Oligometastasis: Expansion of Curative Treatments in the Field of Oncology
Abstract
:1. Introduction
Overview of Oligometastatic Cancer Treatment
2. RT Applications
3. Current Status of Oligometastasis Studies
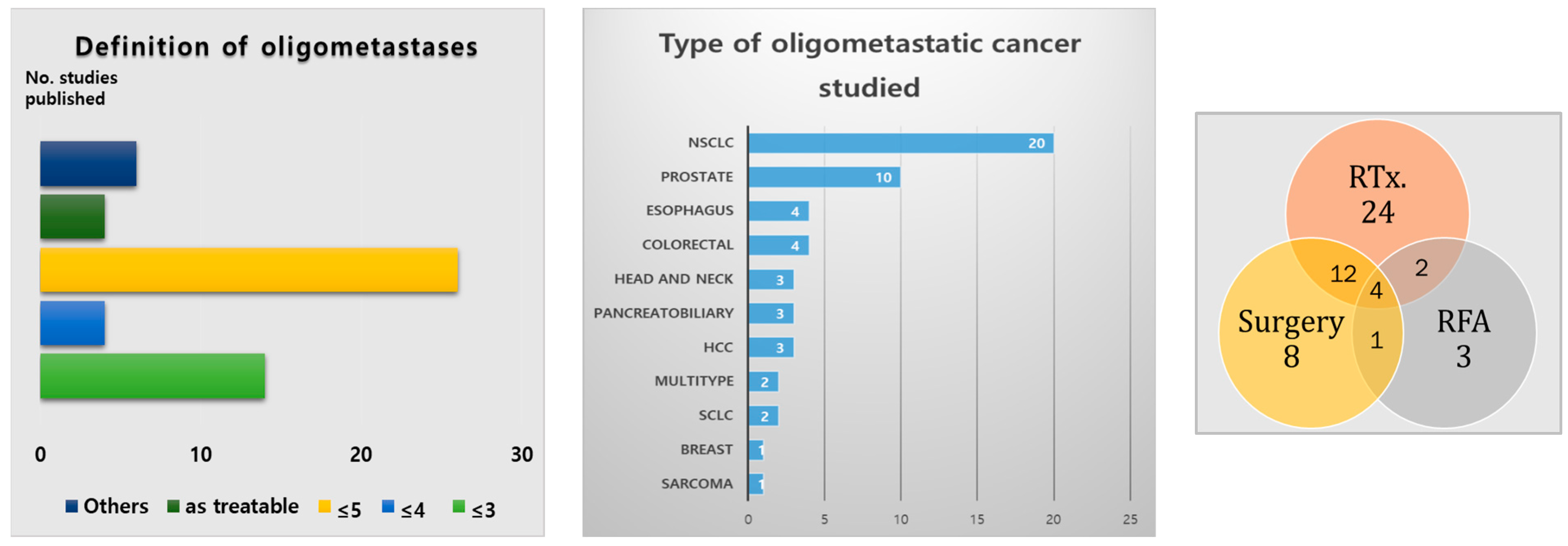
3.1. Results of Literature Analysis on Oligometastatic Treatment
3.2. Combining Immunotherapy with Radiotherapy in Non-Small Cell Lung Cancer (NSCLC)
3.3. Advances in Oligometastatic NSCLC Treatment: Combining Immunotherapy
3.4. Advances in Oligometastatic NSCLC Treatment: Combining Targeted Therapy
3.5. Current Ongoing Studies and Evolving Strategies in Oligometastatic Cancer Management and Classification
4. Current Limitations and Prospects
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rim, C.H.; Cho, W.K.; Lee, J.H.; Kim, Y.S.; Suh, Y.G.; Kim, K.H.; Chie, E.K.; Ahn, Y.C.; Oligometastasis Working Group, Korea Cancer Association. Role of local treatment for oligometastasis: A comparability based meta-analysis. Cancer Res. Treat. 2022, 54, 953–969. [Google Scholar] [CrossRef] [PubMed]
- Chalkidou, A.; Macmillan, T.; Grzeda, M.T.; Peacock, J.; Summers, J.; Eddy, S.; Coker, B.; Patrick, H.; Powell, H.; Berry, L.; et al. Stereotactic ablative body radiotherapy in patients with oligometastatic cancers: A prospective, registry-based, single-arm, observational, evaluation study. Lancet Oncol. 2021, 22, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Hughes, K.S.; Rosenstein, R.B.; Songhorabodi, S.; Adson, M.A.; Ilstrup, D.M.; Fortner, J.G.; Maclean, B.J.; Foster, J.H.; Daly, J.M.; Fitzherbert, D. Resection of the liver for colorectal carcinoma metastases. A multi-institutional study of long-term survivors. Dis. Colon Rectum 1988, 31, 1–4. [Google Scholar] [CrossRef]
- Nordlinger, B.; Guiguet, M.; Vaillant, J.C.; Balladur, P.; Boudjema, K.; Bachellier, P.; Jaeck, D. Surgical resection of colorectal carcinoma metastases to the liver: A prognostic scoring system to improve case selection, based on 1568 patients. Cancer 1996, 77, 1254–1262. [Google Scholar] [CrossRef]
- Pawlik, T.M.; Scoggins, C.R.; Zorzi, D.; Abdalla, E.K.; Andres, A.; Eng, C.; Curley, S.A.; Loyer, E.M.; Muratore, A.; Mentha, G.; et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann. Surg. 2005, 241, 715. [Google Scholar] [CrossRef]
- Pastorino, U.; Buyse, M.; Friedel, G.; Ginsberg, R.J.; Girard, P.; Goldstraw, P.; Johnston, M.; McCormack, P.; Pass, H.; Putnam, J.B.; et al. Long-term results of lung metastasectomy: Prognostic analyses based on 5206 cases. J. Thorac. Cardiovasc. Surg. 1997, 113, 37–49. [Google Scholar] [CrossRef]
- Hellman, S.; Weichselbaum, R.R. Oligometastases. J. Clin. Oncol. 1995, 13, 8–10. [Google Scholar] [CrossRef]
