Protecting Tear-Film Stability under Adverse Environmental Conditions Using a Mucomimetic with a Non-Newtonian Viscosity Agent
Abstract
1. Introduction
2. Methods
2.1. Controlled Environment Chamber (CEC)
2.2. Scheme of Investigation
2.3. Treatment Protocol
- Typical environment (21 °C/40% RH).
- Dry environment (21 °C/5% RH).
2.4. Parameters Assessed
2.5. Non-Invasive Tear Break-Up Time (NITBUT)
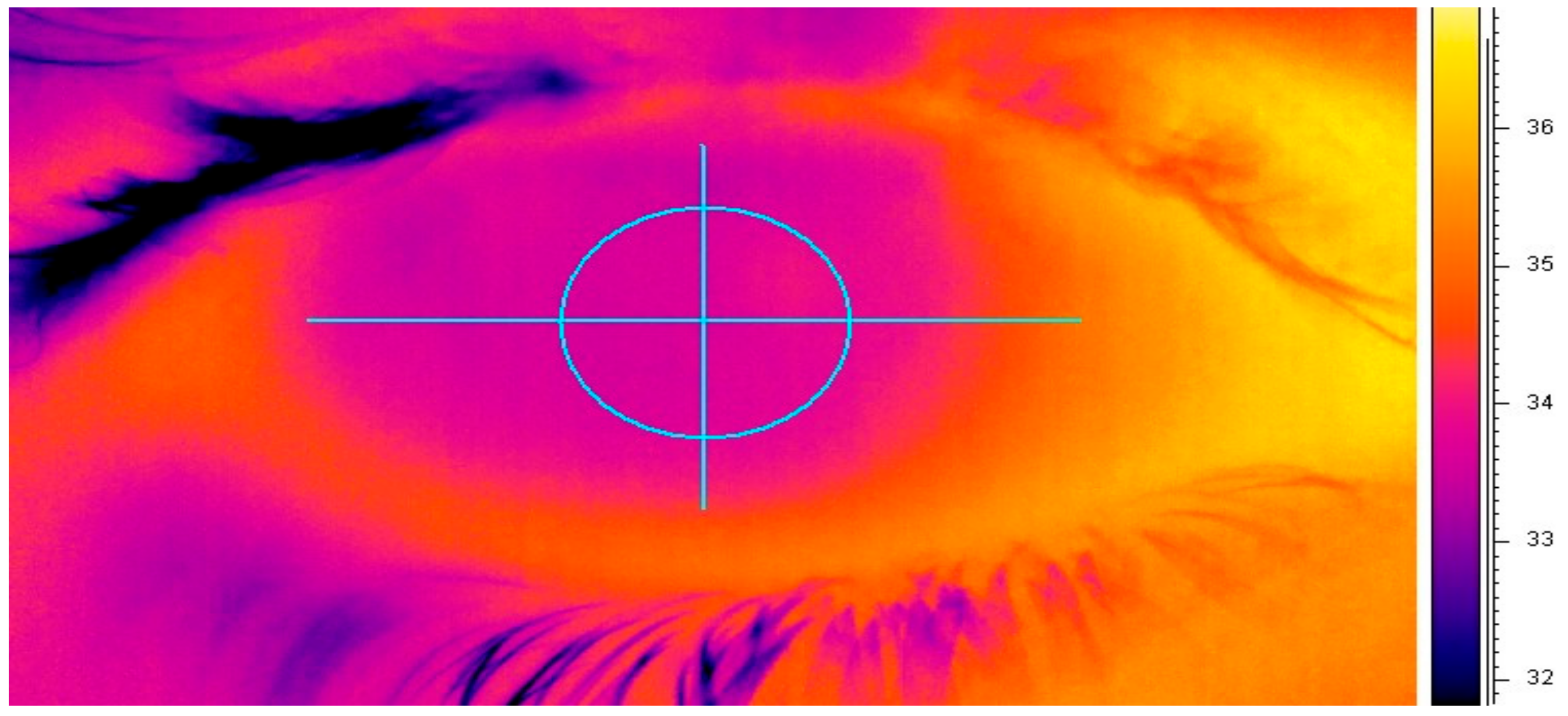
2.6. Ocular Surface Temperature Measurement (OST)
2.7. Tear-Film Evaporation Rate (TFER)
2.8. Statistical Analysis
3. Result
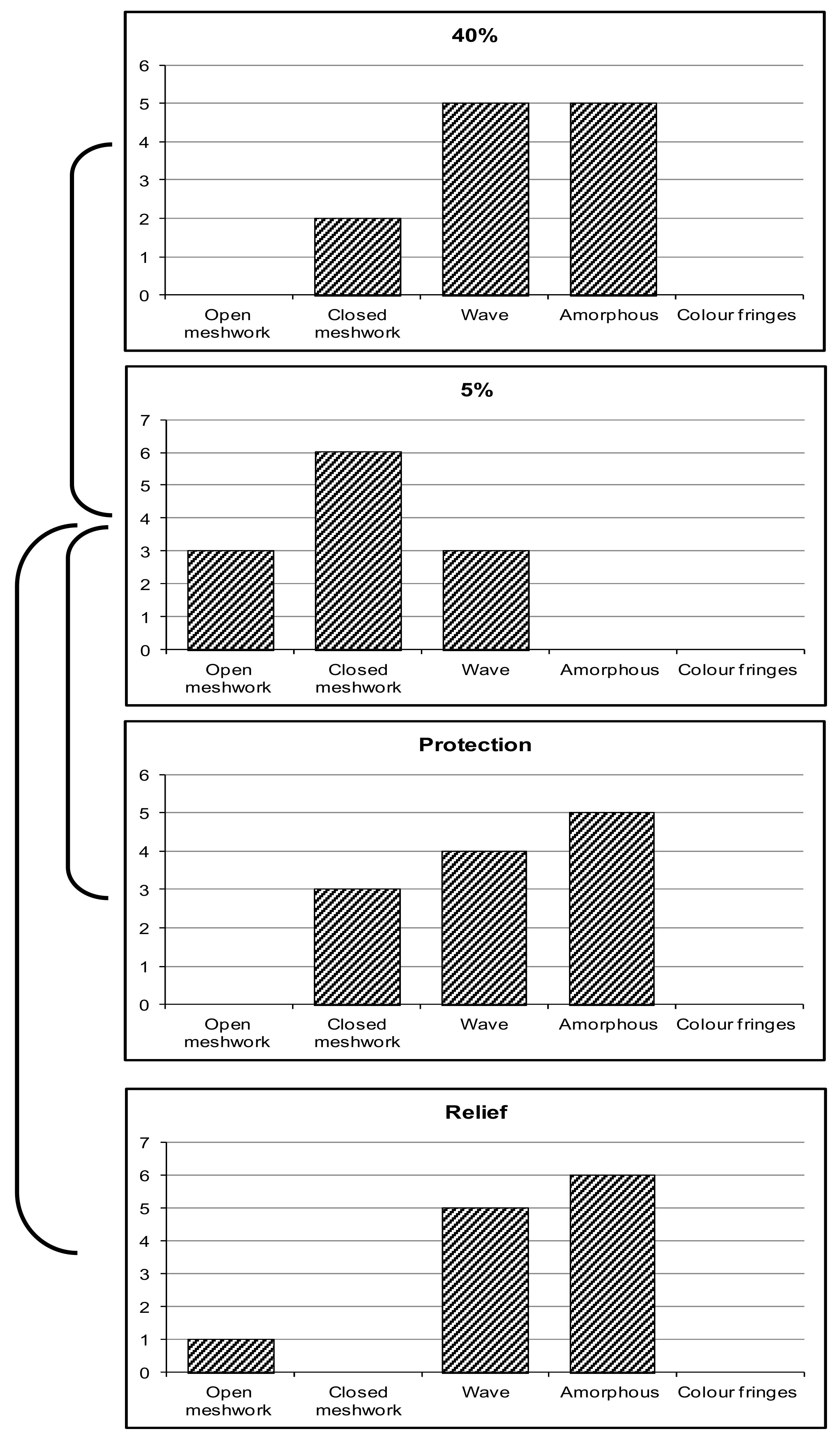
3.1. Lipid-Layer Thickness (LLT)
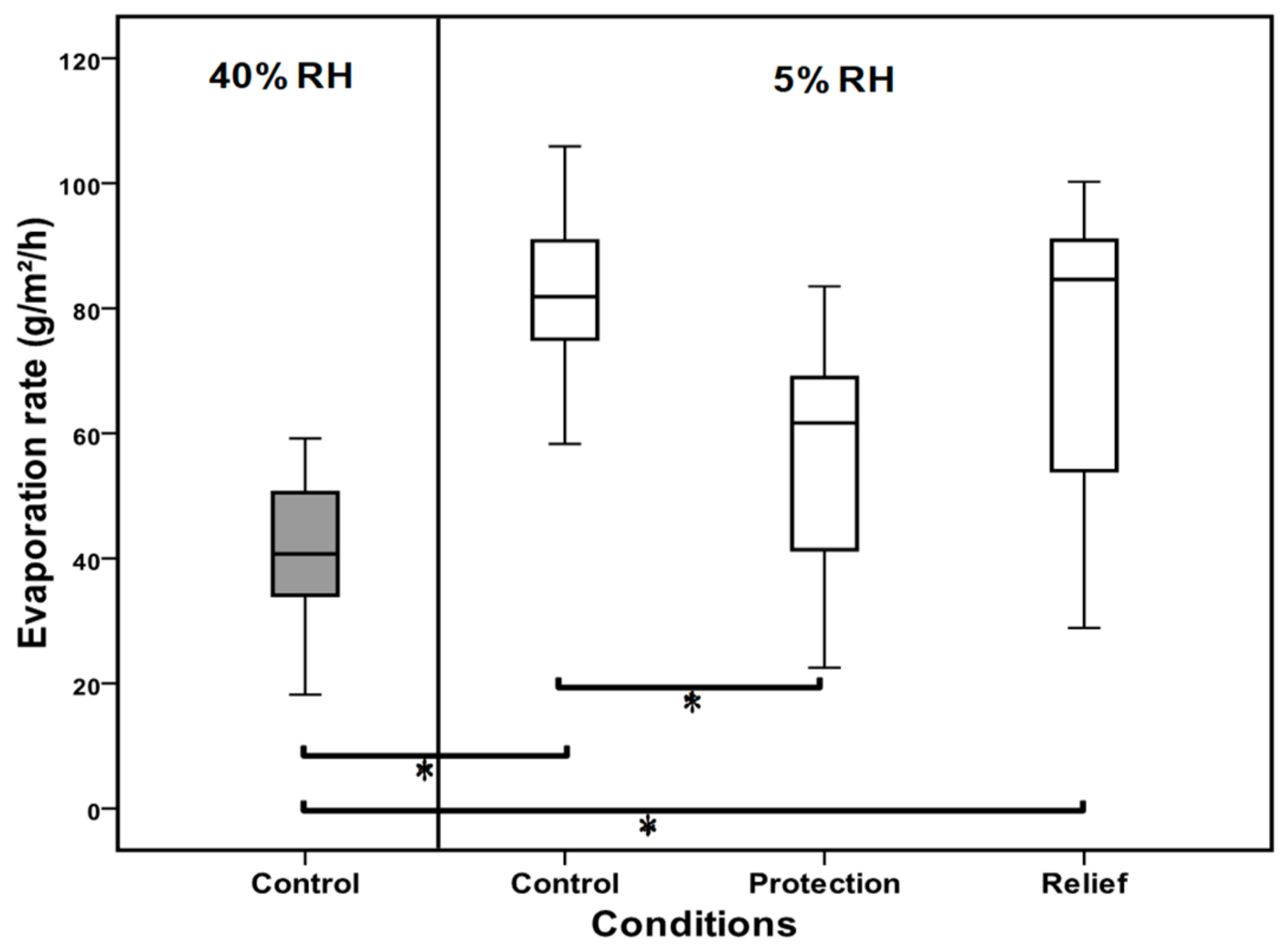
3.2. Evaporation Rate
3.3. Non-Invasive Tear Break-Up Time (NITBUT)
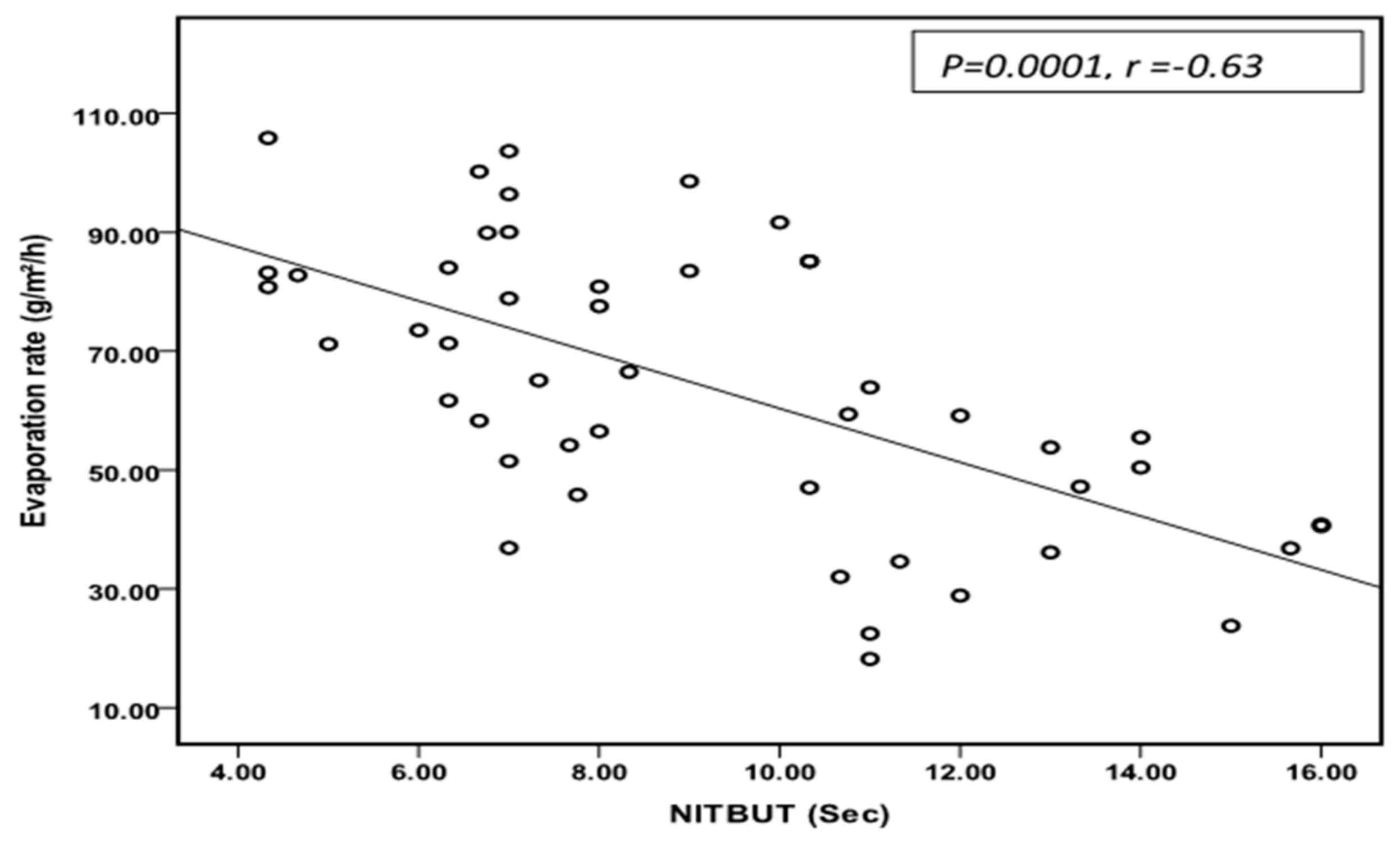
3.4. Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oechsner, M.; Keipert, S. Polyacrylic acid/polyvinylpyrrolidone bipolymeric systems. I. Rheological and mucoadhesive properties of formulations potentially useful for the treatment of dry-eye-syndrome. Eur. J. Pharm. Biopharm. 1999, 47, 113–118. [Google Scholar] [CrossRef]
- Lee, J.W.; Park, J.H.; Robinson, J.R. Bioadhesive-based dosage forms: The next generation. J. Pharm. Sci. 2000, 89, 850–866. [Google Scholar] [CrossRef] [PubMed]
- Bernkop-Schnürch, A. Thiomers: A new generation of mucoadhesive polymers. Adv. Drug Del. Rev. 2005, 57, 1569–1582. [Google Scholar] [CrossRef] [PubMed]
- Avachat, A.M.; Shrotriya, S.N. Tamarind Seed Polysaccharide in Novel Drug Delivery and Biomedical Applications. In Polysaccharide-Based Biomaterials: Delivery of Therapeutics and Biomedical Applications; Royal Society of Chemistry: London, UK, 2022; Volume 13, p. 445. [Google Scholar]
