Cluster Analysis of Subjective Shoulder Stiffness and Muscle Hardness: Associations with Central Sensitization-Related Symptoms
Abstract
:1. Introduction
- High subjective stiffness and pain with high muscle hardness;
- Low subjective stiffness and pain with low muscle hardness;
- High subjective stiffness and pain with low muscle hardness;
- Low subjective stiffness and pain with high muscle hardness.
2. Materials and Methods
2.1. Participants
2.2. Procedure
2.3. Subjective Symptoms of Shoulder Stiffness
2.4. Lifestyle Related to Shoulder Stiffness
2.5. Central Sensitivity Syndromes (CSS)
2.6. Psychological Factors
2.7. EEG
2.8. ANS Activity (Heart Rate Variability)
2.9. Posture Alignment Assessment
2.10. Muscle Hardness
2.11. PPT
2.12. Statistical Analysis
3. Results
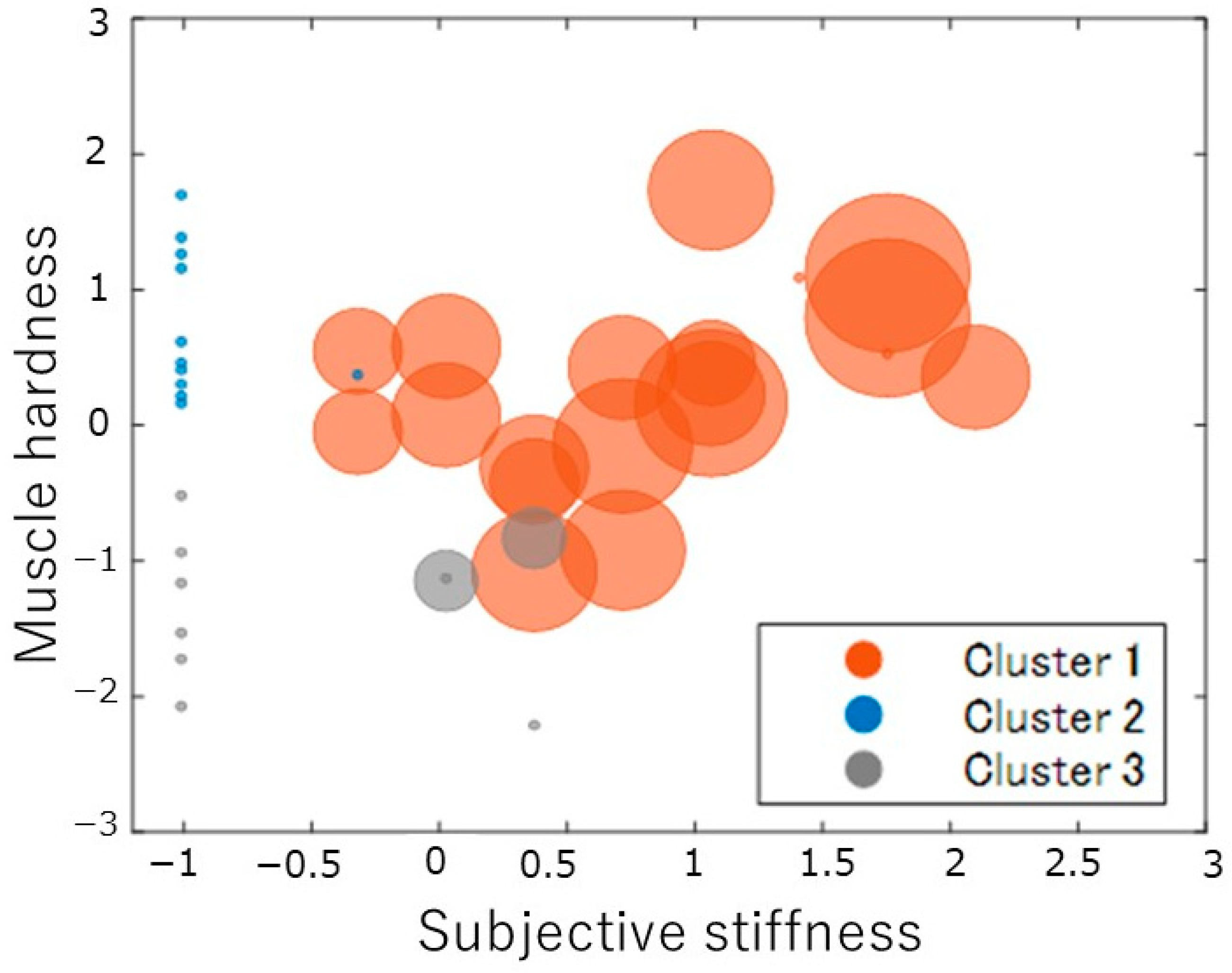
3.1. Results of Cluster Analysis and Multiple Comparisons Based on Subjective Shoulder Stiffness, Subjective Pain, and Shoulder Muscle Stiffness Values
3.2. Comparison of Questionnaire Evaluation Items across Clusters
3.3. Comparison of Posture, Pressure Pain Threshold, ANS Activity, and Brainwave Activity across Clusters
3.4. Correlation Analysis of Subjective Shoulder Stiffness, Pain, and Muscle Stiffness with Other Variables
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Japan Ministry of Health, Labour and Welfare. Comprehensive Survey of Living Conditions 2019. Available online: https://www.mhlw.go.jp/toukei/saikin/hw/k-tyosa/k-tyosa19/dl/04.pdf (accessed on 6 May 2023).
- Yabuki, S. Pathogenesis of the Neck–Shoulder Stiffness (Katakori). Clin. Orthop. Surg. 2007, 42, 413–417. (In Japanese) [Google Scholar]
- Sawada, T.; Matsudaira, K.; Muto, Y.; Koga, T.; Takahashi, M. Potential Risk Factors for Onset of Severe Neck and Shoulder Discomfort (Katakori) in Urban Japanese Workers. Ind. Health 2016, 54, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Ziaeifar, M.; Arab, A.M.; Mosallanezhad, Z.; Nourbakhsh, M.R. Dry Needling versus Trigger Point Compression of the Upper Trapezius: A Randomized Clinical Trial with Two-Week and Three-Month Follow-Up. J. Man. Manip. Ther. 2019, 27, 152–161. [Google Scholar] [CrossRef]
- Onda, A.; Onozato, K.; Kimura, M. Clinical Features of Neck and Shoulder Pain (Katakori) in Japanese Hospital Workers. Fukushima J. Med. Sci. 2022, 68, 79–87. [Google Scholar] [CrossRef]