- Chang, J.Y.; Senan, S.; Paul, M.A.; Mehran, R.J.; Louie, A.V.; Balter, P.; Groen, H.J.; McRae, S.E.; Widder, J.; Feng, L.; et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: A pooled analysis of two randomised trials. Lancet Oncol. 2015, 16, 630–637. [Google Scholar] [CrossRef]
- Tandberg, D.J.; Tong, B.C.; Ackerson, B.G.; Kelsey, C.R. Surgery versus stereotactic body radiation therapy for stage I non–small cell lung cancer: A comprehensive review. Cancer 2018, 124, 667–678. [Google Scholar] [CrossRef]
- Rim, C.H.; Lee, J.S.; Kim, S.Y.; Seong, J. Comparison of radiofrequency ablation and ablative external radiotherapy for the treatment of intrahepatic malignancies: A hybrid meta-analysis. JHEP Rep. 2023, 5, 100594. [Google Scholar] [CrossRef]
- Milano, M.T.; Zhang, H.; Metcalfe, S.K.; Muhs, A.G.; Okunieff, P. Oligometastatic breast cancer treated with curative-intent stereotactic body radiation therapy. Breast Cancer Res. Treat. 2009, 115, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.K.; Kim, M.S.; Kim, J.H.; Yoo, S.Y.; Cho, C.K.; Yang, K.M.; Yoo, H.J.; Seo, Y.S.; Lee, D.H.; Kang, H.J.; et al. Oligometastases confined one organ from colorectal cancer treated by SBRT. Clin. Exp. Metastasis 2010, 27, 273–278. [Google Scholar] [CrossRef]
- Gomez, D.R.; Blumenschein, G.R., Jr.; Lee, J.J.; Hernandez, M.; Ye, R.; Camidge, D.R.; Doebele, R.C.; Skoulidis, F.; Gaspar, L.E.; Gibbons, D.L.; et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: A multicentre, randomised, controlled, phase 2 study. Lancet Oncol. 2016, 17, 1672–1682. [Google Scholar] [CrossRef] [PubMed]
- Gomez, D.R.; Tang, C.; Zhang, J.; Blumenschein, G.R., Jr.; Hernandez, M.; Lee, J.J.; Ye, R.; Palma, D.A.; Louie, A.V.; Camidge, D.R.; et al. Local consolidative therapy vs. maintenance therapy or observation for patients with oligometastatic non–small-cell lung cancer: Long-term results of a multi-institutional, phase II, randomized study. J. Clin. Oncol. 2019, 37, 1558–1565. [Google Scholar] [CrossRef] [PubMed]
- Iyengar, P.; Wardak, Z.; Gerber, D.E.; Tumati, V.; Ahn, C.; Hughes, R.S.; Dowell, J.E.; Cheedella, N.; Nedzi, L.; Westover, K.D.; et al. Consolidative radiotherapy for limited metastatic non–small-cell lung cancer: A phase 2 randomized clinical trial. JAMA Oncol. 2018, 4, e173501. [Google Scholar] [CrossRef] [PubMed]
- Gore, E.M.; Hu, C.; Sun, A.Y.; Grimm, D.F.; Ramalingam, S.S.; Dunlap, N.E.; Higgins, K.A.; Werner-Wasik, M.; Allen, A.M.; Iyengar, P.; et al. Randomized phase II study comparing prophylactic cranial irradiation alone to prophylactic cranial irradiation and consolidative extracranial irradiation for extensive-disease small cell lung cancer (ED SCLC): NRG Oncology RTOG 0937. J. Thorac. Oncol. 2017, 12, 1561–1570. [Google Scholar] [CrossRef]
