The Role of Pre- and Post-Transplant Hydration Status in Kidney Graft Recovery and One-Year Function
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Evaluation of Early Graft Function
2.3. Study Population
2.4. Determining the Volume Status
2.5. Post Hoc Analysis of Relation between Left Ventricular Hypertrophy and Overhydration
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics and Clinical Data before Transplantation
3.2. Postoperative Data on Days 3 and 7 and on Discharge
3.3. Changes in Volemic Status during the Observation Period
3.4. Relation of the Recipient’s Volemic Status and Graft Function after 1 Year
3.5. Post Hoc Analysis of Relation between Left Ventricular Hypertrophy and Overhydration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kasiske, B.L.; Zeier, M.G. KDIGO Clinical practice guideline for the care of kidney transplantation recipients. Am. J. Transplant. 2009, 9 (Suppl. 3), S1–S155. [Google Scholar]
- Perico, N.; Cattaneo, D.; Sayegh, M.H.; Remuzzi, G. Delayed graft function in kidney transplantation. Lancet 2004, 364, 1814. [Google Scholar] [CrossRef] [PubMed]
- Melih, K.V.; Boynuegri, B.; Mustafa, C.; Nilgun, A. Incidence, risk factors, and outcomes of delayed graft function in deceased donor kidney transplantation. Transpl. Proc. 2019, 51, 1096. [Google Scholar] [CrossRef] [PubMed]
- Van Loo, A.A.; Vanholder, R.C.; Bernaert, P.R.; Vermassen, F.E.; van der Vennet, M.; Lameire, N.H. Pretransplantation hemodialysis strategy influences early renal graft function. J. Am. Soc. Nephrol. 1998, 9, 473. [Google Scholar] [CrossRef]
- Siedlecki, A.; Irish, W.; Brennan, D.C. Delayed graft function in kidney transplant. Am. J. Transplant. 2011, 11, 2279. [Google Scholar] [CrossRef] [PubMed]
- Coroas, A.; Oliveira, J.; Sampaio, S.; Borges, C.; Tavares, I.; Pestana, M.; Almeida, M. Sequential body composition analysis by bioimpedance early post-kidney transplantation. Transpl. Int. 2005, 18, 541. [Google Scholar] [CrossRef] [PubMed]
- Gueutin, V.; Ficheux, M.; Châtelet, V.; Lecouf, A.; Henri, P.; de Ligny, B.H.; Ryckelynck, J.-P.; Lobbedez, T. Hydration status of patients with end-stage renal disease after kidney transplantation. Clin. Transplant. 2011, 25, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, A.; Italian CAPD-BIA Study Group. Bioelectric impedance vector distribution in peritoneal dialysis patients with different hydration statuses. Kidney Int. 2004, 65, 1050–1063. [Google Scholar] [CrossRef]