- Cerezo-Téllez, E.; Torres-Lacomba, M.; Mayoral-Del Moral, O.; Sánchez-Sánchez, B.; Dommerholt, J.; Gutiérrez-Ortega, C. Prevalence of Myofascial Pain Syndrome in Chronic Non-Specific Neck Pain: A Population-Based Cross-Sectional Descriptive Study. Pain Med. 2016, 17, 2369–2377. [Google Scholar] [CrossRef] [PubMed]
- Leong, H.T.; Hug, F.; Fu, S.N. Increased Upper Trapezius Muscle Stiffness in Overhead Athletes with Rotator Cuff Tendinopathy. PLoS ONE 2016, 11, e0155187. [Google Scholar] [CrossRef]
- Andersen, L.L.; Hansen, K.; Mortensen, O.S.; Zebis, M.K. Prevalence and Anatomical Location of Muscle Tenderness in Adults with Nonspecific Neck/Shoulder Pain. BMC Musculoskelet. Disord. 2011, 12, 169. [Google Scholar] [CrossRef]
- Ishikawa, H.; Muraki, T.; Morise, S.; Sekiguchi, Y.; Yamamoto, N.; Itoi, E.; Izumi, S.-I. Changes in Stiffness of the Dorsal Scapular Muscles before and after Computer Work: A Comparison between Individuals with and without Neck and Shoulder Complaints. Eur. J. Appl. Physiol. 2017, 117, 179–187. [Google Scholar] [CrossRef]
- Sawada, T.; Okawara, H.; Nakashima, D.; Iwabuchi, S.; Matsumoto, M.; Nakamura, M.; Nagura, T. Reliability of Trapezius Muscle Hardness Measurement: A Comparison between Portable Muscle Hardness Meter and Ultrasound Strain Elastography. Sensors 2020, 20, 7200. [Google Scholar] [CrossRef]
- Horikawa, M. Effect of Visual Display Terminal Height on the Trapezius Muscle Hardness: Quantitative Evaluation by a Newly Developed Muscle Hardness Meter. Appl. Ergon. 2001, 32, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-C.; Hua, S.-H.; Lin, C.-L.; Cheng, C.-H.; Liao, J.-C.; Lin, C.-F. Impact of Prolonged Tablet Computer Usage with Head Forward and Neck Flexion Posture on Pain Intensity, Cervical Joint Position Sense and Balance Control in Mechanical Neck Pain Subjects. J. Med. Biol. Eng. 2020, 40, 372–382. [Google Scholar] [CrossRef]
- Kanda, M.; Kitamura, T.; Suzuki, Y.; Konishi, I.; Watanabe, K.; Sato, N. The Intramuscular Circulation Is Affected by Neck and Shoulder Pain. Adv. Exp. Med. Biol. 2022, 1395, 399–403. [Google Scholar]
- Borstad, J.D. Resting Position Variables at the Shoulder: Evidence to Support a Posture-Impairment Association. Phys. Ther. 2006, 86, 549–557. [Google Scholar] [CrossRef]
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The Revised International Association for the Study of Pain Definition of Pain: Concepts, Challenges, and Compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef]
