Understanding Solid-Based Platelet-Rich Fibrin Matrices in Oral and Maxillofacial Surgery: An Integrative Review of the Critical Protocol Factors and Their Influence on the Final Product
Abstract
1. Introduction
2. Materials and Methods
2.1. Problem Formulation
2.2. Literature Search
2.3. Eligibility Criteria
2.3.1. Inclusion Criteria
2.3.2. Exclusion Criteria
2.4. Study Records
3. Results
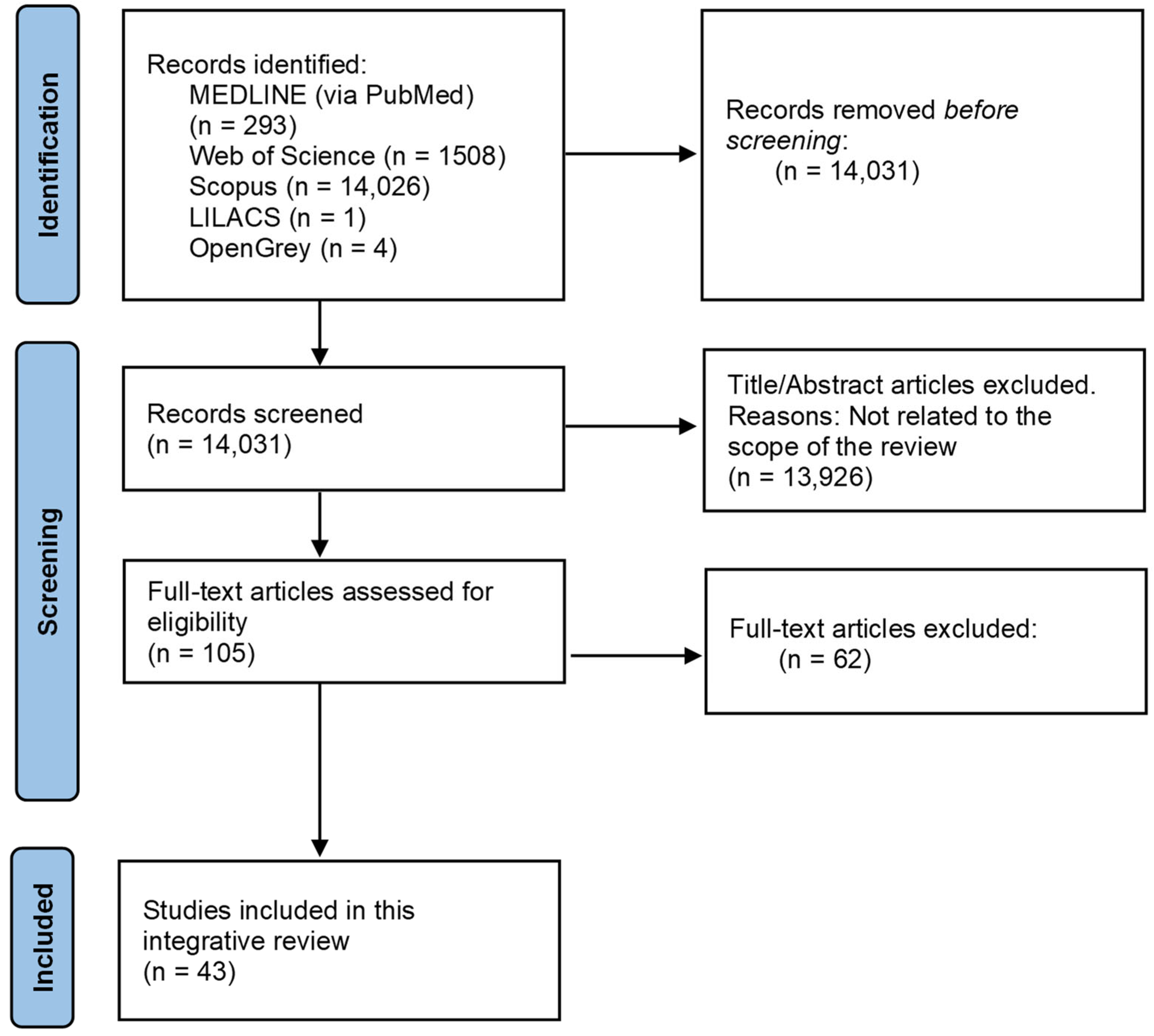
3.1. Study Selection
3.2. Characteristics of the Included Studies and Pooled Results
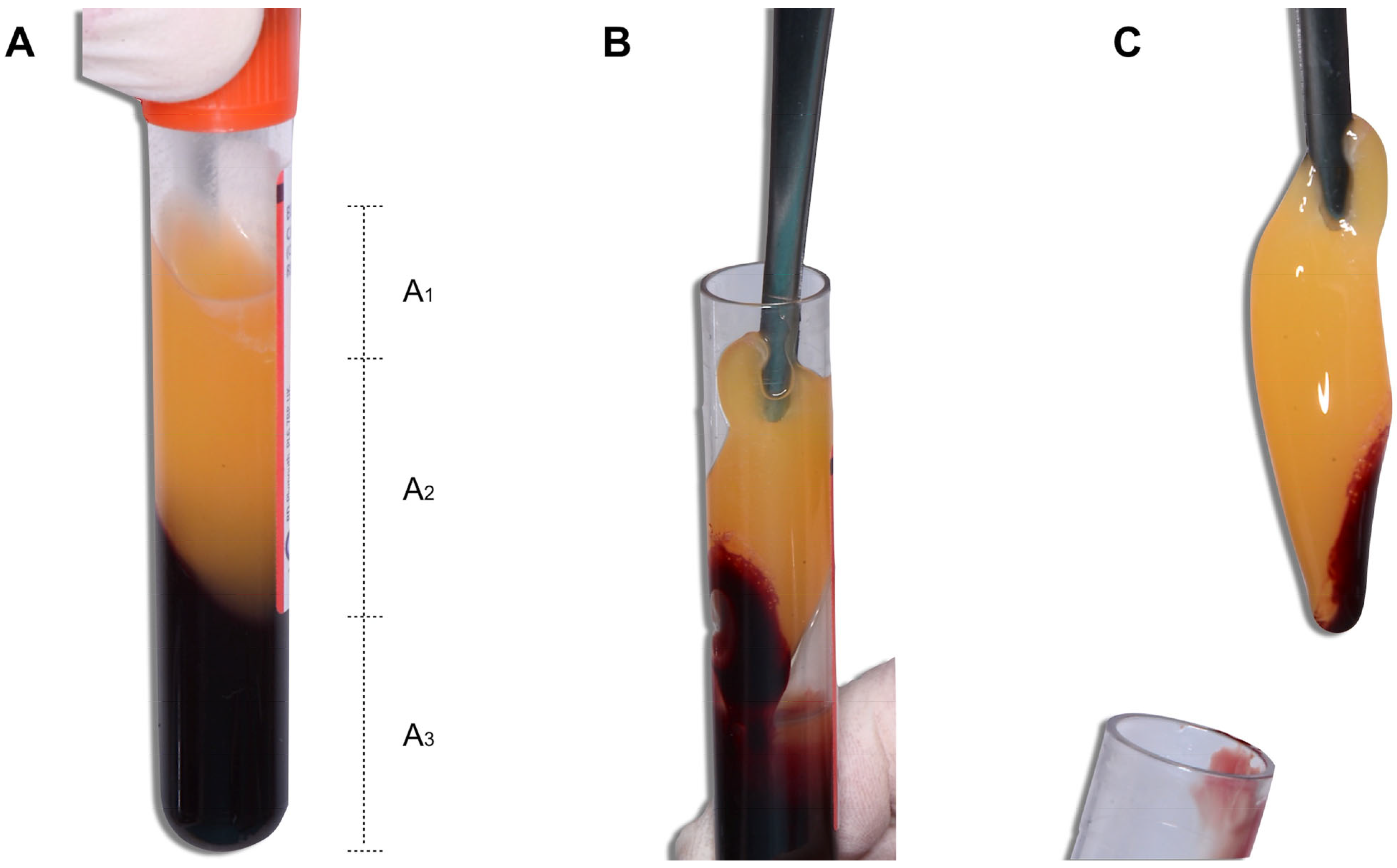
3.2.1. Venipuncture
Influence of the Blood Collection Tube Material on the Macroscopic Level
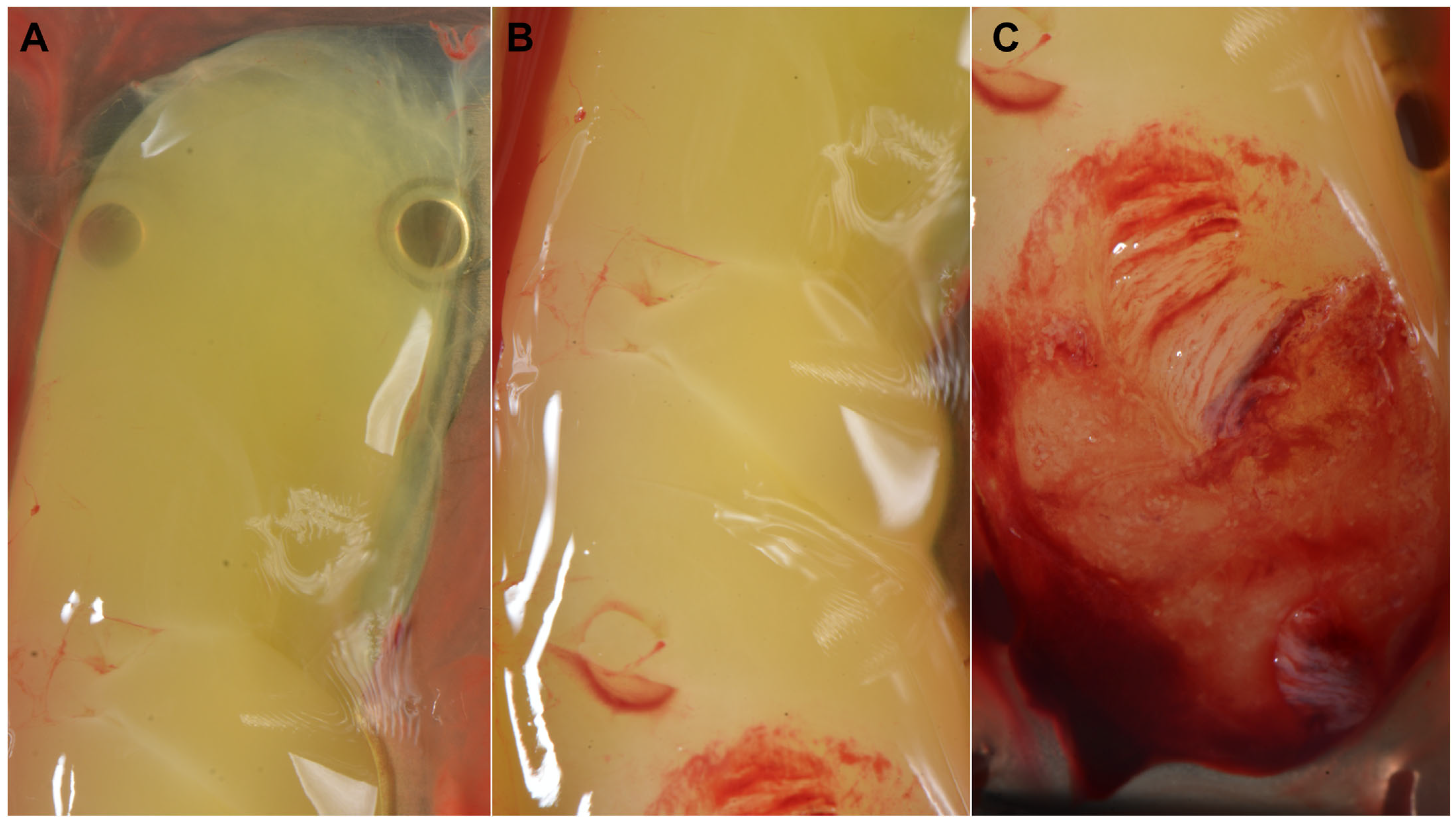
Influence of the Blood Collection Tube Material at the Microscopic Level
Influence of Silica Coatings