- Parker, C.C.; James, N.D.; Brawley, C.D.; Clarke, N.W.; Hoyle, A.P.; Ali, A.; Ritchie, A.W.S.; Attard, G.; Chowdhury, S.; Cross, W.; et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): A randomised controlled phase 3 trial. Lancet 2018, 392, 2353–2366. [Google Scholar] [CrossRef]
- Ost, P.; Reynders, D.; Decaestecker, K.; Fonteyne, V.; Lumen, N.; De Bruycker, A.; Lambert, B.; Delrue, L.; Bultijnck, R.; Claeys, T.; et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: A prospective, randomized, multicenter phase II trial. JCO 2018, 36, 446–453. [Google Scholar] [CrossRef]
- Theelen, W.S.M.E.; Peulen, H.M.U.; Lalezari, F.; van der Noort, V.; de Vries, J.F.; Aerts, J.G.J.V.; Dumoulin, D.W.; Bahce, I.; Niemeijer, A.N.; de Langen, A.J.; et al. Effect of pembrolizumab after stereotactic body radiotherapy vs pembrolizumab alone on tumor response in patients with advanced non–small cell lung cancer: Results of the PEMBRO-RT phase 2 randomized clinical trial. JAMA Oncol. 2019, 5, 1276–1282. [Google Scholar] [CrossRef]
- Welsh, J.; Menon, H.; Chen, D.; Verma, V.; Tang, C.; Altan, M.; Heymach, J.V. Pembrolizumab with or without radiation therapy for metastatic non-small cell lung cancer: A randomized phase I/II trial. J. Immunother. Cancer 2020, 8, e001001. [Google Scholar] [CrossRef]
- Theelen, W.S.; Chen, D.; Verma, V.; Hobbs, B.P.; Peulen, H.M.; Aerts, J.G.; Welsh, J.W. Pembrolizumab with or without radiotherapy for metastatic non-small-cell lung cancer: A pooled analysis of two randomised trials. Lancet Respir. Med. 2021, 9, 467–475. [Google Scholar] [CrossRef]
- Wang, P.; Yin, T.; Zhao, K.; Yu, J.; Teng, F. Efficacy of single-site radiotherapy plus PD-1 inhibitors vs PD-1 inhibitors for oligometastatic non-small cell lung cancer. J. Cancer Res. Clin. Oncol. 2022, 148, 1253–1261. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, Y.; Yang, Z.; Hu, M.; Lu, J.; Zhang, Y.; Han, B. Local consolidative therapy for synchronous oligometastatic non-small cell lung cancer treated with first-line pembrolizumab: A retrospective observational study. Thorac. Cancer 2022, 13, 732–741. [Google Scholar] [CrossRef] [PubMed]
- Bauml, J.M.; Mick, R.; Ciunci, C.; Aggarwal, C.; Davis, C.; Evans, T.; Langer, C.J. Pembrolizumab after completion of locally ablative therapy for oligometastatic non–small cell lung cancer: A phase 2 trial. JAMA Oncol. 2019, 5, 1283–1290. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Zhou, F.; Liu, H.; Jiang, T.; Li, X.; Xu, Y.; Zhou, C. Consolidative local ablative therapy improves the survival of patients with synchronous oligometastatic NSCLC harboring EGFR activating mutation treated with first-line EGFR-TKIs. J. Thorac. Oncol. 2018, 13, 1383–1392. [Google Scholar] [CrossRef]
