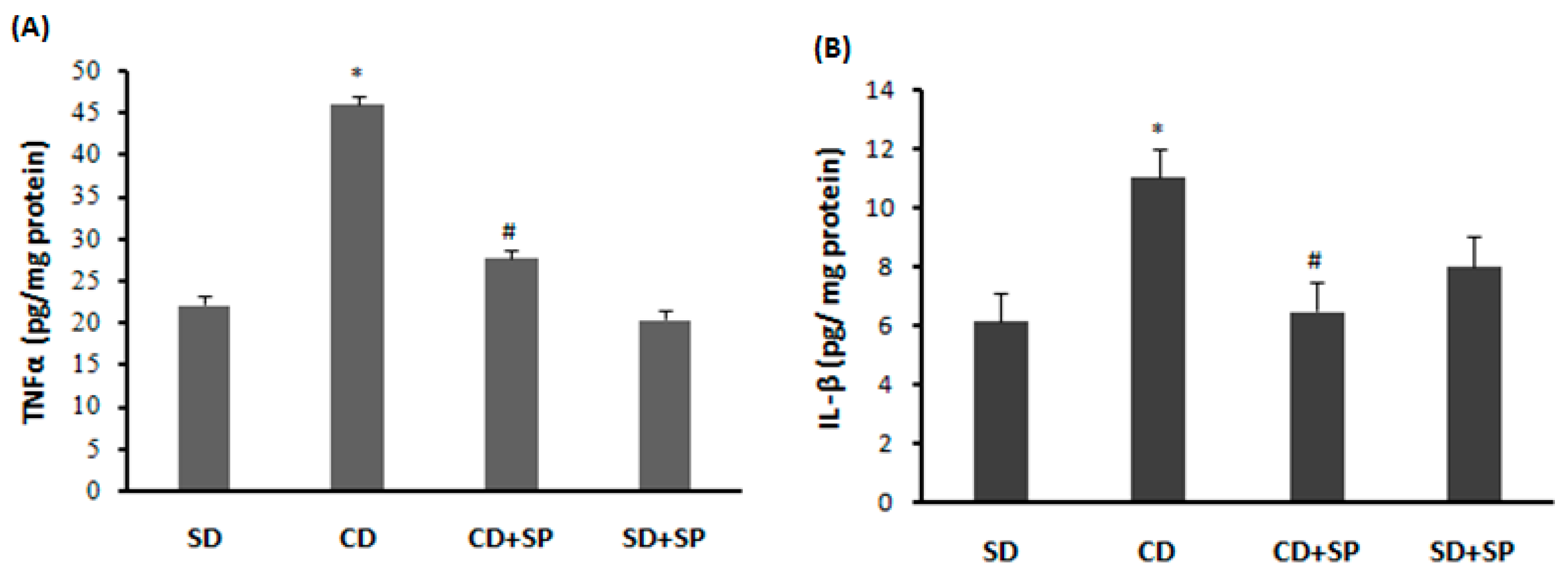
3.9. Effect of SP and a CD on Liver Cytokine Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ataey, A.; Jafarvand, E.; Adham, D.; Moradi-Asl, E. The Relationship Between Obesity, Overweight, and the Human Development Index in World Health Organization Eastern Mediterranean Region Countries. J. Prev. Med. Pub. Health 2020, 53, 98–105. [Google Scholar] [CrossRef]
- Okunogbe, A.; Nugent, R.; Spencer, G.; Ralston, J.; Wilding, J. Economic Impacts of Overweight and Obesity: Current and Future Estimates for Eight Countries. BMJ Glob. Health 2021, 6, e006351. [Google Scholar] [CrossRef]
- Ezzati, M.; Riboli, E. Behavioral and Dietary Risk Factors for Noncommunicable Diseases. N. Engl. J. Med. 2013, 369, 954–964. [Google Scholar] [CrossRef]
- Mitra, M.S.; Donthamsetty, S.; White, B.; Mehendale, H.M. High Fat Diet-Fed Obese Rats Are Highly Sensitive to Doxorubicin-Induced Cardiotoxicity. Toxicol. Appl. Pharmacol. 2008, 231, 413–422. [Google Scholar] [CrossRef]
- Masarone, M.; Rosato, V.; Dallio, M.; Gravina, A.G.; Aglitti, A.; Loguercio, C.; Federico, A.; Persico, M. Role of Oxidative Stress in Pathophysiology of Nonalcoholic Fatty Liver Disease. Oxid. Med. Cell. Longev. 2018, 2018, e9547613. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Tian, R.; She, Z.; Cai, J.; Li, H. Role of Oxidative Stress in the Pathogenesis of Nonalcoholic Fatty Liver Disease. Free Radic. Biol. Med. 2020, 152, 116–141. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The Diagnosis and Management of Nonalcoholic Fatty Liver Disease: Practice Guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328. [Google Scholar] [CrossRef]
- Takaki, A.; Kawai, D.; Yamamoto, K. Multiple Hits, Including Oxidative Stress, as Pathogenesis and Treatment Target in Non-Alcoholic Steatohepatitis (NASH). Int. J. Mol. Sci. 2013, 14, 20704–20728. [Google Scholar] [CrossRef]
- Flachs, P.; Horakova, O.; Brauner, P.; Rossmeisl, M.; Pecina, P.; Franssen-van Hal, N.; Ruzickova, J.; Sponarova, J.; Drahota, Z.; Vlcek, C.; et al. Polyunsaturated Fatty Acids of Marine Origin Upregulate Mitochondrial Biogenesis and Induce β-Oxidation in White Fat. Diabetologia 2005, 48, 2365–2375. [Google Scholar] [CrossRef] [PubMed]
- Castro, H.; Pomar, C.; Picó, C.; Sánchez, J.; Palou, A. Cafeteria Diet over Feeding in Young Male Rats Impairs the Adaptive Response to Fed/Fasted Conditions and Increases Adiposity Independent of Body Weight. Int. J. Obes. 2014, 39, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Darimont, C.; Turini, M.; Epitaux, M.; Zbinden, I.; Richelle, M.; Montell, E.; Ferrer-Martinez, A.; Macé, K. β3-adrenoceptor agonist prevents alterations of muscle diacylglycerol and adipose tissue phospholipids induced by a cafeteria diet. Nutr. Metab. 2004, 1, 4. [Google Scholar] [CrossRef]
- Valdecantos, P.; Pérez-Matute, P.; Martinez, J. Obesity and Oxidative Stress: Role of Antioxidant Supplementation. Rev. Investig. Clínica Organo Hosp. Enfermedades Nutr. 2009, 61, 127–139. [Google Scholar]
- Bonnefont-Rousselot, D. Obésité et stress oxydant. Obésité 2014, 9, 8–13. [Google Scholar] [CrossRef]
- Rigoulet, M.; Yoboue, E.D.; Devin, A. Mitochondrial ROS Generation and Its Regulation: Mechanisms Involved in H2O2 Signaling. Antioxid. Redox Signal. 2011, 14, 459–468. [Google Scholar] [CrossRef]