3.2.2. Influence of the Time from Venipuncture to the Centrifugation of Blood
3.2.3. Blood Centrifugation
Influence of the RCF
- Influence of the RCF on the Macroscopic Characteristics of the PRF
- Influence of the RCF on the Microscopic Characteristics of the PRF
Centrifuge Vibration
Temperature
Angulation of the Tubes during Centrifugation
Centrifugation Duration
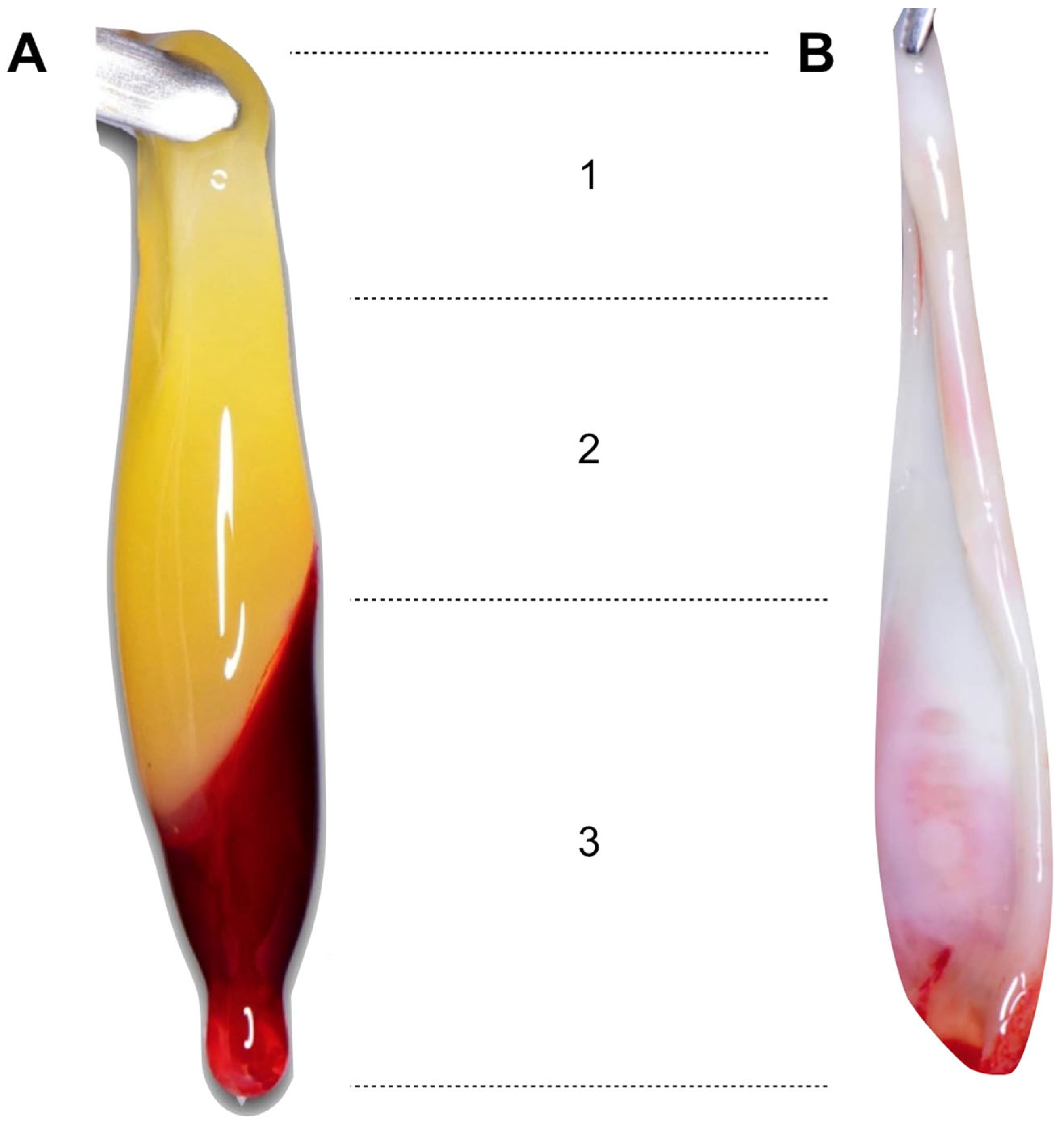
3.2.4. Time from Centrifugation to the Dehydration of the Fibrin Clots
3.2.5. Processing of the Fibrin Clots
3.2.6. Time from the Production of the PRF Membranes to Their Use
4. Discussion
5. Conclusions
- Larger PRF clots were obtained using plain GTs. The material of the blood collection tubes also influenced the distribution of platelets and leukocytes in the fibrin clot.
- The time from venipuncture to centrifugation should ideally be <1 min to achieve larger fibrin clots with a higher density and organisation of the fibrin mesh.
- By adapting the RCF for a given protocol, reproducible results can be obtained. Increasing the RCF increased the size of the fibrin clots with a denser and more polymerised fibrin mesh.
- Once the blood has been centrifuged, a 3–5 min rest period is recommended before removing the fibrin clots, as this has a positive effect on their size and physical properties.
- The use of specific surgical boxes is recommended for the dehydration of the fibrin clots in membranes. It is recommended to compress them for 2 min to achieve optimal dehydration and better physical properties.
- The “face” of the membranes is the area with the highest regenerative potential, so it will be placed towards the bed to be regenerated.