- de Castro, M.A.; Prata, W.M.; Cunha, A.S. Tamarind seed polysaccharide (TSP) uses in ophthalmic drug delivery. Rev. Ciências Farm. Básica E Apl. 2022, 43, 1–10. [Google Scholar] [CrossRef]
- Uccello-Barretta, G.; Nazzi, S.; Balzano, F.; Sansò, M. A nuclear magnetic resonance approach to the comparison of mucoadhesive properties of polysaccharides for ophthalmic uses. Int. J. Pharm. 2011, 406, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Uccello-Barretta, G.; Nazzi, S.; Zambito, Y.; Di Colo, G.; Balzano, F.; Sansò, M. Synergistic interaction between TS-polysaccharide and hyaluronic acid: Implications in the formulation of eye drops. Int. J. Pharm. 2010, 395, 122–131. [Google Scholar] [CrossRef]
- Di Colo, G.; Zambito, Y.; Zaino, C.; Sansò, M. Selected polysaccharides at comparison for their mucoadhesiveness and effect on precorneal residence of different drugs in the rabbit model. Drug Dev. Ind. Pharm. 2009, 35, 941–949. [Google Scholar] [CrossRef]
- Lehr, C.M.; Lee, Y.H.; Lee, V.H.L. Improved ocular penetration of gentamicin by mucoadhesive polymer polycarbophil in the pigmented rabbit. Investig. Ophthalmol. Vis. Sci. 1994, 35, 2809–2814. [Google Scholar]
- Aragona, P.; Di Stefano, G.; Ferreri, F.; Spinella, R.; Stilo, A. Sodium hyaluronate eye drops of different osmolarity for the treatment of dry eye in Sjogren’s syndrome patients. Br. J. Ophthalmol. 2002, 86, 879–884. [Google Scholar] [CrossRef]
- Prabhasawat, P.; Tesavibul, N.; Kasetsuwan, N. Performance profile of sodium hyaluronate in patients with lipid tear deficiency: Randomised, double-blind, controlled, exploratory study. Br. J. Ophthalmol. 2007, 91, 47–50. [Google Scholar] [CrossRef]
- Johnson, M.E.; Murphy, P.J.; Boulton, M. Effectiveness of sodium hyaluronate eyedrops in the treatment of dry eye. Graefes Arch Clin. Exp. Ophthalmol. 2006, 244, 109–112. [Google Scholar] [CrossRef]