- Kim, H.-G.; Cheon, E.-J.; Bai, D.-S.; Lee, Y.H.; Koo, B.-H. Stress and Heart Rate Variability: A Meta-Analysis and Review of the Literature. Psychiatry Investig. 2018, 15, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Katmah, R.; Al-Shargie, F.; Tariq, U.; Babiloni, F.; Al-Mughairbi, F.; Al-Nashash, H. A Review on Mental Stress Assessment Methods Using EEG Signals. Sensors 2021, 21, 5043. [Google Scholar] [CrossRef]
- Ahn, J.W.; Ku, Y.; Kim, H.C. A Novel Wearable EEG and ECG Recording System for Stress Assessment. Sensors 2019, 19, 1991. [Google Scholar] [CrossRef]
- Fontes, M.A.P.; Xavier, C.H.; Marins, F.R.; Limborço-Filho, M.; Vaz, G.C.; Müller-Ribeiro, F.C.; Nalivaiko, E. Emotional Stress and Sympathetic Activity: Contribution of Dorsomedial Hypothalamus to Cardiac Arrhythmias. Brain Res. 2014, 1554, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Alonso, J.F.; Romero, S.; Ballester, M.R.; Antonijoan, R.M.; Mañanas, M.A. Stress Assessment Based on EEG Univariate Features and Functional Connectivity Measures. Physiol. Meas. 2015, 36, 1351–1365. [Google Scholar] [CrossRef]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International Physical Activity Questionnaire: 12-Country Reliability and Validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Nishigami, T.; Mibu, A.; Manfuku, M.; Yono, S.; Shinohara, Y.; Tanabe, A.; Ono, R. Validation of the Japanese Version of the Central Sensitization Inventory in Patients with Musculoskeletal Disorders. PLoS ONE 2017, 12, e0188719. [Google Scholar] [CrossRef]
- Sakairi, Y.; Nakatsuka, K.; Shimizu, T. Development of the Two-Dimensional Mood Scale for Self-Monitoring and Self-Regulation of Momentary Mood States. Jpn. Psychol. Res. 2013, 55, 338–349. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, J.; Gu, C.; Hu, B. Mapping the Frontal Alpha Asymmetry Indicators of Habitual Emotion Regulation: A Data-Driven Approach. Neuroreport 2018, 29, 1288–1292. [Google Scholar] [CrossRef] [PubMed]
- Thammasan, N.; Moriyama, K.; Fukui, K.-I.; Numao, M. Continuous Music-Emotion Recognition Based on Electroencephalogram. IEICE Trans. Inf. Syst. 2016, 99, 1234–1241. [Google Scholar] [CrossRef]
- Jie, X.; Cao, R.; Li, L. Emotion Recognition Based on the Sample Entropy of EEG. Biomed. Mater. Eng. 2014, 24, 1185–1192. [Google Scholar] [CrossRef]
- Tanaka, M.; Shigihara, Y.; Ishii, A.; Funakura, M.; Kanai, E.; Watanabe, Y. Effect of Mental Fatigue on the Central Nervous System: An Electroencephalography Study. Behav. Brain Funct. 2012, 8, 48. [Google Scholar] [CrossRef]