- Cancello, R.; Henegar, C.; Viguerie, N.; Taleb, S.; Poitou, C.; Rouault, C.; Coupaye, M.; Pelloux, V.; Hugol, D.; Bouillot, J.L.; et al. Reduction of Macrophage Infiltration and Chemoattractant Gene Expression Changes in White Adipose Tissue of Morbidly Obese Subjects After Surgery-Induced Weight Loss. Diabetes 2005, 54, 2277–2286. [Google Scholar] [CrossRef]
- Gauvreau, D.; Villeneuve, N.; Deshaies, Y.; Cianflone, K. Novel Adipokines: Links between Obesity and Atherosclerosis. Ann. Endocrinol. 2011, 72, 224–231. [Google Scholar] [CrossRef]
- Jabri, M.A.; Sakly, M.; Marzouki, L.; Sebai, H. Chamomile (Matricaria recutita L.) Decoction Extract Inhibits In Vitro Intestinal Glucose Absorption and Attenuates High Fat Diet-Induced Lipotoxicity and Oxidative Stress. Biomed. Pharmacother. Biomedecine Pharmacother. 2017, 87, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Kulshrestha, A.; Joseph, A.; Jarouliya, U.; Bhadauriya, P.; Prasad, G.; Bisen, P. Spirulina in Health Care Management. Curr. Pharm. Biotechnol. 2008, 9, 400–405. [Google Scholar] [CrossRef]
- Yousefi, R.; Saidpour, A.; Mottaghi, A. The Effects of Spirulina Supplementation on Metabolic Syndrome Components, Its Liver Manifestation and Related Inflammatory Markers: A Systematic Review. Complement. Ther. Med. 2018, 42, 137–144. [Google Scholar] [CrossRef]
- Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Bound Phenolics in Foods, a Review. Food Chem. 2014, 152, 46–55. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, L.; Miron, A.; Klimova, B.; Wan, D.; Kuca, K. The Antioxidant, Immunomodulatory, and Anti-Inflammatory Activities of Spirulina: An Overview. Arch. Toxicol. 2016, 90, 1817–1840. [Google Scholar] [CrossRef]
- Abebe, W.; Agrawal, D. Role of Tyrosine Kinases in Norepinephrine-Induced Contraction of Vascular Smooth Muscle. J. Cardiovasc. Pharmacol. 1995, 26, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Finamore, A.; Palmery, M.; Bensehaila, S.; Peluso, I. Antioxidant, Immunomodulating, and Microbial-Modulating Activities of the Sustainable and Ecofriendly Spirulina. Oxid. Med. Cell. Longev. 2017, 2017, 1–14. [Google Scholar] [CrossRef]
- Karczewski, J.; Śledzińska, E.; Baturo, A.; Jończyk, I.; Maleszko, A.; Samborski, P.; Begier-Krasińska, B.; Dobrowolska-Zachwieja, A. Obesity and Inflammation. Eur. Cytokine Netw. 2018, 29, 83–94. [Google Scholar] [CrossRef]
- Calella, P.; Cerullo, G.; Di Dio, M.; Liguori, F.; Di Onofrio, V.; Gallè, F.; Liguori, G. Antioxidant, Anti-Inflammatory and Immunomodulatory Effects of Spirulina in Exercise and Sport: A Systematic Review. Front. Nutr. 2022, 9, 1048258. [Google Scholar] [CrossRef]
- Al-Qahtani, W.H.; Binobead, M.A. Anti-Inflammatory, Antioxidant and Antihepatotoxic Effects of Spirulina Platensis against d-Galactosamine Induced Hepatotoxicity in Rats. Saudi J. Biol. Sci. 2019, 26, 647–652. [Google Scholar] [CrossRef]
- Jabri, M.-A.; Rtibi, K.; Tounsi, H.; Hosni, K.; Souli, A.; El-Benna, J.; Marzouki, L.; Sakly, M.; Sebai, H. Myrtle Berry Seed Aqueous Extract Inhibits Human Neutrophil Myeloperoxidase in Vitro and Attenuates Acetic Acid-Induced Ulcerative Colitis in Rats. RSC Adv. 2015, 5, 64865–64877. [Google Scholar] [CrossRef]