- Gomes, J.; Amankwah, R.; Powell-Richards, A.; Dua, H. Sodium hyaluronate (hyaluronic acid) promotes migration of human corneal epithelial cells in vitro. Br. J. Ophthalmol. 2004, 88, 821–825. [Google Scholar] [CrossRef]
- Nishida, T.; Nakamura, M.; Mishima, H.; Otori, T. Hyaluronan stimulates corneal epithelial migration. Exp. Eye Res. 1991, 53, 753–758. [Google Scholar] [CrossRef]
- Versura, P.; Profazio, V.; Balducci, N.; Campos, E.C. Efficacy of two-month treatment with Xiloial eyedrops for discomfort from disposable soft contact lenses. Clin. Ophthalmol. 2010, 4, 1035–1041. [Google Scholar] [CrossRef][Green Version]
- Rolando, M.; Valente, C. Establishing the tolerability and performance of tamarind seed polysaccharide (TSP) in treating dry eye syndrome: Results of a clinical study. BMC Ophthalmol. 2007, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Dubashynskaya, N.; Poshina, D.; Raik, S.; Urtti, A.; Skorik, Y.A. Polysaccharides in ocular drug delivery. Pharmaceutics 2019, 12, 22. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Poon, C.S.; Li, X.D.; Luk, F. Indoor air quality investigation on commercial aircraft. Indoor Air 1999, 9, 180–187. [Google Scholar] [CrossRef]
- Wolkoff, P. “Healthy” eye in office-like environments. Environ. Int. 2008, 34, 1204–1214. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Fukayo, S.; Yano, E. Adverse environmental health effects of ultra-low relative humidity indoor air. J. Occup. Health 2003, 45, 133–136. [Google Scholar] [CrossRef]
- Abusharha, A.; Pearce, I.E.; Afsar, T.; Alsaqr, A.; Fagehi, R.; Razak, S. Evaluation of different treatment modalities on the efficacy of hydroxypropyl Guar (HP-Guar) formulation on tear film stability (TFS) in subjects exposed to adverse environmental conditions. BMC Ophthalmol. 2023, 23, 226. [Google Scholar] [CrossRef]