- Once the PRF membranes have been produced, they can be used for the next 300 min by adequately hydrating them from time to time using the GF-rich exudate.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Choukroun, J.; Adda, F.; Schoeffler, C.; Vervelle, A. Une Opportunité En Paro-Implantologie: Le PRF. Implantodontie 2001, 42, 55–62. [Google Scholar]
- Salgado-Peralvo, A.O.; Salgado-García, A.; Arriba-Fuente, L. New Tendencies in Tissue Regeneration: Leucocyte-Rich Platelet-Rich Fibrin. Rev. Esp. Cir. Oral Maxilofac. 2017, 39, 91–98. [Google Scholar] [CrossRef][Green Version]
- Sunitha Raja, V.; Munirathnam Naidu, E. Platelet-Rich Fibrin: Evolution of a Second-Generation Platelet Concentrate. Indian J. Dent. Res. 2008, 19, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Vinaya Kumar, R.; Shubhashini, N. Platelet Rich Fibrin: A New Paradigm in Periodontal Regeneration. Cell Tissue Bank 2013, 14, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Choukroun, J.; Diss, A.; Simonpieri, A.; Girard, M.-O.; Schoeffler, C.; Dohan, S.L.; Dohan, A.J.J.; Mouhyi, J.; Dohan, D.M. Platelet-Rich Fibrin (PRF): A Second-Generation Platelet Concentrate. Part IV: Clinical Effects on Tissue Healing. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 101, e56–e60. [Google Scholar] [CrossRef]
- Mihaylova, Z.; Mitev, V.; Stanimirov, P.; Isaeva, A.; Gateva, N.; Ishkitiev, N. Use of Platelet Concentrates in Oral and Maxillofacial Surgery: An Overview. Acta Odontol. Scand. 2017, 75, 1–11. [Google Scholar] [CrossRef]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.J.; Mouhyi, J.; Gogly, B. Platelet-Rich Fibrin (PRF): A Second-Generation Platelet Concentrate. Part II: Platelet-Related Biologic Features. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 101, e45–e50. [Google Scholar] [CrossRef]
- Pavlovic, V.; Ciric, M.; Jovanovic, V.; Trandafilovic, M.; Stojanovic, P. Platelet-Rich Fibrin: Basics of Biological Actions and Protocol Modifications. Open Med. 2021, 16, 446–454. [Google Scholar] [CrossRef]
- Herrera-Vizcaino, C. Systematic Review of Platelet-Rich Fibrin (PRF) Centrifugation Protocols in Oral and Maxillofacial Surgery and the Introduction of AR2T3: An Easy to Remember Acronym to Correctly Report Vertical and Horizontal PRF Centrifugation. Front. Oral Maxillofac. Med. 2021, 5, 1–20. [Google Scholar] [CrossRef]
- Ghanaati, S.; Booms, P.; Orlowska, A.; Kubesch, A.; Lorenz, J.; Rutkowski, J.; Landes, C.; Sader, R.; Kirkpatrick, C.; Choukroun, J. Advanced Platelet-Rich Fibrin: A New Concept for Cell-Based Tissue Engineering by Means of Inflammatory Cells. J. Oral Implantol. 2014, 40, 679–689. [Google Scholar] [CrossRef]
- Fujioka-Kobayashi, M.; Miron, R.J.; Hernandez, M.; Kandalam, U.; Zhang, Y.; Choukroun, J. Optimized Platelet-Rich Fibrin with the Low-Speed Concept: Growth Factor Release, Biocompatibility, and Cellular Response. J. Periodontol. 2017, 88, 112–121. [Google Scholar] [CrossRef]
- Tunalı, M.; Özdemir, H.; Küçükodacı, Z.; Akman, S.; Yaprak, E.; Toker, H.; Fıratlı, E. A Novel Platelet Concentrate: Titanium-Prepared Platelet-Rich Fibrin. BioMed Res. Int. 2014, 2014, 209548. [Google Scholar] [CrossRef] [PubMed]
- Mourão, C.F.d.A.B.; Valiense, H.; Melo, E.R.; Mourão, N.B.M.F.; Maia, M.D.-C. Obtention of Injectable Platelets Rich-Fibrin (i-PRF) and Its Polymerization with Bone Graft: Technical Note. Rev. Col. Bras. Cir. 2015, 42, 421–423. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.; Cortellini, S.; Temmerman, A.; Li, X.; Pinto, N.; Teughels, W.; Quirynen, M. Characterization of the Leukocyte- and Platelet-Rich Fibrin Block: Release of Growth Factors, Cellular Content, and Structure. Int. J. Oral Maxillofac. Implant. 2019, 34, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Salgado-Peralvo, A.O. Plasma Rico En Fibrina En Odontología: “Protocolo Abierto”; Salgado-Peralvo, A.O., Ed.; Amazon Fulfillment: Wroclaw, Poland, 2020. [Google Scholar]
- Dohan Ehrenfest, D.M.; del Corso, M.; Diss, A.; Mouhyi, J.; Charrier, J.B. Three-Dimensional Architecture and Cell Composition of a Choukroun’s Platelet-Rich Fibrin Clot and Membrane. J. Periodontol. 2010, 81, 546–555. [Google Scholar] [CrossRef]
- Dohan Ehrenfest, D.M.; Rasmusson, L.; Albrektsson, T. Classification of Platelet Concentrates: From Pure Platelet-Rich Plasma (P-PRP) to Leucocyte- and Platelet-Rich Fibrin (L-PRF). Trends Biotechnol. 2009, 27, 158–167. [Google Scholar] [CrossRef]
- Toronto, C.E. Overview of the Integrative Review. In A Step-by-Step Guide to Conducting an Integrative Review; Toronto, C.E., Remington, R., Eds.; Springer Nature: Cham, Switzerland, 2020; pp. 1–10. [Google Scholar]
- Andrade-Aldana, C.; Ugarte-Amenabar, F.; Inostroza-Silva, C.; Diaz-Calderon, P.; Rosenberg-Messina, D.; Pinto-Carrasco, N.; Quirynen, M. The Impact of Gender and Peripheral Blood Parameters on the Characteristics of L-PRF Membranes. J. Oral Biol. Craniofac. Res. 2022, 12, 753–759. [Google Scholar] [CrossRef]