- Clifford, M.N.; Knight, S.; Kuhnert, N. Discriminating between the Six Isomers of Dicaffeoylquinic Acid by LC-MS n. J. Agric. Food Chem. 2005, 53, 3821–3832. [Google Scholar] [CrossRef]
- Gouveia, S.; Castilho, P.C. Characterisation of Phenolic Acid Derivatives and Flavonoids from Different Morphological Parts of Helichrysum Obconicum by a RP-HPLC–DAD-(−)–ESI-MSn Method. Food Chem. 2011, 129, 333–344. [Google Scholar] [CrossRef]
- Bouanane, S.; Benkalfat, N.B.; Baba Ahmed, F.-Z.; Merzouk, H.; Mokhtari, N.S.; Merzouk, S.-A.; Gresti, J.; Tessier, C.; Narce, M. Time Course of Changes in Serum Oxidant/Antioxidant Status in Overfed Obese Rats and Their Offspring. Clin. Sci. 2009, 116, 669–680. [Google Scholar] [CrossRef]
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique; Norecopa: Methuen, MA, USA, 1959. [Google Scholar]
- Hajji, N.; Jabri, M.-A.; Tounsi, H.; Wanes, D.; Ben El Hadj Ali, I.; Boulila, A.; Marzouki, L.; Sebai, H. Phytochemical Analysis by HPLC-PDA/ESI-MS of Globularia Alypum Aqueous Extract and Mechanism of Its Protective Effect on Experimental Colitis Induced by Acetic Acid in Rat. J. Funct. Foods 2018, 47, 220–228. [Google Scholar] [CrossRef]
- Hartree, E.F. Determination of Protein: A Modification of the Lowry Method That Gives a Linear Photometric Response. Anal. Biochem. 1972, 48, 422–427. [Google Scholar] [CrossRef]
- Draper, H.; Hadley, M. Malondialdehyde Determination as Index of Lipid Peroxidation. Methods Enzymol. 1990, 186, 421–431. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue Sulfhydryl Groups. Arch. Biochim. Biophys. 1959, 82, 10–11. [Google Scholar] [CrossRef]
- Sedlak, J.; Lindsay, R.H. Estimation of Total, Protein-Bound, and Nonprotein Sulfhydryl Groups in Tissue with Ellman’s Reagent. Anal. Biochem. 1968, 25, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in Vitro. In Methods in Enzymology; Oxygen Radicals in Biological Systems; Academic Press: Cambridge, MA, USA, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Misra, H.P.; Fridovich, I. The Role of Superoxide Anion in the Autoxidation of Epinephrine and a Simple Assay for Superoxide Dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef]
- Flohé, L.; Günzler, W.A. Assays of Glutathione Peroxidase. In Methods in Enzymology; Oxygen Radicals in Biological Systems; Academic Press: Cambridge, MA, USA, 1984; Volume 105, pp. 114–120. [Google Scholar]
- Dingeon, B.; Ferry, J.P.; Roullet, A. Automatic assay of blood sugar by Trinder’s method. Ann. Biol. Clin. 1975, 33, 3–13. [Google Scholar]
- Payá, M.; Halliwell, B.; Hoult, J.R.S. Interactions of a Series of Coumarins with Reactive Oxygen Species. Biochem. Pharmacol. 1992, 44, 205–214. [Google Scholar] [CrossRef]
- Marklund, S.; Marklund, G. Involvement of the Superoxide Anion Radical in the Autoxidation of Pyrogallol and a Convenient Assay for Superoxide Dismutase. Eur. J. Biochem. 2005, 47, 469–474. [Google Scholar] [CrossRef]
- Fischer, A.H.; Jacobson, K.A.; Rose, J.; Zeller, R. Hematoxylin and Eosin Staining of Tissue and Cell Sections. CSH Protoc. 2008, 2008, pdb.prot4986. [Google Scholar] [CrossRef]
- Manner, S.; Skogman, M.; Goeres, D.; Vuorela, P.; Fallarero, A. Systematic Exploration of Natural and Synthetic Flavonoids for the Inhibition of Staphylococcus Aureus Biofilms. Int. J. Mol. Sci. 2013, 14, 19434–19451. [Google Scholar] [CrossRef]