- Shrestha, B.; Dunn, L. The declaration of Helsinki on medical research involving human subjects: A review of seventh revision. J. Nepal Health Res. Counc. 2019, 17, 548–552. [Google Scholar] [CrossRef]
- Schiffman, R.M.; Christianson, M.D.; Jacobsen, G.; Hirsch, J.D.; Reis, B.L. Reliability and validity of the ocular surface disease index. Arch. Ophthalmol. 2000, 118, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Abusharha, A.; Pearce, I.E.; Afsar, T.; Razak, S. Evaluation of Therapeutic Capability of Emustil Drops against Tear Film Complications under Dry Environmental Conditions in Healthy Individuals. Medicina 2023, 59, 1298. [Google Scholar] [CrossRef]
- Trees, G.R.; Tomlinson, A. Effect of Artificial Tear Solutions and Saline on Tear Film Evaporation. Optom. Vis. Sci. 1990, 67, 886–890. [Google Scholar] [CrossRef]
- Guillon, J.P. Non-invasive Tearscope Plus routine for contact lens fitting. Cont Lens Anterior Eye 1998, 21 (Suppl. 1), S31–S40. [Google Scholar] [CrossRef]
- Hirji, N.; Patel, S.; Callander, M. Human tear film pre-rupture phase time (TP-RPT)—A non-invasive technique for evaluation the pre-corneal tear film using a novel keratometer mire. Ophthalmic Physiol. Opt. 1989, 9, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Mengher, L.S.; Pandher, K.; Bron, A. Non-invasive tear film break-uptime: Sensitivity and specificity. Acta Ophthalmol. 1986, 64, 441–444. [Google Scholar] [CrossRef] [PubMed]
- Cho, P. Reliability of a portable noninvasive tear break-up time test on Hong Kong-Chinese. Optom. Vis. Sci. 1993, 70, 1049–1054. [Google Scholar] [CrossRef]