- Miron, R.J.; Chai, J.; Fujioka-Kobayashi, M.; Sculean, A.; Zhang, Y. Evaluation of 24 Protocols for the Production of Platelet-Rich Fibrin. BMC Oral Health 2020, 20, 310. [Google Scholar] [CrossRef]
- Miron, R.J.; Xu, H.; Chai, J.; Wang, J.; Zheng, S.; Feng, M.; Zhang, X.; Wei, Y.; Chen, Y.; de Almeida Barros Mourão, C.F.; et al. Comparison of Platelet-Rich Fibrin (PRF) Produced Using 3 Commercially Available Centrifuges at Both High (~700 g) and Low (~200 g) Relative Centrifugation Forces. Clin. Oral Investig. 2020, 24, 1171–1182. [Google Scholar] [CrossRef]
- Castro, A.B.; Andrade, C.; Li, X.; Pinto, N.; Teughels, W.; Quirynen, M. Impact of g Force and Timing on the Characteristics of Platelet-Rich Fibrin Matrices. Sci. Rep. 2021, 11, 6038. [Google Scholar] [CrossRef]
- Bonazza, V.; Borsani, E.; Buffoli, B.; Castrezzati, S.; Rezzani, R.; Rodella, L.F. How the Different Material and Shape of the Blood Collection Tube Influences the Concentrated Growth Factors Production. Microsc. Res. Tech. 2016, 79, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S.; Aizawa, H.; Sato, A.; Tsujino, T.; Isobe, K.; Kitamura, Y.; Watanabe, T.; Okudera, H.; Mourão, C.F.; Kawase, T. Concentrated Growth Factor Matrices Prepared Using Silica-Coated Plastic Tubes are Distinguishable from Those Prepared Using Glass Tubes in Platelet Distribution: Application of a Novel near-Infrared Imaging-Based Quantitative Technique. Front. Bioeng. Biotechnol. 2020, 8, 600. [Google Scholar] [CrossRef] [PubMed]
- Tsujino, T.; Takahashi, A.; Yamaguchi, S.; Watanabe, T.; Isobe, K.; Kitamura, Y.; Tanaka, T.; Nakata, K.; Kawase, T. Evidence for Contamination of Silica Microparticles in Advanced Platelet-Rich Fibrin Matrices Prepared Using Silica-Coated Plastic Tubes. Biomedicines 2019, 7, 45. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, S.M. Safety Issues Associated with Platelet-Rich Fibrin Method. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2007, 103, 587. [Google Scholar] [CrossRef]
- Masuki, H.; Isobe, K.; Kawabata, H.; Tsujino, T.; Yamaguchi, S.; Watanabe, T.; Sato, A.; Aizawa, H.; Mourão, C.F.; Kawase, T. Acute Cytotoxic Effects of Silica Microparticles Used for Coating of Plastic Blood-Collection Tubes on Human Periosteal Cells. Odontology 2020, 108, 545–552. [Google Scholar] [CrossRef]
- Komosa, A.; Rzymski, P.; Perek, B.; Ropacka-Lesiak, M.; Lesiak, M.; Siller-Matula, J.M.; Poniedziałek, B. Platelets Redox Balance Assessment: Current Evidence and Methodological Considerations. Vascul. Pharmacol. 2017, 93–95, 6–13. [Google Scholar] [CrossRef]
- Bodega, G.; Alique, M.; Puebla, L.; Carracedo, J.; Ramírez, R.M. Microvesicles: ROS Scavengers and ROS Producers. J. Extracell. Vesicles 2019, 8, 1626654. [Google Scholar] [CrossRef]
- Ye, Z.-W.; Zhang, J.; Townsend, D.M.; Tew, K.D. Oxidative Stress, Redox Regulation and Diseases of Cellular Differentiation. Biochim. Biophys. Acta Gen. Subj. 2015, 1850, 1607–1621. [Google Scholar] [CrossRef]
- Moran, E.C.; Kamiguti, A.S.; Cawley, J.C.; Pettitt, A.R. Cytoprotective Antioxidant Activity of Serum Albumin and Autocrine Catalase in Chronic Lymphocytic Leukaemia. Br. J. Haematol. 2002, 116, 316–328. [Google Scholar] [CrossRef]
- Esfahrood, Z.; Ardakani, M.; Shokri, M.; Shokri, M. Effects of Leukocyte–Platelet-Rich Fibrin and Advanced Platelet-Rich Fibrin on the Viability and Migration of Human Gingival Fibroblasts. J. Indian Soc. Periodontol. 2020, 24, 15. [Google Scholar] [CrossRef]
- Choukroun, J.; Ghanaati, S. Reduction of Relative Centrifugation Force within Injectable Platelet-Rich Fibrin (PRF) Concentrates Advances Patients’ Own Inflammatory Cells, Platelets and Growth Factors: The First Introduction to the Low Speed Centrifugation Concept. Eur. J. Trauma Emerg. Surg. 2018, 44, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Peck, M.; Hiss, D.; Stephen, L. Factors Affecting the Preparation, Constituents, and Clinical Efficacy of Leukocyte- and Platelet-Rich Fibrin (L-PRF). S. Afr. Dent. J. 2016, 71, 298–302. [Google Scholar]
- Miron, R.J.; Pinto, N.R.; Quirynen, M.; Ghanaati, S. Standardization of Relative Centrifugal Forces in Studies Related to Platelet-rich Fibrin. J. Periodontol. 2019, 90, 817–820. [Google Scholar] [CrossRef] [PubMed]
- Tovar, N.; Benalcázar Jalkh, E.B.; Ramalho, I.S.; Rodriguez Colon, R.; Kim, H.; Bonfante, E.A.; Torroni, A.; Coelho, P.G.; Witek, L. Effects of Relative Centrifugation Force on L-PRF: An in Vivo Submandibular Boney Defect Regeneration Study. J. Biomed. Mater. Res. 2021, 109, 2237–2245. [Google Scholar] [CrossRef] [PubMed]
- el Bagdadi, K.; Kubesch, A.; Yu, X.; Al-Maawi, S.; Orlowska, A.; Dias, A.; Booms, P.; Dohle, E.; Sader, R.; Kirkpatrick, C.J.; et al. Reduction of Relative Centrifugal Forces Increases Growth Factor Release within Solid Platelet-Rich-Fibrin (PRF)-Based Matrices: A Proof of Concept of LSCC (Low Speed Centrifugation Concept). Eur. J. Trauma Emerg. Surg. 2019, 45, 467–479. [Google Scholar] [CrossRef]