- Wang, X.S.; Bai, Y.F.; Verma, V.; Yu, R.L.; Tian, W.; Ao, R.; Deng, Y.; Zhu, X.Q.; Liu, H.; Pan, H.X.; et al. Randomized trial of first-line tyrosine kinase inhibitor with or without radiotherapy for synchronous oligometastatic EGFR-mutated non-small cell lung cancer. J. Natl. Cancer Inst. 2023, 115, 742–748. [Google Scholar] [CrossRef]
- Guckenberger, M.; Lievens, Y.; Bouma, A.B.; Collette, L.; Dekker, A.; deSouza, N.M.; Dingemans, A.C.; Fournier, B.; Hurkmans, C.; Lecouvet, F.E.; et al. Characterisation and classification of oligometastatic disease: A European Society for Radiotherapy and Oncology and European Organisation for Research and Treatment of Cancer consensus recommendation. Lancet Oncol. 2020, 21, e18–e28. [Google Scholar] [CrossRef]
- Willmann, J.; Vlaskou Badra, E.; Adilovic, S.; Ahmadsei, M.; Christ, S.M.; van Timmeren, J.E.; Kroeze, S.G.C.; Mayinger, M.; Guckenberger, M.; Andratschke, N. Evaluation of the prognostic value of the ESTRO EORTC classification of oligometastatic disease in patients treated with stereotactic body radiotherapy: A retrospective single center study. Radiother. Oncol. 2022, 168, 256–264. [Google Scholar] [CrossRef]
- Tsai, C.J.; Yang, J.T.; Guttmann, D.M.; Shaverdian, N.; Shepherd, A.F.; Eng, J.; Gelblum, D.; Xu, A.J.; Namakydoust, A.; Iqbal, A.; et al. Consolidative use of radiotherapy to block (CURB) oligoprogression—Interim analysis of the first randomized study of stereotactic body radiotherapy in patients with oligoprogressive metastatic cancers of the lung and breast. Int. J. Radiat. Oncol. 2021, 111, 1325–1326. [Google Scholar] [CrossRef]
- Lussier, Y.A.; Khodarev, N.N.; Regan, K.; Corbin, K.; Li, H.; Ganai, S.; Khan, S.A.; Gnerlich, J.L.; Darga, T.E.; Fan, H.; et al. Oligo- and polymetastatic progression in lung metastasis(es) patients is associated with specific microRNAs. PLoS ONE 2012, 7, e50141. [Google Scholar] [CrossRef]
- Wong, A.C.; Watson, S.P.; Pitroda, S.P.; Son, C.H.; Das, L.C.; Stack, M.E.; Uppal, A.; Oshima, G.; Khodarev, N.N.; Salama, J.K.; et al. Clinical and molecular markers of long-term survival after oligometastasis-directed stereotactic body radiotherapy (SBRT). Cancer 2016, 122, 2242–2250. [Google Scholar] [CrossRef] [PubMed]
- Hanssen, A.; Riebensahm, C.; Mohme, M.; Joosse, S.A.; Velthaus, J.L.; Berger, L.A.; Bernreuther, C.; Glatzel, M.; Loges, S.; Lamszus, K.; et al. Frequency of circulating tumor cells (CTC) in patients with brain metastases: Implications as a risk assessment marker in oligo-metastatic disease. Cancers 2018, 10, 527. [Google Scholar] [CrossRef] [PubMed]