- Abusharha, A.A.; Pearce, E.I. The Effect of Low Humidity on the Human Tear Film. Cornea 2012, 32, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Morgan, P.B.; Soh, M.P.; Efron, N.; Tullo, A.B. Potential applications of ocular thermography. Optom. Vis. Sci. 1993, 70, 568–576. [Google Scholar] [CrossRef]
- Armstrong, R.A. Statistical guidelines for the analysis of data obtained from one or both eyes. Ophthalmic Physiol. Opt. 2013, 33, 7–14. [Google Scholar] [CrossRef]
- Wolkoff, P. Ocular discomfort by environmental and personal risk factors altering the precorneal tear film. Toxicol. Lett. 2010, 199, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Wolkoff, P.; Nojgaard, J.K.; Troiano, P.; Piccoli, B. Eye complaints in the office environment: Precorneal tear film integrity influenced by eye blinking efficiency. Occup. Environ. Med. 2005, 62, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Norback, D.; Lindgren, T.; Wieslander, G. Changes in ocular and nasal signs and symptoms among air crew in relation to air humidification on intercontinental flights. Scand. J. Work. Environ. Health 2006, 32, 138–144. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Norback, D.; Wieslander, G.; Nordstrom, K.; Walinder, R.; Venge, P. The effect of air humidification on symptoms and nasal patency, tear film stability, and biomarkers in nasal lavage: A 6 weeks’ longitudinal study. Indoor Built Environ. 2000, 9, 28. [Google Scholar] [CrossRef]
- Lang, P.; Masci, G.; Dentini, M.; Crescenzi, V.; Cooke, D.; Gidley, M.; Fanutti, C.; Reid, J. Tamarind seed polysaccharide: Preparation, characterisation and solution properties of carboxylated, sulphated and alkylaminated derivatives. Carbohydr. Polym. 1992, 17, 185–198. [Google Scholar] [CrossRef]