- Corradini, E.; Foglia, P.; Giansanti, P.; Gubbiotti, R.; Samperi, R.; Laganà, A. Flavonoids: Chemical Properties and Analytical Methodologies of Identification and Quantitation in Foods and Plants. Nat. Prod. Res. 2011, 25, 469–495. [Google Scholar] [CrossRef]
- Cartea, M.E.; Francisco, M.; Soengas, P.; Velasco, P. Phenolic Compounds in Brassica Vegetables. Molecules 2010, 16, 251–280. [Google Scholar] [CrossRef]
- De la Rosa, L.A.; Moreno-Escamilla, J.O.; Rodrigo-García, J.; Alvarez-Parrilla, E. Phenolic Compounds. In Postharvest Physiology and Biochemistry of Fruits and Vegetables; Elsevier: Amsterdam, The Netherlands, 2019; pp. 253–271. [Google Scholar]
- Liu, M.; Zhu, K.; Yao, Y.; Chen, Y.; Guo, H.; Ren, G.; Yang, X.; Li, J. Antioxidant, Anti-inflammatory, and Antitumor Activities of Phenolic Compounds from White, Red, and Black Chenopodium Quinoa Seed. Cereal Chem. 2020, 97, 703–713. [Google Scholar] [CrossRef]
- Aguilera, Y.; Martin-Cabrejas, M.A.; González de Mejia, E. Phenolic Compounds in Fruits and Beverages Consumed as Part of the Mediterranean Diet: Their Role in Prevention of Chronic Diseases. Phytochem. Rev. 2016, 15, 405–423. [Google Scholar] [CrossRef]
- Sobeh, M.; Esmat, A.; Petruk, G.; Abdelfattah, M.A.; Dmirieh, M.; Monti, D.M.; Abdel-Naim, A.B.; Wink, M. Phenolic Compounds from Syzygium Jambos (Myrtaceae) Exhibit Distinct Antioxidant and Hepatoprotective Activities in Vivo. J. Funct. Foods 2018, 41, 223–231. [Google Scholar] [CrossRef]
- Golay, A. Rôle Des Graisses Alimentaires Dans Le Développement de l’obésité. OCL Ol. Corps Gras Lipides 1998, 5, 205–207. [Google Scholar]
- Laissouf, A.; Nassima, M.-S.; Merzouk, H.; Nouzha, B. Dietary flaxseed oil supplementation improves the oxidant/antioxidant status in obese aged rats. IJMPS 2013, 3, 87–94. [Google Scholar]
- Armitage, J.; Taylor, P.; Poston, L. Experimental Models of Development Programming: Consequences of Exposure to an Energy Rich Diet during Development. J. Physiol. 2005, 565, 3–8. [Google Scholar] [CrossRef]
- Nagaoka, S.; Shimizu, K.; Kaneko, H.; Shibayama, F.; Morikawa, K.; Kanamaru, Y.; Ayako; Hirahashi, T.; Kato, T. A Novel Protein C-Phycocyanin Plays a Crucial Role in the Hypocholesterolemic Action of Spirulina Platensis Concentrate in Rats. J. Nutr. 2005, 135, 2425–2430. [Google Scholar] [CrossRef]
- You, J.; Lee, Y.; Kim, K.; Kim, S.; Chang, K. Ethanol Extract of Lotus (Nelumbo Nucifera) Root Exhibits an Anti-Adipogenic Effect in Human Pre-Adipocytes and Anti-Obesity and Anti-Oxidant Effects in Rats Fed a High-Fat Diet. Nutr. Res. N. Y. 2014, 34, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Lee, M.-S.; Shin, Y.; Kim, C.-T.; Kim, I.-H.; Kim, Y.; Kim, Y. Anti-Obesity and Anti-Inflammatory Effects of High Hydrostatic Pressure Extracts of Ginseng in High-Fat Diet Induced Obese Rats. J. Funct. Foods 2014, 10, 169–177. [Google Scholar] [CrossRef]
- Karmase, A.; Birari, R.; Bhutani, K. Evaluation of Anti-Obesity Effect of Aegle Marmelos Leaves. Phytomedicine Int. J. Phytother. Phytopharm. 2013, 20, 805–812. [Google Scholar] [CrossRef] [PubMed]
- González-Arceo, M.; Gómez-Zorita, S.; Aguirre, L.; Portillo, M.P. Effect of Microalgae and Macroalgae Extracts on Non-Alcoholic Fatty Liver Disease. Nutrients 2021, 13, 2017. [Google Scholar] [CrossRef] [PubMed]