- Payen, D.; de Pont, A.C.; Sakr, Y.; Spies, C.; Reinhart, K.; Vincent, J.L. A positive fluid balance is associated with a worse outcome in patients with acute renal failure. Crit. Care 2008, 12, R74. [Google Scholar] [CrossRef]
- Reisinger, N.; Koratala, A. Quantitative Lung Ultrasonography for the Nephrologist: Applications in Dialysis and Heart Failure. Kidney360 2021, 3, 176–184. [Google Scholar] [CrossRef]
- Yarlagadda, S.G.; Coca, S.G.; Garg, A.X.; Doshi, M.; Poggio, E.; Marcus, R.J.; Parikh, C.R. Marked variation in the definition and diagnosis of delayed graft function: A systematic review. Nephrol. Dial. Transplant. 2008, 23, 2995–3003. [Google Scholar] [CrossRef]
- Decruyenaere, P.; Decruyenaere, A.; Vermassen, F.; Peters, P. Twenty-two competing definitions for delayed graft function after kidney transplantation: A single-centre comparison. ESOT 2015, 28, 230. [Google Scholar]
- Mallon, D.H.; Summers, D.M.; Bradley, J.A.; Pettigrew, G.J. Defining delayed graft function after renal transplantation: Simplest is best. Transplantation 2013, 96, 885–889. [Google Scholar] [CrossRef] [PubMed]
- Boom, H.; Mallat, M.J.; de Fijter, J.W.; Zwinderman, A.H.; Paul, L.C. Delayed graft function influences renal function, but not survival. Kidney Int. 2000, 58, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, E.; Ruiz, J.C.; Pinera, C.; Fernandez-Fresnedo, G.; Escallada, R.; Palomar, R.; Cotorruelo, J.G.; Zubimendi, J.A.; Martin de Francisco, A.L.; Arias, M. Creatinine reduction ratio on post-transplant day two as a criterion in defining delayed graft function. Am. J. Transplant. 2004, 4, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Hall, I.E.; Yarlagadda, S.G.; Coca, S.G.; Wang, Z.; Doshi, M.; Devarajan, P.; Han, W.K.; Marcus, R.J.; Parikh, C.R. IL-18 and urinary NGAL predict dialysis and graft recovery after kidney transplantation. J. Am. Soc. Nephrol. 2010, 21, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Zoccali, C.; Torino, C.; Tripepi, R.; Tripepi, G.; D’Arrigo, G.; Postorino, M.; Gargani, L.; Sicari, R.; Picano, E.; Mallamaci, F. Pulmonary Congestion Predicts Cardiac Events and Mortality in ESRD. J. Am. Soc. Nephrol. 2013, 24, 639–646. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef] [PubMed]