- Kobayashi, E.; Flückiger, L.; Fujioka-Kobayashi, M.; Sawada, K.; Sculean, A.; Schaller, B.; Miron, R.J. Comparative Release of Growth Factors from PRP, PRF, and Advanced-PRF. Clin. Oral Investig. 2016, 20, 2353–2360. [Google Scholar] [CrossRef]
- Dohan-Ehrenfest, D.M.; Kang, B.S.; del Corso, M.; Nally, M.; Quirynen, M.; Wang, H.L.; Pinto, N. The Impact of Centrifuge Characteristics and Centrifugation Protocols on the Cells, Growth Factors and Fibrin Architecture of a Leukocyte- and Platelet-Rich Fibrin (L-PRF) Clot and Membrane. Part 1: Evaluation of the Vibration Shocks of 4 Models of Table Centrifuges for L-PRF. POSEIDO J. 2014, 2, 129–139. [Google Scholar]
- Dohan Ehrenfest, D.M.; Pinto, N.R.; Pereda, A.; Jiménez, P.; del Corso, M.; Kang, B.-S.; Nally, M.; Lanata, N.; Wang, H.-L.; Quirynen, M. The Impact of the Centrifuge Characteristics and Centrifugation Protocols on the Cells, Growth Factors, and Fibrin Architecture of a Leukocyte- and Platelet-Rich Fibrin (L-PRF) Clot and Membrane. Platelets 2018, 29, 171–184. [Google Scholar] [CrossRef]
- Pinto, N.R.; Pereda, A.; Jiménez, P.; del Corso, M.; Kang, B.S.; Wang, H.L.; Quirynen, M.; Dohan-Ehrenfest, D.M. The Impact of the Centrifuge Characteristics and Centrifugation Protocols on the Cells, Growth Factors and Fibrin Architecture of a Leukocyte- and Platelet-Rich Fibrin (L-PRF) Clot and Membrane. Part 2: Macroscopic, Photonic Microscopy and Scanning Electron Microscopy Analysis of 4 Kinds of L-PRF Clots and Membranes. POSEIDO J. 2014, 2, 141–154. [Google Scholar]
- Crisci, A.; Lombardi, D.; Serra, E.; Lombardi, G.; Cardillo, F.; Crisci, M. Standardized Protocol Proposed for Clinical Use of L-PRF and the Use of L-PRF Wound Box®. J. Unexplored Med. Data 2017, 2, 77. [Google Scholar] [CrossRef]
- Miron, R.J.; Chai, J.; Zheng, S.; Feng, M.; Sculean, A.; Zhang, Y. A Novel Method for Evaluating and Quantifying Cell Types in Platelet Rich Fibrin and an Introduction to Horizontal Centrifugation. J. Biomed. Mater. Res. A 2019, 107, 2257–2271. [Google Scholar] [CrossRef]
- Block Scientific Horizontal versus Fixed Angle Centrifugation of Blood Specimens. Available online: https://blockscientific.com/feature-horizontal-versus-fixed-angle-centrifugation-of-blood-specimens (accessed on 30 October 2022).
- Fujioka-Kobayashi, M.; Kono, M.; Katagiri, H.; Schaller, B.; Zhang, Y.; Sculean, A.; Miron, R.J. Histological Comparison of Platelet Rich Fibrin Clots Prepared by Fixed-Angle versus Horizontal Centrifugation. Platelets 2021, 32, 413–419. [Google Scholar] [CrossRef]
- Wei, Y.; Cheng, Y.; Wang, Y.; Zhang, X.; Miron, R.J.; Zhang, Y. The Effect of Resting and Compression Time Post-Centrifugation on the Characteristics of Platelet Rich Fibrin (PRF) Membranes. Clin. Oral. Investig. 2022, 26, 5281–5288. [Google Scholar] [CrossRef] [PubMed]
- Dohan-Ehrenfest, D.M. How to Optimize the Preparation of Leukocyte- and Platelet-Rich Fibrin (L-PRF, Choukroun´s Technique) Clots and Membranes: Introducing the PRF Box. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2010, 110, 278–280. [Google Scholar] [CrossRef]
- Kobayashi, M.; Kawase, T.; Horimizu, M.; Okuda, K.; Wolff, L.F.; Yoshie, H. A Proposed Protocol for the Standardized Preparation of PRF Membranes for Clinical Use. Biologicals 2012, 40, 323–329. [Google Scholar] [CrossRef]
- Dohan Ehrenfest, D.M.; de Peppo, G.M.; Doglioli, P.; Sammartino, G. Slow Release of Growth Factors and Thrombospondin-1 in Choukroun’s Platelet-Rich Fibrin (PRF): A Gold Standard to Achieve for All Surgical Platelet Concentrates Technologies. Growth Factors 2009, 27, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Thanasrisuebwong, P.; Surarit, R.; Bencharit, S.; Ruangsawasdi, N. Influence of Fractionation Methods on Physical and Biological Properties of Injectable Platelet-Rich Fibrin: An Exploratory Study. Int. J. Mol. Sci. 2019, 20, 1657. [Google Scholar] [CrossRef]
- Bai, M.-Y.; Chuang, M.-H.; Lin, M.-F.; Tang, S.-L.; Wong, C.-C.; Chan, W.P. Relationships of Age and Sex with Cytokine Content and Distribution in Human Platelet Fibrin Gels. Sci. Rep. 2018, 8, 10642. [Google Scholar] [CrossRef]
- Miron, R.J.; Chai, J.; Zhang, P.; Li, Y.; Wang, Y.; Mourão, C.F.d.A.B.; Sculean, A.; Kobayashi, M.F.; Zhang, Y. Novel Method for Harvesting Concentrated Platelet-Rich Fibrin (C-PRF) with a 10-Fold Increase in Platelet and Leukocyte Yields. Clin. Oral Investig. 2020, 24, 2819–2828. [Google Scholar] [CrossRef]
- Fujioka-Kobayashi, M.; Katagiri, H.; Kono, M.; Schaller, B.; Zhang, Y.; Sculean, A.; Miron, R.J. Improved Growth Factor Delivery and Cellular Activity Using Concentrated Platelet-Rich Fibrin (C-PRF) When Compared with Traditional Injectable (i-PRF) Protocols. Clin. Oral Investig. 2020, 24, 4373–4383. [Google Scholar] [CrossRef]
- Su, C.Y.; Kuo, Y.P.; Tseng, Y.H.; Su, C.-H.; Burnouf, T. In Vitro Release of Growth Factors from Platelet-Rich Fibrin (PRF): A Proposal to Optimize the Clinical Applications of PRF. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 108, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Soloviev, D.A.; Hazen, S.L.; Szpak, D.; Bledzka, K.M.; Ballantyne, C.M.; Plow, E.F.; Pluskota, E. Dual Role of the Leukocyte Integrin AMß2 in Angiogenesis. J. Immunol. 2014, 193, 4712–4721. [Google Scholar] [CrossRef]