- Koo, S.Y.; Hwang, J.-H.; Yang, S.-H.; Um, J.-I.; Hong, K.W.; Kang, K.; Pan, C.-H.; Hwang, K.T.; Kim, S.M. Anti-Obesity Effect of Standardized Extract of Microalga Phaeodactylum Tricornutum Containing Fucoxanthin. Mar. Drugs 2019, 17, 311. [Google Scholar] [CrossRef]
- El-Baz, F.K.; Salama, A.; Salama, R.A.A. Therapeutic Effect of Dunaliella Salina Microalgae on Thioacetamide- (TAA-) Induced Hepatic Liver Fibrosis in Rats: Role of TGF-β and MMP9. BioMed Res. Int. 2019, 2019, e7028314. [Google Scholar] [CrossRef]
- Alfadda, A.A.; Sallam, R.M. Reactive Oxygen Species in Health and Disease. J. Biomed. Biotechnol. 2012, 2012, 1–14. [Google Scholar] [CrossRef]
- Charradi, K.; Elkahoui, S.; Karkouch, I.; Limam, F.; Hamdaoui, G.; Hassine, F.; El May, M.; May, A.; Aouani, E. Grape Seed and Skin Extract Alleviates High-Fat Diet-Induced Renal Lipotoxicity and Prevents Copper Depletion in Rat. Appl. Physiol. Nutr. Metab. Physiol. Appliquée Nutr. Métabolisme 2013, 38, 259–267. [Google Scholar] [CrossRef]
- Violi, F.; Basili, S.; Nigro, C.; Pignatelli, P. Role of NADPH Oxidase in Atherosclerosis. Future Cardiol. 2009, 5, 83–92. [Google Scholar] [CrossRef]
- Chang, C.; Tzeng, T.-F.; Liou, S.-S.; Liu, I.-M. Kaempferol Regulates the Lipid-Profile in High-Fat Diet-Fed Rats through an Increase in Hepatic PPAR α Levels. Planta Med. 2011, 77, 1876–1882. [Google Scholar] [CrossRef]
- Feingold, K.; Grunfeld, C. Introduction to Lipids and Lipoproteins; MDText. com. Inc.: South Dartmouth, MA, USA, 2020; undefined 2015. [Google Scholar]
- Iwata, K.; Inayama, T.; Kato, T. Effects of Spirulina Platensis on Plasma Lipoprotein Lipase Activity in Fructose-Induced Hyperlipidemic Rats. J. Nutr. Sci. Vitaminol. 1990, 36, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Mazokopakis, E.; Starakis, I.; Papadomanolaki, M.; Mavroeidi, N.; Emmanuel, S. The Hypolipidaemic Effects of Spirulina (Arthrospira platensis) Supplementation in a Cretan Population: A Prospective Study. J. Sci. Food Agric. 2014, 94, 432–437. [Google Scholar] [CrossRef]
- Azab, S.; Abdel Daim, M.; Eldahshan, O. Phytochemical, Cytotoxic, Hepatoprotective and Antioxidant Properties of Delonix Regia Leaves Extract. Med. Chem. Res. 2013, 22, 4269–4277. [Google Scholar] [CrossRef]
- Payen, J.-L.; Muscari, F.; Vibert, É.; Ernst, O.; Pelletier, G. Lithiase biliaire. Presse Médicale 2011, 40, 567–580. [Google Scholar] [CrossRef] [PubMed]
- Parikh, P.; Mani, U.; Iyer, U. Role of Spirulina in the Control of Glycemia and Lipidemia in Type 2 Diabetes Mellitus. J. Med. Food 2001, 4, 193–199. [Google Scholar] [CrossRef]
- Bhattacharrya, S.; Mehta, P. The Hepatoprotective Potential of Spirulina and Vitamin C Supplemention in Cisplatin Toxicity. Food Funct. 2011, 3, 164–169. [Google Scholar] [CrossRef]
- Moor, V.; Biapa, P.; Legrand, N.; Moukette, B.; Sando, Z.; Kenfack, C.; Ateba Amana, B.; Matip, M.; Pieme, A.; Jeanne, N. Hypolipidemic Effect and Activation of Lecithin Cholesterol Acyl Transferase (LCAT) by Aqueous Extract of Spirulina Platensis during Toxicological Investigation. BMC Nutr. 2017, 3, 25. [Google Scholar] [CrossRef]
- Bucher, S.; Le Guillou, D.; Allard, J.; Pinon, G.; Begriche, K.; Tête, A.; Sergent, O.; Lagadic-Gossmann, D.; Fromenty, B. Possible Involvement of Mitochondrial Dysfunction and Oxidative Stress in a Cellular Model of NAFLD Progression Induced by Benzo[a]Pyrene/Ethanol CoExposure. Oxid. Med. Cell. Longev. 2018, 2018, 4396403. [Google Scholar] [CrossRef]
