Technical Note on Vestibuloplasty around Dental Implants Using Erbium YAG Laser-Assisted Periosteal Fenestration (LA-PF)
Abstract
:1. Introduction
2. Case Report
2.1. Surgical Procedures of Er:YAG Laser-Assisted Periosteal Fenestration (LA-PF)
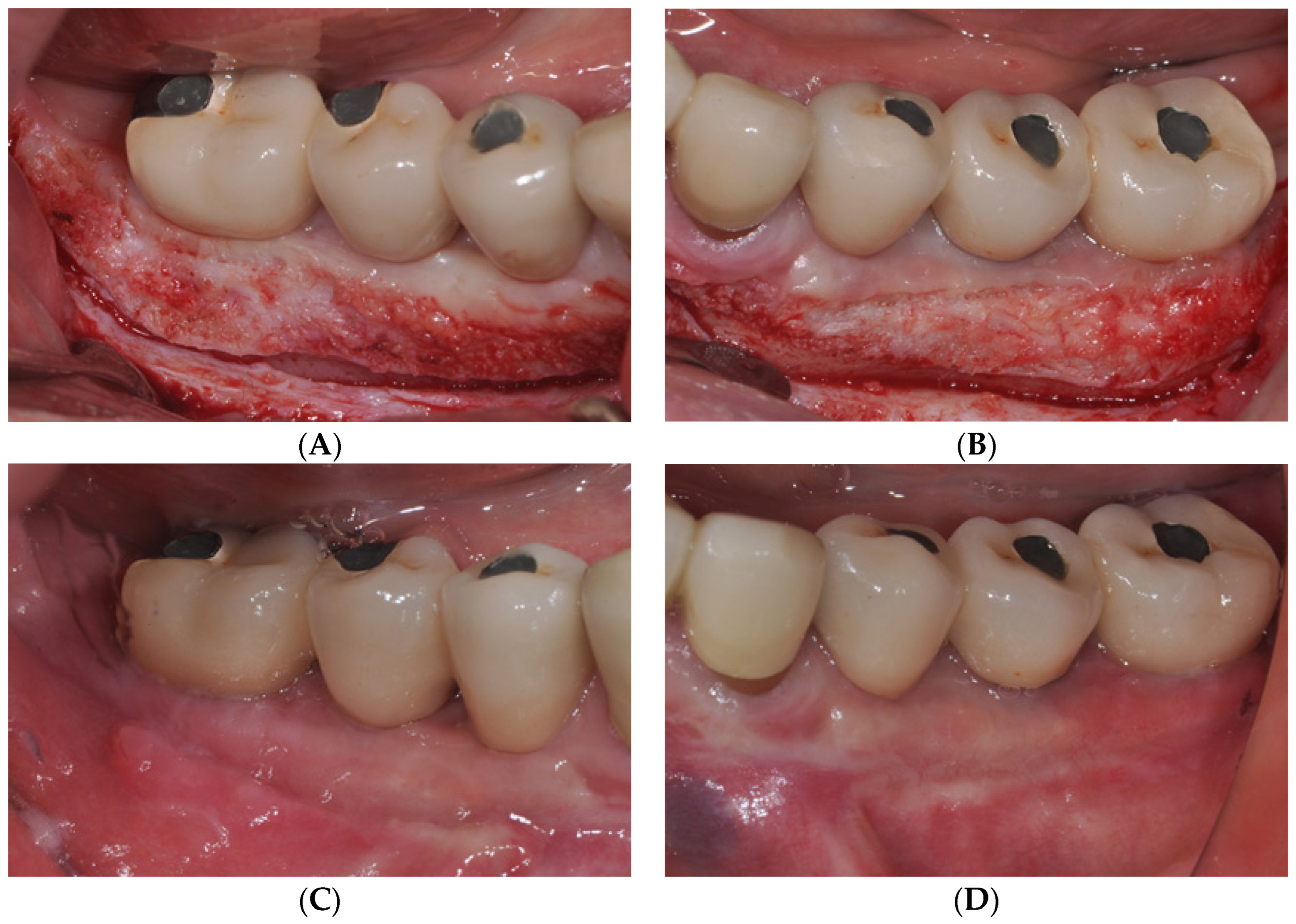
2.2. Case
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wennström, J.L.; Derks, J. Is there a need for keratinized mucosa around implants to maintain health and tissue stability? Clin. Oral Implant. Res. 2012, 23, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.H.; Chan, H.L.; Wang, H.L. The significance of keratinized mucosa on implant health: A systematic review. J. Periodontol. 2013, 84, 1755–1767. [Google Scholar] [CrossRef]
- Tavelli, L.; Barootchi, S.; Avila-Ortiz, G.; Urban, I.A.; Giannobile, W.V.; Wang, H.L. Peri-implant soft tissue phenotype modification and its impact on peri-implant health: A systematic review and network meta-analysis. J. Periodontol. 2021, 92, 21–44. [Google Scholar] [CrossRef]
- Sculean, A.; Romanos, G.; Schwarz, F.; Ramanauskaite, A.; Keeve, P.L.; Khoury, F.; Koo, K.-T.; Raluca, C. Soft-tissue management as part of the surgical treatment of periimplantitis: A narrative review. Implant. Dent. 2019, 28, 210–216. [Google Scholar] [CrossRef]
- Khoury, F.; Keeve, P.L.; Ramanauskaite, A.; Schwarz, F.; Koo, K.-T.; Sculean, A.; Romanos, G. Surgical treatment of peri-implantitis—Consensus report of working group. Int. Dent. J. 2019, 69, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Alpan, A.L.; Cin, G.T. Comparison of hyaluronic acid, hypochlorous acid, and flurbiprofen on postoperative morbidity in palatal donor area: A randomized controlled clinical trial. Clin. Oral Investig. 2023, 27, 2735–2746. [Google Scholar] [CrossRef]
- Chen, Y.; Lai, Y.; Yen, J.; Lin, Y.; Chen, H.; Lee, S. Long-term evaluation of peri-implant keratinized mucosa stability after free epithelialized graft and keratinized mucosa shifting procedures: A retrospective study up to 13 years. Clin. Oral Implant. Res. 2023, 21, 1083–1093. [Google Scholar] [CrossRef]
- Lee, W.P.; Kwon, Y.S. Vestibuloplasty around dental implants using modified periosteal fenestration (mPF): Case series. Implantology 2020, 24, 22–30. [Google Scholar] [CrossRef]
- Park, D.Y.; Lee, W.P. Vestibuloplasty around teeth and dental implants using simplified periosteal fenestration (sPF): Case Reports. J. Kor. Dent. Assoc. 2020, 59, 20–27. [Google Scholar]