- Srivastava, H.; Singh, P. Structure of the polysaccharide from tamarind kernel. Carbohydr. Res. 1967, 4, 326–342. [Google Scholar] [CrossRef]
- Saettone, M.F.; Burgalassi, S.; Giannaccini, B.; Boldrini, E.; Bianchini, P.; Luciani, G. Ophthalmic solutions viscosified with tamarind seed polysaccharide. PCT Int. Appl. WO 1997, 97, 787. [Google Scholar]
- De Smedt, S.; Lauwers, A.; Demeester, J.; Engelborghs, Y.; De Mey, G.; Du, M. Structural information on hyaluronic acid solutions as studied by probe diffusion experiments. Macromolecules 1994, 27, 141–146. [Google Scholar] [CrossRef]
- Bansal, J.; Kedige, S.D.; Anand, S. Hyaluronic acid: A promising mediator for periodontal regeneration. Indian J. Dent. Res. 2010, 21, 575. [Google Scholar]
- Kogan, G.; Šoltés, L.; Stern, R.; Gemeiner, P. Hyaluronic acid: A natural biopolymer with a broad range of biomedical and industrial applications. Biotechnol. Lett. 2007, 29, 17–25. [Google Scholar] [CrossRef]
- Atkins, E.; Sheehan, J. Structure for hyaluronic acid. Nature 1972, 235, 253–254. [Google Scholar] [CrossRef]
- Nakamura, M.; Hikida, M.; Nakano, T.; Ito, S.; Hamano, T.; Kinoshita, S. Characterization of water retentive properties of hyaluronan. Cornea 1993, 12, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Craig, J.P.; Tomlinson, A. Importance of the lipid layer in human tear film stability and evaporation. Optom. Vis. Sci. 1997, 74, 8–13. [Google Scholar] [CrossRef]
- Bron, A.J.; Tiffany, J.M.; Gouveia, S.M.; Yokoi, N.; Voon, L.W. Functional aspects of the tear film lipid layer. Exp. Eye Res. 2004, 78, 347–360. [Google Scholar] [CrossRef]
- McCann, L.C.; Tomlinson, A.; Pearce, E.I.; Papa, V. Effectiveness of Artificial Tears in the Management of Evaporative Dry Eye. Cornea 2012, 31, 1. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T. Topical mucosal adhesive dosage forms. Med. Res. Rev. 1986, 6, 227–242. [Google Scholar] [CrossRef]