- Imoto, D.; Hirano, S.; Mukaino, M.; Saitoh, E.; Otaka, Y. A novel gait analysis system for detecting abnormal hemiparetic gait patterns during robot-assisted gait training: A criterion validity study among healthy adults. Front. Neurorobot. 2022, 16, 1047376. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.-H.; Cho, M.-U.; Park, C.-W.; Kim, S.-Y.; Kim, M.-J.; Hong, B.; Kong, Y.-K. A Comparison Study of Posture and Fatigue of Neck According to Monitor Types (Moving and Fixed Monitor) by Using Flexion Relaxation Phenomenon (FRP) and Craniovertebral Angle (CVA). Int. J. Environ. Res. Public Health 2020, 17, 6345. [Google Scholar] [CrossRef]
- Kocur, P.; Wilski, M.; Lewandowski, J.; Łochyński, D. Female Office Workers With Moderate Neck Pain Have Increased Anterior Positioning of the Cervical Spine and Stiffness of Upper Trapezius Myofascial Tissue in Sitting Posture. PM&R 2019, 11, 476–482. [Google Scholar]
- Cuesta-Vargas, A.I.; Neblett, R.; Nijs, J.; Chiarotto, A.; Kregel, J.; van Wilgen, C.P.; Pitance, L.; Knezevic, A.; Gatchel, R.J.; Mayer, T.G.; et al. Establishing Central Sensitization–Related Symptom Severity Subgroups: A Multicountry Study Using the Central Sensitization Inventory. Pain Med. 2020, 21, 2430–2440. [Google Scholar] [CrossRef] [PubMed]
- Geenen, R.; Bijlsma, J.W.J. Deviations in the Endocrine System and Brain of Patients with Fibromyalgia: Cause or Consequence of Pain and Associated Features? Ann. N. Y. Acad. Sci. 2010, 1193, 98–110. [Google Scholar] [CrossRef]
- Shigetoh, H.; Tanaka, Y.; Koga, M.; Osumi, M.; Morioka, S. The Mediating Effect of Central Sensitization on the Relation between Pain Intensity and Psychological Factors: A Cross-Sectional Study with Mediation Analysis. Pain Res. Manag. 2019, 2019, 3916135. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, Y.; Takamoto, K.; Nishimaru, H.; Taguchi, T.; Urakawa, S.; Sakai, S.; Ono, T.; Nishijo, H. Compression at Myofascial Trigger Point on Chronic Neck Pain Provides Pain Relief through the Prefrontal Cortex and Autonomic Nervous System: A Pilot Study. Front. Neurosci. 2017, 11, 186. [Google Scholar] [CrossRef]
- Ge, H.-Y.; Fernández-de-las-Peñas, C.; Arendt-Nielsen, L. Sympathetic Facilitation of Hyperalgesia Evoked from Myofascial Tender and Trigger Points in Patients with Unilateral Shoulder Pain. Clin. Neurophysiol. 2006, 117, 1545–1550. [Google Scholar] [CrossRef] [PubMed]
- Brosschot, J.F.; Verkuil, B.; Thayer, J.F. Generalized Unsafety Theory of Stress: Unsafe Environments and Conditions, and the Default Stress Response. Int. J. Environ. Res. Public Health 2018, 15, 464. [Google Scholar] [CrossRef]