- Lebow, E.S.; Murciano-Goroff, Y.; Razavi, P.; Reis-Filho, J.S.; Flynn, J.; Zhang, Z.; Tu, H.Y.; Bertucci, C.; Lim, L.P.; Li, M.; et al. Circulating tumor DNA as a biomarker in oligometastatic non-small cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, S174. [Google Scholar] [CrossRef]
- Sud, S.; Hall, J.; Tan, X.; Roberts, O.; Green, R.; Park, S.J.; Poellmann, M.; Bu, J.; Hong, S.; Wang, A.Z.; et al. Prospective characterization of circulating tumor cell kinetics in patients with oligometastatic disease receiving definitive radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, S58–S59. [Google Scholar] [CrossRef]
- Barnum, K.J.; Weiss, S.A. Prognostic and predictive biomarkers in oligometastatic disease. Cancer J. 2020, 26, 100–107. [Google Scholar] [CrossRef]
- Rashdan, S.; Iyengar, P.; Minna, J.D.; Gerber, D.E. Narrative review: Molecular and genetic profiling of oligometastatic non-small cell lung cancer. Transl. Lung Cancer Res. 2021, 10, 3351–3368. [Google Scholar] [CrossRef]
- Mehrens, D.; Unterrainer, M.; Corradini, S.; Niyazi, M.; Manapov, F.; Westphalen, C.B.; Froelich, M.F.; Wildgruber, M.; Seidensticker, M.; Ricke, J.; et al. Cost-effectiveness analysis of local treatment in oligometastatic disease. Front. Oncol. 2021, 11, 667993. [Google Scholar] [CrossRef]
- Verma, V.; Yegya-Raman, N.; Sprave, T.; Han, G.; Kantarjian, H.M.; Welsh, J.W.; Chang, J.Y.; Lin, S.H. A systematic review of the cost-effectiveness of stereotactic radiation therapy for cancer oligometastases. Int. J. Radiat. Oncol. Biol. Phys. 2022, 114, 977–988. [Google Scholar] [CrossRef]
- Chie, E.K.; Rim, C.H.; Cho, W.K.; Ahn, Y.C. Barriers in oligometastasis care, an radiation oncologist’s perspective. Cancer Res. Treat. 2023, 55, 1063–1064. [Google Scholar] [CrossRef]

| Author, Publication Year | Patient Recruit | No. of Patients | Target Disease | Study Design | Outcomes | Reference |
|---|---|---|---|---|---|---|
| Hughes et al., 1988 | 1948–1985 | 697 | Colorectal cancer with liver metastasis | Multicenter retrospective | 5-year survival: 24.5% | [3] |
| Nordlinger et al., 1996 | 1968–1990 | 1568 | Colorectal cancer with liver metastasis | Multicenter retrospective | 5-year survival: 28% | [4] |
| Pawlik et al., 2005 | 1990–2004 | 557 | Colorectal cancer with liver metastasis | Multicenter retrospective | Median OS: 74.3 months 5-year survival: 58% | [5] |
| Pastorino et al., 1997 | 1991–1995 | 5206 | Advanced solid tumor with lung metastasis | Multicenter retrospective | 5, 10, and 15-year survival: 36%, 26%, and 22%, respectively | [6] |
| Author, Publication Year | Patient Recruit | No. of Patients | Target Disease | Study Design | Comparison | Outcomes (Months) | Reference |
|---|---|---|---|---|---|---|---|
| Gomez et al., 2016 | 2012–2016 | 49 | NSCLC, ≤3 Mets | RCT | RTx. or surgery vs. standard maintenance | PFS 11.9 vs. 3.9 | [13] |