- Smudla, A.; Trimmel, D.; Szabó, G.; Fazakas, J. Systolic Blood Pressure Pattern: The Tick Mark Signal of Delayed Renal Graft Function. Transplant. Proc. 2019, 51, 1226–1230. [Google Scholar] [CrossRef]
- Campos, L.; Parada, B.; Furriel, F.; Castelo, D.; Moreira, P.; Mota, A. Do intraoperative hemodynamic factors of the recipient influence renal graft function? Transplant. Proc. 2012, 44, 1800–1803. [Google Scholar] [CrossRef]
- Germain, M.J.; Greco, B.A.; Hodgins, S.; Chapagain, B.; Thadhani, R.; Wojciechowski, D.; Crisalli, K.; Nathanson, B.H.; Chait, Y. Assessing the accuracy of estimated dry weight in dialysis patients post-transplantation: The kidney knows best. J. Nephrol. 2021, 34, 2093–2097. [Google Scholar] [CrossRef] [PubMed]
- Panuccio, V.; Tripepi, R.; Parlongo, G.; Mafrica, A.; Caridi, G.; Catalano, F.; Marino, F.; Tripepi, G.; Mallamaci, F.; Zoccali, C. Lung ultrasound to detect and monitor pulmonary congestion in patients with acute kidney injury in nephrology wards: A pilot study. J. Nephrol. 2020, 33, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Vitturi, N.; Dugo, M.; Soattin, M.; Simoni, F.; Maresca, L.; Zagatti, R.; Maresca, M.C. Lung ultrasound during hemodialysis: The role in the assessment of volume status. Int. Urol. Nephrol. 2014, 46, 169–174. [Google Scholar] [CrossRef]
- Douglas, I.S.; Alapat, P.M.; Corl, K.A.; Exline, M.C.; Forni, L.G.; Holder, A.L.; Kaufman, D.A.; Khan, A.; Levy, M.M.; Martin, G.S.; et al. Fluid response evaluation in sepsis hypotension and shock: A randomized clinical trial. Chest 2020, 158, 1431–1445. [Google Scholar] [CrossRef] [PubMed]
- Argaiz, E.R.; Rola, P.; Haycock, K.H.; Verbrugge, F.H. Fluid management in acute kidney injury: From evaluating fluid responsiveness towards assessment of fluid tolerance. Eur. Heart J. Acute Cardiovasc. Care 2022, 11, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Picano, E.; Frassi, F.; Agricola, E.; Gligorova, S.; Gargani, L.; Mottola, G. Ultrasound lung comets: A clinically useful sign of extravascular lung water. J. Am. Soc. Echocardiogr. 