- Fujii, T.; Matsudaira, K.; Yoshimura, N.; Hirai, M.; Tanaka, S. Associations between Neck and Shoulder Discomfort (Katakori) and Job Demand, Job Control, and Worksite Support. Mod. Rheumatol. 2013, 23, 1198–1204. [Google Scholar] [CrossRef] [PubMed]
- Aikawa, R.; Kishino, T.; Shibasaki, S.; Harashima, K.; Nakajima, S.; Ohnishi, H.; Watanabe, T. Relationship between trapezius muscle hardness and transverse cervical artery flow in association with neck and upper-back stiffness. Clin. Physiol. Funct. Imaging 2020, 40, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Ahern, G.L.; Schwartz, G.E. Differential Lateralization for Positive and Negative Emotion in the Human Brain: EEG Spectral Analysis. Neuropsychologia 1985, 23, 745–755. [Google Scholar] [CrossRef]
- Nilsen, K.B.; Sand, T.; Westgaard, R.H.; Stovner, L.J.; White, L.R.; Bang Leistad, R.; Helde, G.; Rø, M. Autonomic Activation and Pain in Response to Low-Grade Mental Stress in Fibromyalgia and Shoulder/Neck Pain Patients. Eur. J. Pain 2007, 11, 743–755. [Google Scholar] [CrossRef]
- Billman, G.E. The LF/HF Ratio Does Not Accurately Measure Cardiac Sympatho-Vagal Balance. Front. Physiol. 2013, 4, 26. [Google Scholar] [CrossRef]
- Anan, T.; Kajiki, S.; Oka, H.; Fujii, T.; Kawamata, K.; Mori, K.; Matsudaira, K. Effects of an Artificial Intelligence-Assisted Health Program on Workers With Neck/Shoulder Pain/Stiffness and Low Back Pain: Randomized Controlled Trial. JMIR mHealth uHealth 2021, 9, e27535. [Google Scholar] [CrossRef] [PubMed]

| Variable | Overall (n = 40) | Cluster 1 (n = 19) | Cluster 2 (n = 11) | Cluster 3 (n = 10) |
|---|---|---|---|---|
| Age (years) | 20.6 ± 0.6 | 20.5 ± 0.5 | 20.5 ± 0.5 | 21.0 ± 0.7 |
| Height (cm) | 165.4 ± 8.1 | 163.7 ± 8.1 | 167.4 ± 9.1 | 166.5 ± 7.6 |
| Weight (kg) | 55.7 ± 3.3 | 54.6 ± 10.9 | 56.3 ± 11.6 | 57.3 ± 7.1 |
| BMI | 20.3 ± 2.7 | 20.7 ± 2.0 | 20.0 ± 3.4 | 20.2 ± 2.7 |
| VDT task time (hour/day) | 3.4 ± 0.5 | 3.4 ± 0.5 | 3.4 ± 0.5 | 3.3 ± 0.7 |
| Sleeping time (hour/day) | 5.8 ± 1.2 | 5.8 ± 0.9 | 6.0 ± 0.9 | 5.9 ± 1.1 |
| Physical exercise habit: mild load (times/week) | 4.4 ± 2.3 | 4.1 ± 2.1 | 4.3 ± 2.7 | 5.0 ± 2.3 |
| Physical exercise habit: moderate load (times/week) | 1.8 ± 1.7 | 1.8 ± 2.0 | 1.6 ± 1.9 | 1.8 ± 1.3 |
| Physical exercise habit: severe load (times/week) | 0.6 ± 1.1 | 0.6 ± 1.2 | 0.6 ± 1.1 | 0.4 ± 0.7 |
| Subjective stiffness | 2.9 ± 2.9 | 5.3 ± 2.1 | 0.2 ± 0.6 | 1.4 ± 1.8 |
| Pain | 1.6 ± 2.1 | 3.3 ± 2.0 | 0 ± 0 | 0.2 ± 0.4 |