- Rakic, D.; Joksimovic Jovic, J.; Jakovljevic, V.; Zivkovic, V.; Nikolic, M.; Sretenovic, J.; Nikolic, M.; Jovic, N.; Bicanin Ilic, M.; Arsenijevic, P.; et al. High Fat Diet Exaggerate Metabolic and Reproductive PCOS Features by Promoting Oxidative Stress: An Improved EV Model in Rats. Medicina 2023, 59, 1104. [Google Scholar] [CrossRef]
- Mahadev, K.; Motoshima, H.; Wu, X.; Ruddy, J.; Arnold, R.; Cheng, G.; Lambeth, J.; Goldstein, B. The NAD(P)H Oxidase Homolog Nox4 Modulates Insulin-Stimulated Generation of H2O2 and Plays an Integral Role in Insulin Signal Transduction. Mol. Cell. Biol. 2004, 24, 1844–1854. [Google Scholar] [CrossRef]
- Lih-Brody, L.; Powell, S.; Collier, K.; Reddy, G.; Cerchia, R.; Kahn, E.; Weissman, G.; Katz, S.; Floyd, R.; McKinley, M.; et al. Increased Oxidative Stress and Decreased Antioxidant Defenses in Mucosa of Inflammatory Bowel Disease. Dig. Dis. Sci. 1996, 41, 2078–2086. [Google Scholar] [CrossRef] [PubMed]
- Reimund, J.-M. Stress Oxydant Au Cours Des Syndromes Inflammatoires Chroniques. Nutr. Clin. Metab. 2002, 16, 275–284. [Google Scholar] [CrossRef]
- Davì, G.; Guagnano, M.; Ciabattoni, G.; Basili, S.; Falco, A.; Marinopiccoli, M.; Nutini, M.; Sensi, S.; Patrono, C. Platelet Activation in Obese Women: Role of Inflammation and Oxidant Stress. JAMA J. Am. Med. Assoc. 2002, 288, 2008–2014. [Google Scholar] [CrossRef] [PubMed]
- Riss, J.; Decorde, K.; Sutra, T.; Delage, M.; Baccou, J.C.; Jouy, N.; Brune, J.-P.; Oreal, H.; Cristol, J.; Rouanet, J.-M. Phycobiliprotein C-Phycocyanin from Spirulina Platensis Is Powerfully Responsible for Reducing Oxidative Stress and NADPH Oxidase Expression Induced by an Atherogenic Diet in Hamsters. J. Agric. Food Chem. 2007, 55, 7962–7967. [Google Scholar] [CrossRef]
- Dartsch, P. Antioxidant Potential of SelectedSpirulina Platensis Preparations. Phytother. Res. PTR 2008, 22, 627–633. [Google Scholar] [CrossRef]
- Reddy, B.; Yuvaraj, N.; Vazhoor, B.; Ramnath, V.; Philomina, P.T.; Mc, S. Antioxidant and Hypolipidemic Effects of Spirulina and Natural Carotenoids in Broiler Chicken. Indian Vet. J. 2004, 81, 383–386. [Google Scholar]
- Miranda, M.; Cintra, R.; Barros, S.B.M.; Filho, J. Antioxidant Activity of the Microalga Spirulina Maxima. Braz. J. Med. Biol. Res. 1998, 31, 1075–1079. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, Y.; Zhang, R.; Cai, T.; Cai, Y. Medical Application of Spirulina Platensis Derived C-Phycocyanin. Evid. Based Complement. Alternat. Med. 2016, 2016, 1–14. [Google Scholar] [CrossRef]
- Belay, A. The Potential Application of Spirulina (Arthrospira) as a Nutritional and Therapeutic Supplement in Health Management. J. Am. Nutraceutical. Assoc. 2002, 5, 24. [Google Scholar]
- Dröge, W. Free Radicals in the Physiological Control of Cell Function. Physiol. Rev. 2002, 82. [Google Scholar] [CrossRef]
- Bhathena, S.; Ali, A.A.; Haudenschild, C.; Latham, P.; Ranich, T.; Hansen, C.; Velasquez, M. Dietary Flaxseed Meal Is More Protective Than Soy Protein Concentrate Against Hypertriglyceridemia and Steatosis of the Liver in an Animal Model of Obesity. J. Am. Coll. Nutr. 2003, 22, 157–164. [Google Scholar] [CrossRef]