- Monje, A.; Blasi, G. Significance of keratinized mucosa/gingiva on peri-implant and adjacent periodontal conditions in erratic maintenance compliers. J. Periodontol. 2019, 90, 445–453. [Google Scholar] [CrossRef]
- Lee, W.P.; Lee, K.H.; Yu, S.J.; Kim, B.O. A retrospective comparison of 3 approaches of vestibuloplasty around mandibular molar implants: Apically positioned flap versus free gingival graft versus modified periosteal fenestration. J. Periodontal. Implant. Sci. 2021, 51, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Kon, S.; Pustiglioni, F.E.; Novaes, A.B.; Ruben, M.P.; De Araujo, N.S. Split Thickness Flap, Apically Replaced, With Protected Linear Periosteal Fenestration: A Clinical and Histological Study in Dogs. J. Periodontol. 1978, 49, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Khattak, B.P.; Yadav, N.; Misra, S.; Sharma, A. Comparative evaluation of the relative efficacy of the free mucosal graft and periosteal fenestration for increasing the vestibular depth—A clinical study. Contemp. Clin. Dent. 2014, 5, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Li, K.T.; Fang, C.Y.; Hsia, Y.J.; He, C.H. A combo approach for vestibuloplasty in the edentulous maxilla. J. Dent. Sci. 2023, 18, 1439–1441. [Google Scholar] [CrossRef] [PubMed]
- Pisevska, S.G.; Simjanovska, L.; Markovska, M.; Petreska, M.P.; Chadikovska, E. ER:YAG LASER: Mininal invasive technique for vestibuloplasty in the lower jaw. J. Morphol. Sci. 2019, 2, 37–42. [Google Scholar]
- Wigdor, H.A.; Walsh, J.T., Jr.; Featherstone, J.D.B.; Visuri, S.R.; Fried, D.; Waldvogel, J.L. Lasers in dentistry. Lasers Surg. Med. 1995, 16, 103–133. [Google Scholar] [CrossRef]
- Mier, M.; Armida, M. Laser therapy and its applications in dentistry. Pract. Odontol. 1989, 10, 9–16. [Google Scholar]
- Inchingolo, A.M.; Malcangi, G.; Ferrara, I.; Viapiano, F.; Netti, A.; Buongiorno, S.; Latini, G.; Azzollini, D.; De Leonardis, N.; de Ruvo, E.; et al. Laser Surgical Approach of Upper Labial Frenulum: A Systematic Review. Int. J. Environ. Res. Public Health 2023, 20, 1302. [Google Scholar] [CrossRef]
- Tarullo, A.; Laino, L.; Tarullo, A.; Inchingolo, F.; Flace, P.; Inchingolo, A.M.; Inchingolo, A.D.; Dipalma, G.; Podo Brunetti, S.; Cagiano, R. Use of a Diode Laser in an Excisional Biopsy of Two Spoon like Neoformations on the Tongue Tip. Acta Biomed. 2011, 82, 63–68. [Google Scholar]