2006, 19, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Agricola, E.; Bove, T.; Oppizzi, M.; Marino, G.; Zangrillo, A.; Margonato, A.; Picano, E. “Ultrasound comet-tail images”: A marker of pulmonary edema: A comparative study with wedge pressure and extravascular lung water. Chest 2005, 127, 1690–1695. [Google Scholar] [CrossRef] [PubMed]
- Loutradis, C.; Papadopoulos, C.E.; Sachpekidis, V.; Ekart, R.; Krunic, B.; Karpetas, A.; Bikos, A.; Tsouchnikas, I.; Mitsopoulos, E.; Papagianni, A.; et al. Lung Ultrasound-Guided Dry Weight Assessment and Echocardiographic Measures in Hypertensive Hemodialysis Patients: A Randomized Controlled Study. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2020, 75, 11–20. [Google Scholar] [CrossRef]
- Vlahu, C.A.; Lemkes, B.A.; Struijk, D.G.; Koopman, M.G.; Krediet, R.T.; Vink, H. Damage of the endothelial glycocalyx in dialysis patients. J. Am. Soc. Nephrol. 2012, 23, 1900–1908. [Google Scholar] [CrossRef]
- Van den Berg, B.M.; Vink, H.; Spaan, J.A.E. The endothelial glycocalyx protects against myocardial edema. Circ. Res. 2003, 92, 592–594. [Google Scholar] [CrossRef]
- Snoeijs, M.G.; Vink, H.; Voesten, N.; Christiaans, M.H.; Daemen, J.W.; Peppelenbosch, A.G.; Tordoir, J.H.; Peutz-Kootstra, C.J.; Buurman, W.A.; Schurink, G.W.; et al. Acute ischemic injury to the renal microvasculature in human kidney transplantation. Am. J. Physiol. Renal Physiol. 2010, 299, F1134–F1140. [Google Scholar] [CrossRef]
- Liew, H.; Roberts, M.A.; McMahon, L.P. Markers of the endothelial glycocalyx are improved following kidney transplantation. Kidney Blood Press. Res. 2021, 46, 581–587. [Google Scholar] [CrossRef]

| IGF 1 | SGF + DGF 1 | ||
|---|---|---|---|
| Recipients | n = 51 | n = 47 | p |
| Gender male | 26 (51%) | 25 (53.2%) | 0.827 |
| Age (years) | 50.65 (12.07) | 47.94 (12.57) | 0.279 |
| Duration of kidney replacement therapy (months) | 22.00 (9.0–49.0) | 20.00 (9.0–37.0) | 0.326 |
| Residual diuresis (mL/24 h) | 500 (0.0–1000.0) | 600 (0.0–1500.0) | 0.413 |
| Creatinine (μmol/L) | 774 (618.0–960.0) | 646 (526.0–839.0) | 0.032 |
| Urea (mmol/L) | 19.4 (13.2–24.7) | 17.2 (11.80–23.30) | 0.413 |