- Ebrahimi-Mameghani, M.; Sadeghi, Z.; Abbasalizad Farhangi, M.; Vaghef-Mehrabany, E.; Aliashrafi, S. Glucose Homeostasis, Insulin Resistance and Inflammatory Biomarkers in Patie’nts with Non-Alcoholic Fatty Liver Disease: Beneficial Effects of Supplementation with Microalgae Chlorella Vulgaris: A Double- Blind Placebo- Controlled Randomized Clinical Trial. Clin. Nutr. 2016, 36, 1001–1006. [Google Scholar] [CrossRef]
- Haimeur, A.; MIMOUNI, V.; Ulmann, L.; Martineau, A.-S.; Messaouri, H.; Pineau-Vincent, F.; Gérard, T.; Meskini, N. Fish Oil and Microalga Omega-3 as Dietary Supplements: A Comparative Study on Cardiovascular Risk Factors in High-Fat Fed Rats. Lipids 2016, 51, 1037–1049. [Google Scholar] [CrossRef]
- Zafrani, E. Non-Alcoholic Fatty Liver Disease: An Emerging Pathological Spectrum. Virchows Arch. Int. J. Pathol. 2004, 444, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Piya, M.; McTernan, P.; Kumar, S. Adipokine Inflammation and Insulin Resistance: The Role of Glucose, Lipids and Endotoxin. J. Endocrinol. 2012, 216, T1–T15. [Google Scholar] [CrossRef]
- Dal-Ros, S.; Van der Werf, R.; Walter, C.; Bietiger, W.; Seyfritz, E.; Mura, C.; Peronet, C.; Legrandois, J.; Werner, D.; Ennahar, S.; et al. P234 Apports bénéfiques des antioxydants naturels dans la prévention du diabète de type 2 chez l’animal. Diabetes Metab. 2014, 40, A83. [Google Scholar] [CrossRef]
- Gonzalez, R.; Rodríguez, S.; Romay, C.; González, A.; Armesto, J.; Remirez, D.; Merino, N. Anti-Inflammatory Activity of Phycocyanin Extract in Acetic Acid-Induced Colitis in Rats. Pharmacol. Res. Off. J. Ital. Pharmacol. Soc. 1999, 39, 1055–1059. [Google Scholar] [CrossRef]
- Manconi, M.; Pendás, J.; Ledón, N.; Moreira, T.; Sinico, C.; Saso, L.; Fadda, A. Phycocyanin Liposomes for Topical Anti-Inflammatory Activity: In-Vitro in-Vivo Studies. J. Pharm. Pharmacol. 2009, 61, 423–430. [Google Scholar] [CrossRef]
- Nishimura, S.; Manabe, I.; Nagasaki, M.; Eto, K.; Yamashita, H.; Ohsugi, M.; Otsu, M.; Hara, K.; Ueki, K.; Sugiura, S.; et al. CD8+ Effector T Cells Contribute to Macrophage Recruitment and Adipose Tissue Inflammation in Obesity. Nat. Med. 2009, 15, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Grover, P.; Bhatnagar, A.; Kumari, N.; Narayan Bhatt, A.; Kumar Nishad, D.; Purkayastha, J. C-Phycocyanin-a Novel Protein from Spirulina Platensis- In Vivo Toxicity, Antioxidant and Immunomodulatory Studies. Saudi J. Biol. Sci. 2021, 28, 1853–1859. [Google Scholar] [CrossRef] [PubMed]
- Romay, C.; Gonzalez, R.; Ledon, N.; Remirez, D.; Rimbau, V. C-Phycocyanin: A Biliprotein with Antioxidant, Anti-Inflammatory and Neuroprotective Effects. Curr. Protein Pept. Sci. 2003, 4, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Barnes, G.; Yang, Q.; Tan, G.; Yang, D.; Chou, C.; Sole, J.; Nichols, A.; Ross, J.; Tartaglia, L.; et al. Chronic Inflammation in Fat Plays a Crucial Role in the Development of Obesity-Related Insulin Resistance. J. Clin. Invest. 2004, 112, 1821–1830. [Google Scholar] [CrossRef]
- Romay, C.; Delgado Hernandez, R.; Remirez, D.; Gonzalez, R.; Rojas, A. Effects of Phycocyanin Extract on Tumor Necrosis Factor-α And Nitrite Levels in Serum of Mice Treated with Endotoxin. Arzneimittelforschung 2001, 51, 733–736. [Google Scholar] [CrossRef] [PubMed]