| Muscle hardness (N) | 34.7 ± 5.7 | 36.2 ± 3.9 | 38.9 ± 3.1 | 27.1 ± 3.1 |
| PPT (N) | 28.7 ± 11.3 | 31.1 ± 14.5 | 27.6 ± 7.5 | 25.3 ± 6.4 |
| CVA (degree) | 55.3 ± 6.1 | 55.5 ± 5.7 | 53.6 ± 6.3 | 56.9 ± 7.1 |
| Spine angle (degree) | 152.7 ± 6.6 | 152.6 ± 5.8 | 153.8 ± 6.8 | 151.6 ± 8.3 |
| TDMS: pleasure | 7.9 ± 4.5 | 7.6 ± 5.3 | 7.7 ± 4.4 | 8.5 ± 3.4 |
| TDMS: arousal | −2.9 ± 3.4 | −2.89 ± 3.4 | −3.00 ± 4.5 | −2.9 ± 2.6 |
| TDMS: stability | 5.6 ± 2.3 | 5.5 ± 2.5 | 5.5 ± 2.4 | 5.7 ± 2.1 |
| TDMS: vitality | 2.6 ± 3.2 | 2.6 ± 3.5 | 2.5 ± 3.8 | 2.8 ± 2.2 |
| Distress | 3.5 ± 2.3 | 4.1 ± 2.6 | 3.1 ± 1.9 | 2.7 ± 2.1 |
| CSI | 21.8 ± 11.5 | 27.9 ± 10.9 | 13.0 ± 9.4 | 19.7 ± 8.5 |
| Fp1 alpha power (ln μV2) | 1.4 ± 0.1 | 1.4 ± 0.1 | 1.5 ± 0.1 | 1.4 ± 0.1 |
| Fp1 beta power (ln μV2) | 1.6 ± 0.2 | 1.6 ± 0.2 | 1.7 ± 0.2 | 1.7 ± 0.3 |
| Fp1 alpha/beta ratio | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 |
| Fp2 alpha power (ln μV2) | 1.4 ± 0.1 | 1.4 ± 0.1 | 1.5 ± 0.1 | 1.4 ± 0.1 |
| Fp2 beta power (ln μV2) | 1.6 ± 0.2 | 1.6 ± 0.1 | 1.7 ± 0.2 | 1.7 ± 0.3 |
| Fp2 alpha/beta ratio | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 |
| LF power (ln ms2) | 2.6 ± 0.5 | 2.7 ± 0.4 | 2.5 ± 0.4 | 2.6 ± 0.5 |
| HF power (ln ms2) | 2.6 ± 0.5 | 2.8 ± 0.3 | 2.5 ± 0.3 | 2.6 ± 0.6 |
| LF/HF ratio | −0.003 ± 0.3 | −0.04 ± 0.4 | 0.03 ± 0.2 | −0.01 ± 0.3 |
| Variable | Overall | Subjective Stiffness Group | Non-Subjective Stiffness Group | ||||
|---|---|---|---|---|---|---|---|
| Subjective Stiffness | Pain | Muscle Hardness | Subjective Stiffness | Pain | Muscle Hardness | Muscle Hardness | |
| Age | −0.15 | −0.03 | −0.25 | −0.19 | 0.04 | −0.26 | −0.26 |
| Height | −0.05 | −0.06 | −0.01 | 0.14 | 0.22 | −0.21 | 0.22 |
| Weight | 0.06 | 0.04 | −0.12 | 0.32 | 0.35 | −0.19 | −0.04 |
| BMI | 0.10 | −0.04 | 0.02 | 0.19 | −0.17 | 0.25 | −0.24 |
| VDT task time | 0.25 | 0.17 | 0.13 | −0.18 | −0.25 | −0.13 | 0.46 |
| Sleeping time | −0.01 | −0.06 | 0.06 | −0.07 | −0.08 | 0.03 | 0.10 |
| Physical exercise habit: mild load | −0.20 | −0.14 | −0.25 | −0.24 | −0.01 | −0.30 | −0.20 |
| Physical exercise habit: moderate load | 0.03 | 0.23 | −0.20 | 0.22 | 0.65 ** | −0.12 | −0.33 |
| Physical exercise habit: severe load | 0.08 | 0.14 | −0.02 | 0.11 | 0.33 | −0.06 | 0.03 |
| Subjective stiffness | 1 | 0.71 ** | 0.18 | 1 | 0.41 * | 0.45 * | 1 |
| Pain | 0.71 ** | 1 | 0.16 | 0.41 * | 1 | 0.35 | 0.31 |
| Muscle hardness | 0.18 | 0.16 | 1 | 0.45 * | 0.35 | 1 | −0.25 |
| PPT | 0.29 | 0.25 | 0.07 | 0.27 | 0.26 | −0.05 | −0.07 |