- Sunwoo, Y.; Chou, C.; Takeshita, J.; Murakami, M.; Tochihara, Y. Physiological and subjective responses to low relative humidity. J. Physiol. Anthropol. Appl. Hum. Sci. 2006, 25, 7–14. [Google Scholar] [CrossRef]
- Ousler, G.W.; Hagberg, K.W.; Schindelar, M.; Welch, D.; Abelson, M.B. The Ocular Protection Index. Cornea 2008, 27, 509–513. [Google Scholar] [CrossRef]
- Lemp, M.A.; Baudouin, C.; Baum, J.; Dogru, M.; Foulks, G.N.; Kinoshita, S.; Laibson, P.; McCulley, J.; Murube, J.; Pflugfelder, S.C.; et al. The definition and classification of dry eye disease: Report of the Definition and Classification Subcommittee of the international Dry Eye WorkShop. Ocul. Surf. 2007, 5, 75–92. [Google Scholar]
- Galor, A.; Britten-Jones, A.C.; Feng, Y.; Ferrari, G.; Goldblum, D.; Gupta, P.K.; Merayo-Lloves, J.; Na, K.-S.; Naroo, S.A.; Nichols, K.K. TFOS Lifestyle: Impact of lifestyle challenges on the ocular surface. Ocul. Surf. 2023, 28, 262–303. [Google Scholar] [CrossRef]
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.-K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K. TFOS DEWS II definition and classification report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Paschides, C.A.; Stefaniotou, M.; Papageorgiou, J.; Skourtis, P.; Psilas, K. Ocular surface and environmental changes. Acta Ophthalmol. Scand. 1998, 76, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Wyon, D.P.; Fang, L.; Lagercrantz, L.; Fanger, P.O. Experimental determination of the limiting criteria for human exposure to low winter humidity indoors (RP-1160). HvacR Res. 2006, 12, 201–213. [Google Scholar] [CrossRef]