- Kazakova, R.; Tomov, G.; Vlahova, A.; Zlatev, S.; Dimitrova, M.; Kazakov, S.; Corsalini, M.; Forte, M.; Di Venere, D.; Dell’olio, F.; et al. Assessment of Healing after Diode Laser Gingivectomy Prior to Prosthetic Procedures. Appl. Sci. 2023, 13, 5527. [Google Scholar] [CrossRef]
- Verma, S.K.; Maheshwari, S.; Singh, R.K.; Chaudhari, P.K. Laser in Dentistry: An Innovative Tool in Modern Dental Practice. Natl. J. Maxillofac. Surg. 2012, 3, 124–132. [Google Scholar] [CrossRef]
- Harashima, T.; Kinoshita, J.I.; Kimura, Y.; Brugnera, A.; Zanin, F.; Pecora, J.D.; Matsumoto, K. Morphological Comparative Studyon Ablation of Dental Hard Tissues at Cavity Preparation by Er:YAG and Er,Cr:YSGG Lasers. Photomed. Laser Surg. 2005, 23, 52–55. [Google Scholar] [CrossRef] [PubMed]
- De Benedittis, M.; Petruzzi, M.; Pastore, L.; Inchingolo, F.; Serpico, R. Nd:YAG Laser for Gingivectomy in Sturge-Weber Syndrome. J. Oral Maxillofac. Surg. 2007, 65, 314–316. [Google Scholar] [CrossRef] [PubMed]
- Tomov, G.T. Laser surgery in everyday practice with Er: YAG laser LiteTouch. Dent. Trib. 2011, 2, 6–9. [Google Scholar]
- Tomov, G.T.; Bachurska, S.Y.; Tashkova, D.A.; Ivanov, G.P. Pathomorphological distinction between Er:YAG and diode lasers on the excisional biopsy of the oral mucosa. Russ. Open Med. J. 2013, 2, 0107. [Google Scholar] [CrossRef]
- Suter, V.G.A.; Altermatt, H.J.; Bornstein, M.M. A randomized controlled clinical and histopathological trial comparing excisional biopsies of oral fibrous hyperplasias using CO2 and Er:YAG laser. Lasers Med. Sci. 2017, 32, 573–581. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, K.-O.; Lee, W.-P. Technical Note on Vestibuloplasty around Dental Implants Using Erbium YAG Laser-Assisted Periosteal Fenestration (LA-PF). Medicina 2023, 59, 1884. https://doi.org/10.3390/medicina59101884
Lim K-O, Lee W-P. Technical Note on Vestibuloplasty around Dental Implants Using Erbium YAG Laser-Assisted Periosteal Fenestration (LA-PF). Medicina. 2023; 59(10):1884. https://doi.org/10.3390/medicina59101884
Chicago/Turabian StyleLim, Kyeong-Ok, and Won-Pyo Lee. 2023. "Technical Note on Vestibuloplasty around Dental Implants Using Erbium YAG Laser-Assisted Periosteal Fenestration (LA-PF)" Medicina 59, no. 10: 1884. https://doi.org/10.3390/medicina59101884
APA StyleLim, K.-O., & Lee, W.-P. (2023). Technical Note on Vestibuloplasty around Dental Implants Using Erbium YAG Laser-Assisted Periosteal Fenestration (LA-PF). Medicina, 59(10), 1884. https://doi.org/10.3390/medicina59101884