| CVA | −0.05 | −0.05 | −0.14 | 0.02 | −0.06 | −0.05 | −0.05 |
| Spine angle | −0.12 | −0.12 | 0.10 | 0.06 | 0.01 | 0.27 | 0.27 |
| TDMS: pleasure | −0.16 | −0.02 | −0.14 | −0.23 | 0.06 | −0.06 | −0.24 |
| TDMS: arousal | 0.08 | 0.07 | −0.05 | 0.27 | 0.18 | −0.12 | 0.02 |
| TDMS: stability | −0.19 | 0.01 | −0.09 | −0.42 * | 0.02 | 0.01 | −0.21 |
| TDMS: vitality | −0.05 | 0.08 | −0.12 | −0.02 | 0.20 | −0.12 | −0.13 |
| Daily conscious psychological stress | 0.44 ** | 0.27 | 0.22 | 0.50 * | 0.17 | 0.13 | 0.35 |
| CSI | 0.59 ** | 0.48 ** | 0.06 | 0.42 * | 0.15 | 0.07 | 0.07 |
| Fp1 α power | −0.13 | −0.23 | 0.05 | −0.01 | −0.12 | −0.05 | 0.19 |
| Fp1 β power | 0.10 | 0.13 | 0.11 | 0.14 | 0.22 | 0.18 | 0.01 |
| Fp1 α/β ratio | −0.22 | −0.33 * | −0.09 | −0.19 | −0.38 | −0.30 | 0.17 |
| Fp2 α power | −0.13 | −0.14 | 0.07 | 0.03 | 0.05 | −0.02 | 0.20 |
| Fp2 β power | 0.08 | 0.09 | 0.11 | 0.16 | 0.2 | 0.17 | 0.01 |
| Fp2 α/β ratio | −0.18 | −0.18 | −0.04 | −0.18 | −0.21 | −0.23 | 0.16 |
| LF power | 0.15 | 0.03 | 0.04 | 0.24 | 0.1 | 0.18 | −0.23 |
| HF power | 0.12 | 0.10 | −0.06 | 0.38 | 0.34 | 0.24 | −0.61 |
| LF/HF ratio | 0.03 | −0.11 | 0.14 | −0.25 | −0.41 | −0.10 | 0.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komoto, N.; Sakebayashi, H.; Imagawa, N.; Mizuno, Y.; Nakata, I.; Shigetoh, H.; Kodama, T.; Miyazaki, J. Cluster Analysis of Subjective Shoulder Stiffness and Muscle Hardness: Associations with Central Sensitization-Related Symptoms. Medicina 2023, 59, 1831. https://doi.org/10.3390/medicina59101831
Komoto N, Sakebayashi H, Imagawa N, Mizuno Y, Nakata I, Shigetoh H, Kodama T, Miyazaki J. Cluster Analysis of Subjective Shoulder Stiffness and Muscle Hardness: Associations with Central Sensitization-Related Symptoms. Medicina. 2023; 59(10):1831. https://doi.org/10.3390/medicina59101831
Chicago/Turabian StyleKomoto, Natsuna, Hanako Sakebayashi, Naoto Imagawa, Yuji Mizuno, Ibuki Nakata, Hayato Shigetoh, Takayuki Kodama, and Junya Miyazaki. 2023. "Cluster Analysis of Subjective Shoulder Stiffness and Muscle Hardness: Associations with Central Sensitization-Related Symptoms" Medicina 59, no. 10: 1831. https://doi.org/10.3390/medicina59101831
APA StyleKomoto, N., Sakebayashi, H., Imagawa, N., Mizuno, Y., Nakata, I., Shigetoh, H., Kodama, T., & Miyazaki, J. (2023). Cluster Analysis of Subjective Shoulder Stiffness and Muscle Hardness: Associations with Central Sensitization-Related Symptoms. Medicina, 59(10), 1831. https://doi.org/10.3390/medicina59101831