- Kawazoe, T.; Kim, H.H. Tissue Augmentation by White Blood Cell-Containing Platelet-Rich Plasma. Cell Transplant. 2012, 21, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Bennardo, F.; Gallelli, L.; Palleria, C.; Colosimo, M.; Fortunato, L.; De Sarro, G.; Giudice, A. Can Platelet-Rich Fibrin Act as a Natural Carrier for Antibiotics Delivery? A Proof-of-Concept Study for Oral Surgical Procedures. BMC Oral Health 2023, 23, 134. [Google Scholar] [CrossRef]
- Polak, D.; Clemer-Shamai, N.; Shapira, L. Incorporating Antibiotics into Platelet-Rich Fibrin: A Novel Antibiotics Slow-Release Biological Device. J. Clin. Periodontol. 2019, 46, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Salgado-Peralvo, A.O. Historia Clínica y Exploración En Implantología Oral; Salgado-Peralvo, A.O., Ed.; Amazon Fulfillment: Wroclaw, Poland, 2019. [Google Scholar]
- Sudrajat, N.; Nurhidayat, B.R.; Putra, O.S.; Loebis, R.; Zuhria, I. Relation between Blood Parameters and Platelet-Rich Fibrin Membrane Size for Ocular Graft. NeuroQuantology 2022, 20, 454–459. [Google Scholar] [CrossRef]
- Miron, R.J.; Dham, A.; Dham, U.; Zhang, Y.; Pikos, M.A.; Sculean, A. The Effect of Age, Gender, and Time between Blood Draw and Start of Centrifugation on the Size Outcomes of Platelet-Rich Fibrin (PRF) Membranes. Clin. Oral Investig. 2019, 23, 2179–2185. [Google Scholar] [CrossRef] [PubMed]
- Kurachi, K.; Kurachi, S. Genetic Mechanisms of Age Regulation of Blood Coagulation. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 902–906. [Google Scholar] [CrossRef]
- Mari, D.; Ogliari, G.; Castaldi, D.; Vitale, G.; Bollini, E.M.; Lio, D. Hemostasis and Ageing. Immun. Ageing 2008, 5, 12. [Google Scholar] [CrossRef]
- Amin, H.; Mohsin, S.; Aslam, M.; Hussain, S.; Saeed, T.; Ullah, M.I.; Sami, W. Coagulation Factors and Antithrombin Levels in Young and Elderly Subjects in Pakistani Population. Blood Coagul. Fibrinolysis 2012, 23, 745–750. [Google Scholar] [CrossRef]
- Canonico, M.; Brailly-Tabard, S.; Gaussem, P.; Setiao, J.; Rouaud, O.; Ryan, J.; Carcaillon, L.; Guiochon-Mantel, A.; Scarabin, P.-Y. Endogenous Oestradiol as a Positive Correlate of Plasma Fibrinogen among Older Postmenopausal Women: A Population-Based Study (the Three-City Cohort Study). Clin. Endocrinol. 2012, 77, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Vorster, H.; Jerling, J.; Steyn, K.; Badenhorst, C.; Slazus, W.; Venter, C.; Jooste, P.; Bourne, L. Plasma Fibrinogen of Black South Africans: The BRISK Study. Public Health Nutr. 1998, 1, 169–176. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ockerman, A.; Braem, A.; EzEldeen, M.; Castro, A.; Coucke, B.; Politis, C.; Verhamme, P.; Jacobs, R.; Quirynen, M. Mechanical and Structural Properties of Leukocyte- and Platelet-rich Fibrin Membranes: An in Vitro Study on the Impact of Anticoagulant Therapy. J. Periodontal Res. 2020, 55, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Dohan Ehrenfest, D.M.; Andia, I.; Zumstein, M.A.; Zhang, C.-Q.; Pinto, N.R.; Bielecki, T. Classification of Platelet Concentrates (Platelet-Rich Plasma-PRP, Platelet-Rich Fibrin-PRF) for Topical and Infiltrative Use in Orthopedic and Sports Medicine: Current Consensus, Clinical Implications and Perspectives. Muscles Ligaments Tendons J. 2014, 4, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Salgado-Peralvo, A.O.; Mateos-Moreno, M.V.; Uribarri, A.; Kewalramani, N.; Peña-Cardelles, J.F.; Velasco-Ortega, E. Treatment of Oroantral Communication with Platelet-Rich Fibrin: A Systematic Review. J. Stomatol. Oral Maxillofac. Surg. 2022, 123, e367–e375. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.; Choukroun, J.; Ghanaati, S. Controversies Related to Scientific Report Describing G-Forces from Studies on Platelet-Rich Fibrin: Necessity for Standardization of Relative Centrifugal Force Values. Int. J. Growth Factors Stem Cells Dent. 2018, 1, 80. [Google Scholar] [CrossRef]


| Author | Year | Type of Study | Phase of the PRF Protocol Analysed | |||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |||
| Pavlovic et al. [8] | 2021 | Literature review | X | |||||
| Herrera-Vizcaina, C [9] | 2021 | Systematic review | X | |||||
| Ghanaati et al. [10] | 2014 | Comparative in vitro study | X | |||||
| Fujioka-Kobayashi et al. [11] | 2017 | Comparative in vitro study | X | |||||
| Tunali et al. [12] | 2014 | Comparative in vitro study | X | |||||
| Dohan Ehrenfest et al. [16] | 2010 | Comparative in vitro study | X | |||||
| Andrade-Aldana et al. [19] | 2022 | Comparative in vitro study | X | |||||
| Miron et al. [20] | 2020 | Comparative in vitro study | X | |||||
| Miron et al. [21] | 2020 | Comparative in vitro study | X | X | X | |||
| Castro et al. [22] | 2021 | Comparative in vitro study | X | X | X | X | X | |