| Gomez et al., 2019 | 2012–2016 | 49 | NSCLC, ≤3 Mets | RCT | RTx. Or surgery vs. standard maintenance | PFS 14.2 vs. 4.4 OS 41.2 vs. 17.0 | [14] |
| Iyengar et al., 2018 | 2014–2016 | 29 | NSCLC, ≤6 lesions Including primary, ≤3 Met lung or liver | RCT | SABR + CTx vs. CTx | PFS 9.7 vs. 3.5 OS not reached | [15] |
| Gore et al., 2017 | 2010–2015 | 86 | SCLC, extended disease | RCT | PCI and cRT | 3-/12-month rate of progression 14.5%/75% vs. 53.3%/79.6% | [16] |
| Parker et al., 2018 | 2013–2016 | 2061 | Prostate cancer, newly diagnosed metastatic | RCT | SOC and RTx vs. SOC | failure-free survival 17 vs. 13 no survival advantage | [17] |
| Ost et al., 2017 | 2012–2015 | 62 | Prostate cancer, asymptomatic, biochemical recurrence after 1st treatment, ≤3 extracranial Met lesion on PET-CT, and serum testosterone levels > 50 ng/mL | RCT | MDT vs. surveillance | ADT-free survival 21 vs. 13 | [18] |
| Author, Publication Year | Patient Recruit | No. of Patients | Target Disease | Study Design | Comparison | Outcomes (Months) | Reference |
|---|---|---|---|---|---|---|---|
| Wang et al., 2021 | 2018–2020 | 152 | NSCLC, <4 Mets | Single-center retrospective | ICI + RT vs. ICI | PFS 13.8 vs. 8.9 | [22] |
| Chen et al., 2022 | 2015–2020 | 231 | NSCLC, ≤5 Mets | Single-center retrospective | LCT vs. non-LCT | PFS 13.97 vs. 10.08 OS 30.67 vs. 21.97 | [23] |
| Bauml et al., 2019 | 2015–2017 | 51 | NSCLC, ≤4 Mets | Single-arm phase 2 trial | LAT followed by pembrolizumab | PFS 19.1 months (significantly greater than the historical median of 6.6 months) | [24] |
| Xu et al., 2018 | 2010–2016 | 145 | NSCLC, ≤5 Mets, EGFR mutant | Single-center retrospective | All-LAT vs. part-LAT vs. non-LAT | PFS 20.6, 15.6, and 13.9 OS 40.9, 34.1, and 30.8 | [25] |
| Wang et al., 2023 | 2016–2019 | 133 | NSCLC, ≤5 Mets, EGFR mutant | RCT | TKI + RT vs. TKI | PFS 20.2 vs. 12.5 OS 25.5 vs. 17.4 | [26] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, A.R.; Rim, C.H. Oligometastasis: Expansion of Curative Treatments in the Field of Oncology. Medicina 2023, 59, 1934. https://doi.org/10.3390/medicina59111934
Lim AR, Rim CH. Oligometastasis: Expansion of Curative Treatments in the Field of Oncology. Medicina. 2023; 59(11):1934. https://doi.org/10.3390/medicina59111934
Chicago/Turabian StyleLim, Ah Reum, and Chai Hong Rim. 2023. "Oligometastasis: Expansion of Curative Treatments in the Field of Oncology" Medicina 59, no. 11: 1934. https://doi.org/10.3390/medicina59111934
APA StyleLim, A. R., & Rim, C. H. (2023). Oligometastasis: Expansion of Curative Treatments in the Field of Oncology. Medicina, 59(11), 1934. https://doi.org/10.3390/medicina59111934