- Pena-Verdeal, H.; Garcia-Queiruga, J.; García-Resúa, C.; Yebra-Pimentel, E.; Giráldez, M.J. Osmolality and pH of commercially available contact lens care solutions and eye drops. Contact Lens Anterior Eye 2021, 44, 101379. [Google Scholar] [CrossRef]


| EVAP (g/m2/h) | NITBUT (s) | OST (°C) | |
|---|---|---|---|
| 40% RH | 40.93 ± 12.49 | 13.00 ± 2.1 | 33.99 ± 0.66 |
| 5% RH | 82.42 ± 14.66 | 6.00 ± 1.82 | 33.70 ± 0.44 |
| Protection | 56.45 ± 18.13 | 8.00 ± 1.94 | 33.50 ± 0.41 |
| Relief | 75.38 ± 22.84 | 8.00 ± 2.41 | 33.92 ± 0.59 |
| LLT 40% | LLT 5% | LLT Protection | LLT Relief | |
|---|---|---|---|---|
| LLT 40% | 0.007 | 0.725 | 0.803 | |
| LLT 5% | 0.011 | 0.004 | ||
| LLT Protection | 0.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abusharha, A.; Pearce, E.I.; Afsar, T.; Razak, S. Protecting Tear-Film Stability under Adverse Environmental Conditions Using a Mucomimetic with a Non-Newtonian Viscosity Agent. Medicina 2023, 59, 1862. https://doi.org/10.3390/medicina59101862
Abusharha A, Pearce EI, Afsar T, Razak S. Protecting Tear-Film Stability under Adverse Environmental Conditions Using a Mucomimetic with a Non-Newtonian Viscosity Agent. Medicina. 2023; 59(10):1862. https://doi.org/10.3390/medicina59101862
Chicago/Turabian StyleAbusharha, Ali, E. Ian Pearce, Tayyaba Afsar, and Suhail Razak. 2023. "Protecting Tear-Film Stability under Adverse Environmental Conditions Using a Mucomimetic with a Non-Newtonian Viscosity Agent" Medicina 59, no. 10: 1862. https://doi.org/10.3390/medicina59101862
APA StyleAbusharha, A., Pearce, E. I., Afsar, T., & Razak, S. (2023). Protecting Tear-Film Stability under Adverse Environmental Conditions Using a Mucomimetic with a Non-Newtonian Viscosity Agent. Medicina, 59(10), 1862. https://doi.org/10.3390/medicina59101862