| Bonazza et al. [23] | 2016 | Comparative in vitro study | X | |||||
| Yamaguchi et al. [24] | 2020 | Comparative in vitro study | X | |||||
| Tsujino et al. [25] | 2019 | Comparative in vitro study | X | |||||
| O’Connell, SM [26] | 2007 | Letter to the editor | X | |||||
| Masuki et al. [27] | 2020 | Comparative in vitro study | X | |||||
| Komosa et al. [28] | 2017 | Literature review | X | |||||
| Bodega et al. [29] | 2019 | Literature review | X | |||||
| Ye et al. [30] | 2015 | Literature review | X | |||||
| Moran et al. [31] | 2002 | Comparative in vitro study | X | |||||
| Esfahrood et al. [32] | 2020 | Comparative in vitro study | X | |||||
| Choukroun et al. [33] | 2018 | Comparative in vitro study | X | |||||
| Salgado-Peralvo et al. [15] | 2020 | Book | X | |||||
| Peck et al. [34] | 2016 | Literature review | X | |||||
| Miron et al. [35] | 2019 | Guest editorial | X | |||||
| Tovar et al. [36] | 2021 | Animal in vitro study | X | |||||
| El Bagdadi et al. [37] | 2019 | Comparative in vitro study | X | |||||
| Kobayashi et al. [38] | 2016 | Comparative in vitro study | X | |||||
| Dohan-Ehrenfest et al. [39] | 2014 | Comparative in vitro study | X | |||||
| Dohan-Ehrenfest et al. [40] | 2018 | Comparative in vitro study | X | |||||
| Pinto et al. [41] | 2014 | Comparative in vitro study | X | |||||
| Crisci et al. [42] | 2017 | Animal in vitro study | X | X | ||||
| Miron et al. [43] | 2019 | Comparative in vitro study | X | |||||
| Block Scientific [44] | 2019 | Web page | X | |||||
| Fujioka-Kobayashi et al. [45] | 2021 | Comparative in vitro study | X | X | ||||
| Wei et al. [46] | 2022 | Comparative in vitro study | X | X | ||||
| Dohan-Ehrenfest et al. [47] | 2010 | Letter to the editor | X | X | X | |||
| Kobayashi et al. [48] | 2012 | Comparative in vitro study | X | |||||
| Dohan-Ehrenfest et al. [49] | 2009 | Comparative in vitro study | X | X | ||||
| Thanasrisuebwong et al. [50] | 2019 | Comparative in vitro study | X | |||||
| Bai et al. [51] | 2018 | Animal in vitro study | X | |||||
| Miron et al. [52] | 2020 | Comparative in vitro study | X | |||||
| Fujioka-Kobayashi et al. [53] | 2020 | Comparative in vitro study | X | |||||
| Su et al. [54] | 2009 | Comparative in vitro study | X | |||||
| Acronym | Protocol (Commercial Firm) | RPM 1 | RCFClot 2 | RCFmax | Duration | Material of the Blood Collection Tubes |
|---|---|---|---|---|---|---|
| CGF | Concentrated growth factors (Medifuge®, Silfradent™, Sofia, Italy) | 2700 | UNS 3 | UNS | 2 min | Plain PTs 5 |
| 2400 | UNS | UNS | 4 min | |||
| 2700 | UNS | UNS | 4 min | |||
| 3000 | UNS | UNS | 3 min | |||
| T-PRF | Titanium platelet-rich fibrin | 2800 | UNS | ~400 g 4 | 12 min | Grade IV titanium tubes |
| L-PRF | Leukocyte- and platelet-rich fibrin (Intra-Lock™, Boca Raton, FL, USA) | 2700 | ~408 g | ~653 g | 12 min | scPTs 6 |
| A-PRF | Advanced platelet-rich fibrin (Process™ for PRF, Nice, France) | 1500 | ~193 g | ~276 g | 14 min | Plain GTs 7 |
| A-PRF+ | Advanced platelet-rich fibrin plus (Process™ for PRF, Nice, France) | 1300 | ~145 g | ~208 g | 8 min | Plain GTs |
| i-PRF | Inyectable platelet-rich fibrin (Intra-Lock™, Boca Raton, FL, USA) | 700 | UNS | ~60 g | 3 min | Plain PTs |
| C-PRF | Concentrated platelet-rich fibrin (Not registered) | UNS | ~408 g | UNS | 12 min | Plain PTs |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salgado-Peralvo, Á.-O.; Kewalramani, N.; Pérez-Jardón, A.; Pato-Mourelo, J.; Castro-Calderón, A.; Arriba-Fuente, L.; Pérez-Sayáns, M. Understanding Solid-Based Platelet-Rich Fibrin Matrices in Oral and Maxillofacial Surgery: An Integrative Review of the Critical Protocol Factors and Their Influence on the Final Product. Medicina 2023, 59, 1903. https://doi.org/10.3390/medicina59111903
Salgado-Peralvo Á-O, Kewalramani N, Pérez-Jardón A, Pato-Mourelo J, Castro-Calderón A, Arriba-Fuente L, Pérez-Sayáns M. Understanding Solid-Based Platelet-Rich Fibrin Matrices in Oral and Maxillofacial Surgery: An Integrative Review of the Critical Protocol Factors and Their Influence on the Final Product. Medicina. 2023; 59(11):1903. https://doi.org/10.3390/medicina59111903
Chicago/Turabian StyleSalgado-Peralvo, Ángel-Orión, Naresh Kewalramani, Alba Pérez-Jardón, Jesús Pato-Mourelo, Adriana Castro-Calderón, Lorenzo Arriba-Fuente, and Mario Pérez-Sayáns. 2023. "Understanding Solid-Based Platelet-Rich Fibrin Matrices in Oral and Maxillofacial Surgery: An Integrative Review of the Critical Protocol Factors and Their Influence on the Final Product" Medicina 59, no. 11: 1903. https://doi.org/10.3390/medicina59111903
APA StyleSalgado-Peralvo, Á.-O., Kewalramani, N., Pérez-Jardón, A., Pato-Mourelo, J., Castro-Calderón, A., Arriba-Fuente, L., & Pérez-Sayáns, M. (2023). Understanding Solid-Based Platelet-Rich Fibrin Matrices in Oral and Maxillofacial Surgery: An Integrative Review of the Critical Protocol Factors and Their Influence on the Final Product. Medicina, 59(11), 1903. https://doi.org/10.3390/medicina59111903