| Sodium (mmol/L) | 136 (134.0–138.0) | 137 (134.0–139.0) | 0.609 |
| Serum total protein (g/L) | 72.65 (6.51) | 73.70 (5.96) | 0.414 |
| Hemoglobin (g/L) | 123 (114–129) | 123 (114.0–130.0) | 0.613 |
| CRP (mg/L) | 5 (1.5–5.0) | 3 (1.32–5.0) | 0.305 |
| BMI (kg/m2) | 25.18 (4.69) | 26.27 (5.75) | 0.349 |
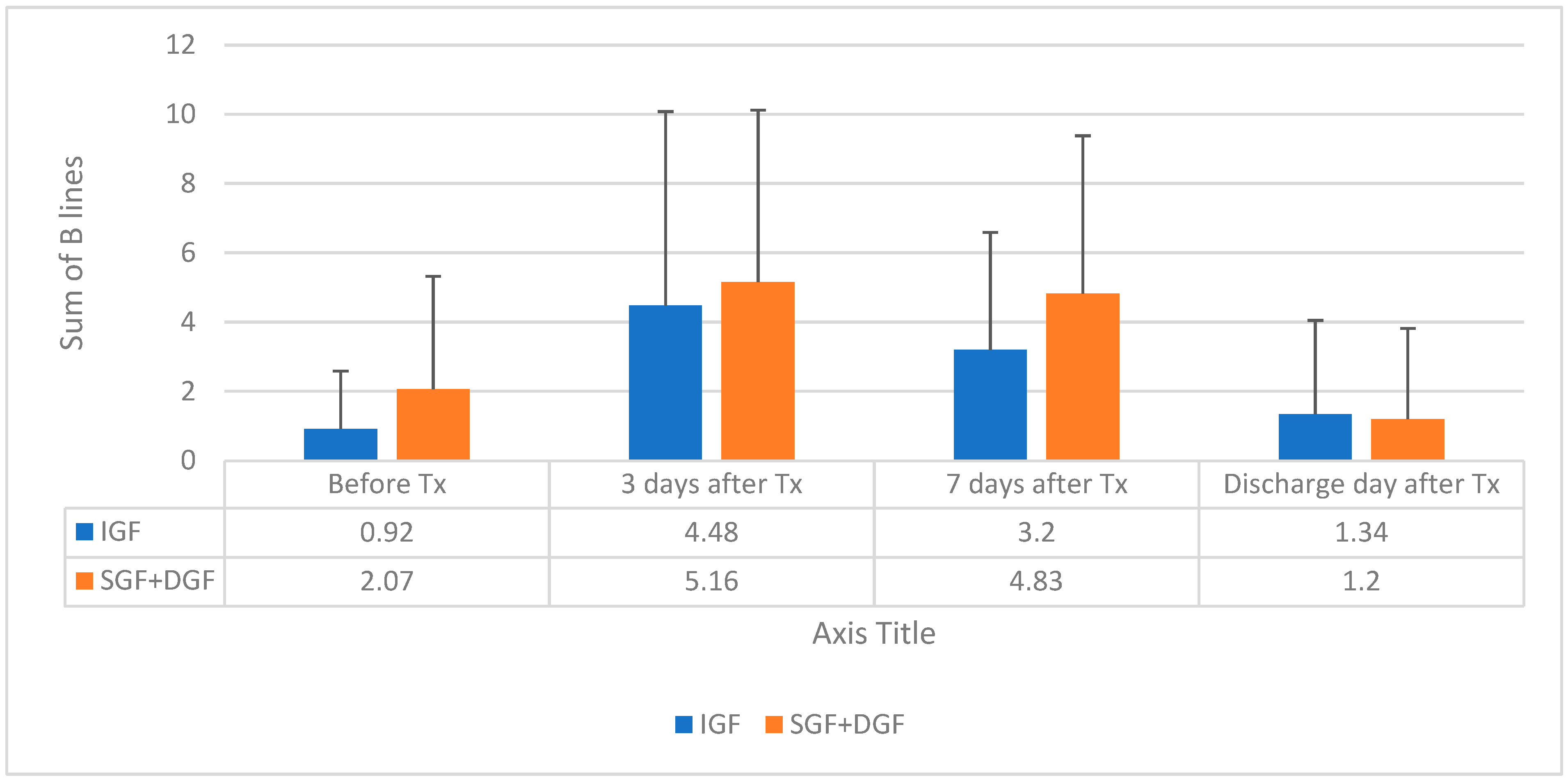
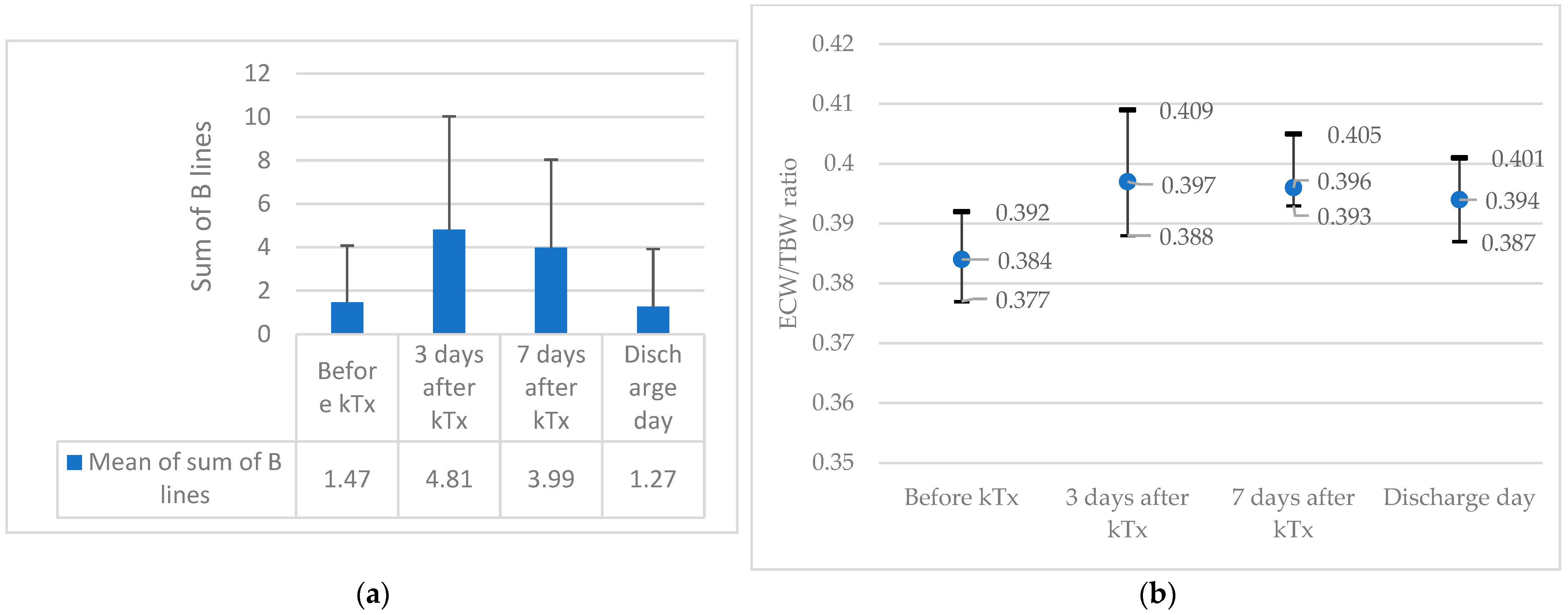
| Sum of BL 2 | 0.92 (1.67) | 2.07 (3.25) | 0.037 |
| ECW/TBW ratio 3 | 0.381 (0.375–0.389) | 0.381 (0.374–0.390) | 0.646 |
| Amount of fluid per transplantation day (mL/kg) | 33.22 (11.75) | 33.05 (14.61) | 0.952 |
| Donor data | |||
| Age (years) | 46 (32.0–56.0) | 54 (48.0–60.0) | 0.003 |
| Expanded criteria donor 4 | 5 (20%) | 6 (25%) | 0.347 |
| Diuresis per hour (mL/h) | 112.5 (87.5–166.67) | 116.67 (95.83–165.0) | 0.91 |
| Cold ischemic time of transplanted kidney (min) | 840 (720.0–1020.0) | 960 (800.0–1080.0) | 0.044 |
| Model | Odds Ratio (95% CI) | p |
|---|---|---|
| 1. Unadjusted analysis | ||
| More than 1 BL 1 | 2.514 (1.079–5.858) | 0.033 |
| 2. Adjusted analysis | ||
| Cold ischemic time (minutes) | 1.001 (0.999–1.002) | 0.372 |
| Donor age (years) | 1.038 (1.004–1.073) | 0.026 |
| More than 1 BL 1 | 1.169 (0.941–1.452) | 0.159 |
| IGF 1 | SGF+DGF 1 | ||
|---|---|---|---|
| Recipients | n = 51 | n = 47 | p |
| mGFR (mL/min/1.73 m2) | 54.0 (29.0–71.0) | 16.0 (8.0–34.5) | 0.001 |
| Serum Sodium (mmol/L) | 137.0 (134.0–139.0) | 136.0 (133.0–139.0) | 0.125 |
| Serum albumin (g/L) | 31.0 (30.0–33.38) | 31.0 (30.0–34.0) | 0.664 |
| Hemoglobin (g/L) | 94.79 (16.37) | 92.74 (12.78) | 0.592 |
| CRP (mg/L) | 17.2 (7.5–26.0) | 27.0 (11.9–46.56) | 0.013 |
| Sum of BL 2 | 4.48 (5.6) | 5.16 (4.96) | 0.54 |
| ECW/TBW ratio 3 | 0.394 (0.385–0.404) | 0.395 (0.387–0.404) | 0.538 |
| Diuresis per 24 h (mL/24 h) | 3622.45 (1623.53) | 2475.39 (1534.92) | 0.001 |
| Fluid therapy per 24 h (mL/kg/24 h) | 46.86 (17.68) | 39.55 (17.42) | 0.068 |
| IGF 1 | SGF + DGF 1 | ||
|---|---|---|---|
| Recipients | n = 51 | n = 47 | p |
| mGFR (mL/min/1.73 m2) | 64.94 (29.66) | 30.38 (20.48) | 0.001 |
| Serum Sodium (mmol/L) | 136.0 (135.0–138.0) | 136.0 (135.0–138.0) | 0.72 |
| Serum albumin (g/L) | 33.0 (31.0–35.0) | 33.0 (29.5–36.0) | 0.646 |