- Katsuura-Kamano, S.; Imamura, T.; Bando, N.; Yamanishi, R. β-Carotene and β-Cryptoxanthin but Not Lutein Evoke Redox and Immune Changes in RAW264 Murine Macrophages. Mol. Nutr. Food Res. 2009, 53, 1396–1405. [Google Scholar] [CrossRef]
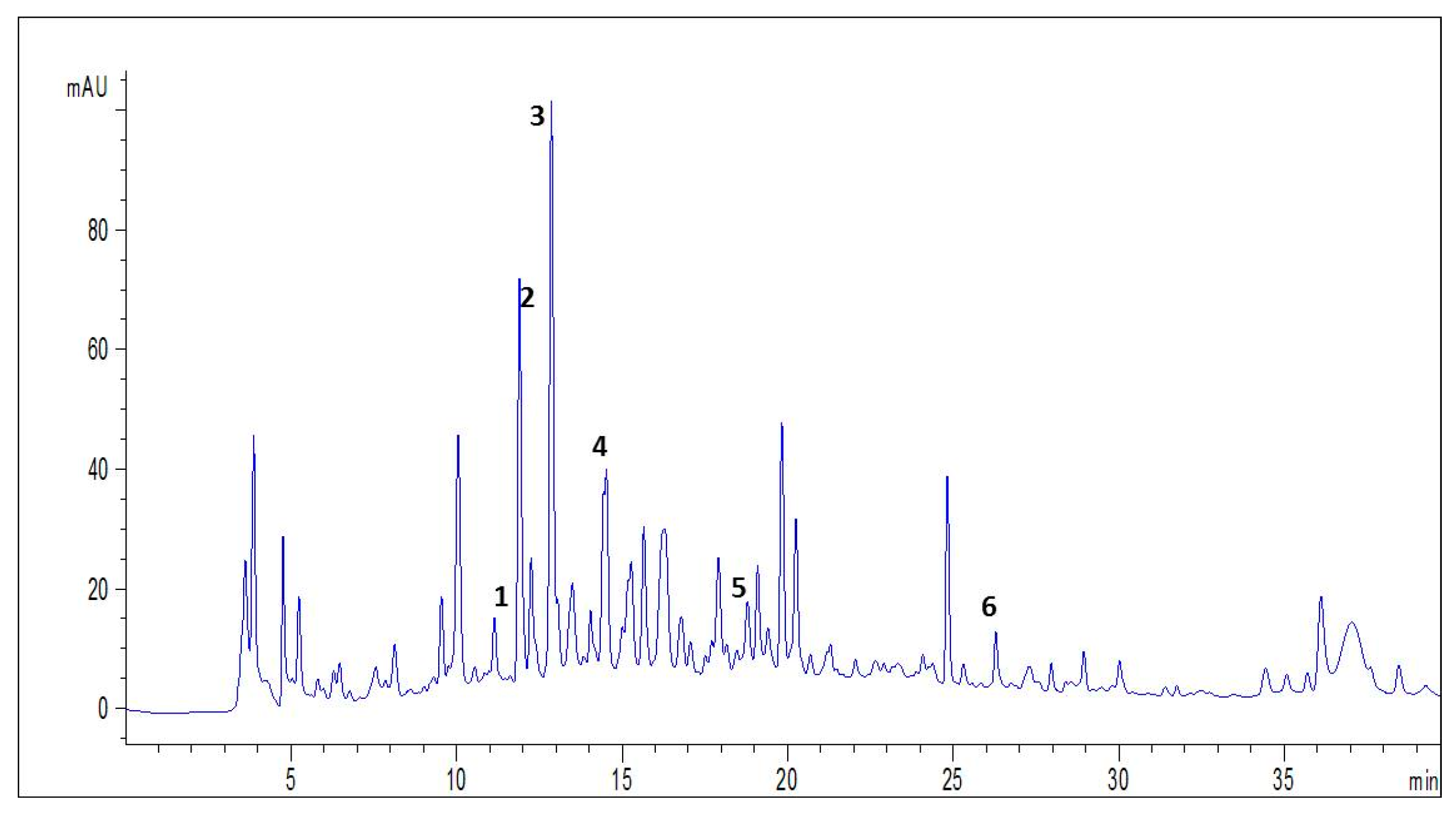

| NO | Compounds | Molecular Formula | Molecular Mass | [M − H] −m/z | Retention Time (min) | % Composition |
|---|---|---|---|---|---|---|
| 1 | Resorcinol | C6H6O2 | 110 | 109 | 11,554 | 26.14 |
| 2 | Chlorogenic Acid | C16H18O9 | 354 | 353 | 11,996 | 13.34 |
| 3 | Catechin | C15H14O6 | 290 | 289 | 12,561 | 26.48 |
| 4 | Syringic Acid | C9H10O5 | 198 | 197 | 14,609 | 5.59 |
| 5 | Sinapic Acid | C11H12O5 | 170 | 169 | 18,840 | 18.33 |
| 6 | Quercetin | C15H10O7 | 302 | 301 | 26,161 | 10.09 |
| SD | CD | CD + SP | SD + SP | |
|---|---|---|---|---|
| Final Body weight (g) | 268.16 ± 3.73 | 301.13 ± 7.31 * | 270.87 ± 3.27 # | 261.61 ± 2.84 |
| Body weigh gain (g) | 26.37 ± 2.01 | 52 ± 2.14 * | 23.5 ± 2.14 # | 16.71 ± 1.98 * |
| Liver weight (g) | 5.01 ± 1.11 | 9.22 ± 0.29 * | 7.73 ± 0.23 # | 6.53 ± 0.34 # |
| Total abdominal fat weight (g) | 4.21 ± 0.24 | 13.49 ± 1.92 * | 8.57 ± 0.44 # | 4.78 ± 0.17# |
| Food intake (g) | 110.29 ± 8.18 | 140.94 ± 4.79 * | 124.11 ± 4.96 # | 111.97 ± 4.68 # |
| Pretreatment | TC (mM/L) | TG (mM/L) | HDL-C (mM/L) | LDL-C (mM/L) | ||||
|---|---|---|---|---|---|---|---|---|
| Plasma | Liver | Plasma | Liver | Plasma | Liver | Plasma | Liver | |
| SD | 0.95 ± 0.06 | 1.18 ± 0.08 | 0.62 ± 0.06 | 2.05 ± 0,21 | 0.55 ± 0.03 | 0.61 ± 0.06 | 0.52 ± 0.05 | 0.62 ± 0.06 |
| CD | 1.83 ± 0.04 * | 2.16 ± 0.15 * | 1.03 ± 0.07 * | 3.85 ± 0.36 * | 0.33 ± 0.03 * | 0.54 ± 0.03 | 0.81 ± 0.04 * | 0.66 ± 0.05 |
| CD + SP | 1.14 ± 0.03 # | 1.34 ± 0.14 # | 0.65 ± 0.05 # | 2.46 ± 0.24 # | 0.51 ± 0.03 # | 0.59 ± 0.03 | 0.57 ± 0.02 # | 0.63 ± 0.04 |
| SD + SP | 1.01 ± 0.11 # | 1.19 ± 0.06 # | 0.62 ± 0.07 # | 2.09 ± 0.22 # | 0.57 ± 0.02 # | 0.60 ± 0.01 | 0.49 ± 0.03 # | 0.54 ± 0.04 |
| GLY (Mm/L) | ASAT (U/L) | ALAT (U/L) | Bilirubin D (µM/L) | |
|---|---|---|---|---|
| SD | 5.53 ± 0.20 | 147.66 ± 6.25 | 51.5 ± 4.35 | 0.16 ± 0.04 |
| CD | 7.89 ± 0.12 * | 229.71 ± 12.43 * | 92.85 ± 2.94 * | 0.27 ± 0.02 * |
| CD + SP | 6.42 ± 0.21 # | 166.83 ± 10.38 # | 57.33 ± 2.13 # | 0.17 ± 0.02 # |
| SD + SP | 6.04 ± 0.19 # | 156.66 ± 15.32 # | 50.5 ± 2.66 # | 0.14 ± 0.05 # |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arrari, F.; Jabri, M.-A.; Ayari, A.; Dakhli, N.; Ben Fayala, C.; Boubaker, S.; Sebai, H. Chromatographic Analyses of Spirulina (Arthrospira platensis) and Mechanism of Its Protective Effects against Experimental Obesity and Hepatic Steatosis in Rats. Medicina 2023, 59, 1823. https://doi.org/10.3390/medicina59101823
Arrari F, Jabri M-A, Ayari A, Dakhli N, Ben Fayala C, Boubaker S, Sebai H. Chromatographic Analyses of Spirulina (Arthrospira platensis) and Mechanism of Its Protective Effects against Experimental Obesity and Hepatic Steatosis in Rats. Medicina. 2023; 59(10):1823. https://doi.org/10.3390/medicina59101823
Chicago/Turabian StyleArrari, Fatma, Mohamed-Amine Jabri, Ala Ayari, Nouha Dakhli, Chayma Ben Fayala, Samir Boubaker, and Hichem Sebai. 2023. "Chromatographic Analyses of Spirulina (Arthrospira platensis) and Mechanism of Its Protective Effects against Experimental Obesity and Hepatic Steatosis in Rats" Medicina 59, no. 10: 1823. https://doi.org/10.3390/medicina59101823