| Hemoglobin (g/L) | 102.08 (12.48) | 96.62 (13.69) | 0.042 |
| CRP (mg/L) | 5.0 (2.03–5.0) | 6.35 (3.88–16.43) | 0.002 |
| Sum of BL 2 | 3.2 (3.39) | 4.83(4.55) | 0.064 |
| ECW/TBW ratio 3 | 0.396 (0.390–0.406) | 0.396 (0.390–0.404) | 0.503 |
| Diuresis per 24 h (mL/24 h) | 3622.45 (1623.53) | 2475.06 (1534.92) | 0.001 |
| Fluid therapy per 24 h (mL/kg/24 h) | 54.68 (18.13) | 38.83 (34.58) | 0.001 |
| IGF 1 | SGF+DGF 1 | ||
|---|---|---|---|
| Recipients | n = 51 | n = 47 | p |
| mGFR (mL/min/1.73 m2) | 64.5 (24.23) | 41.57 (17.79) | 0.001 |
| Serum Sodium (mmol/L) | 136.0 (134.0–137.25) | 137.0 (135.0–138.0) | 0.092 |
| Serum albumin (g/L) | 34.3 (31.0–37.0) | 34.4 (32.2–37.0) | 0.482 |
| Hemoglobin (g/L) | 112.84 (13.77) | 102.49 (13.50) | 0.001 |
| CRP (mg/L) | 5 (1.19–5.0) | 4.7 (1.0–5.0) | 0.919 |
| Sum of BL 2 | 1.34 (2.71) | 1.20 (2.62) | 0.823 |
| ECW/TBW ratio 3 | 0.396 (0.390–0.406) | 0.396 (0.393–0.407) | 0.973 |
| Diuresis per 24 h (mL/24 h) | 3479.41 (963.8) | 3066.3 (1093.72) | 0.051 |
| Fluid therapy per 24 h (mL/kg/24 h) | 44.5 (12.63) | 40.87 (13.88) | 0.261 |
| Model | Odds Ratio (95% CI) | p |
|---|---|---|
| Adjusted analysis | ||
| Cold ischemic time (minutes) | 1.000 (0.998–1.002) | 0.848 |
| Donor age (years) | 0.936 (0.885–0.990) | 0.021 |
| More than 1 BL 1 | 1.090 (0.279–4.264) | 0.902 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bura, A.; Kaupe, V.; Karpaviciute, J.; Stankuviene, A.; Vaiciunas, K.; Bumblyte, I.A.; Vaiciuniene, R. The Role of Pre- and Post-Transplant Hydration Status in Kidney Graft Recovery and One-Year Function. Medicina 2023, 59, 1931. https://doi.org/10.3390/medicina59111931
Bura A, Kaupe V, Karpaviciute J, Stankuviene A, Vaiciunas K, Bumblyte IA, Vaiciuniene R. The Role of Pre- and Post-Transplant Hydration Status in Kidney Graft Recovery and One-Year Function. Medicina. 2023; 59(11):1931. https://doi.org/10.3390/medicina59111931
Chicago/Turabian StyleBura, Andrejus, Vaiva Kaupe, Justina Karpaviciute, Asta Stankuviene, Kestutis Vaiciunas, Inga Arune Bumblyte, and Ruta Vaiciuniene. 2023. "The Role of Pre- and Post-Transplant Hydration Status in Kidney Graft Recovery and One-Year Function" Medicina 59, no. 11: 1931. https://doi.org/10.3390/medicina59111931





