Oral Manifestations of Graft vs. Host Disease: A Comprehensive Review for Best Practice in Dentistry
Abstract
:1. Introduction
- Graft containing supply of immunocompetent cells [4]
- Immunological dissimilarity between host and donor
- Immunosuppressed host.
1.1. How It Works: Allo-HSCT
- Resolution of the disease
- Allogeneic stem cell engraftment (production of the necessary space for the infusion)
- Bone marrow restoration by the infused cells after a period of aplasia (which takes about 100 days)
- Elimination of remaining diseased cells thanks to the graft-versus-tumor effect (GvT): allogeneically transplanted human hematopoietic cells can eliminate residual tumor cells in the recipient by an immune-mediated mechanism [3].
- Bone marrow (BM).
- Peripheral blood stem cells (PBSC), which is the most innovative and most used technique to date.
- Umbilical cord blood, which, however, does not provide a quantity of stem cells sufficient to treat an adult.
1.2. Pre-HSCT Dentistry Videat
Epidemiology
1.3. Acute and Chronic GVHD
- Persistent, if characterized by the persistence of signs and symptoms of classic aGvHD initiated before day 100 post-transplant;
- Recurrent, if it arises as a classic form that resolves but recurs after day 100 post-transplant;
- De novo, if it occurs after day 100 post-transplant without any signs or symptoms in the previous time frame.
1.3.1. aGvHD Pathogenesis
- Afferent phase: this concerns the phase prior to HSC transplantation, i.e., conditioning. The use of high-dose chemotherapy and radiotherapy induces tissue damage in the patient, with the secretion of numerous pro-inflammatory cytokines such as IL-1 and TNF-alpha. These will lead to an overexpression of adhesion molecules and antigens on MHCs. This event will facilitate the recognition of these cells as non-self by donor immune cells once transplantation has taken place [17].
- Induction and expansion phase: after transplantation, the recognition of host antigens by donor T cells, which, once activated, will undergo proliferation and differentiation [18]. The contact of CD4+ cells with class II MCHs and CD8+ cells with class I MCHs will cause the production of several cytokines with a decisive role in the development of the disease, such as IL-2 and IFN-gamma. Under the effect of these cytokines, lymphocytes become active and proliferate and T-helper lymphocytes will be prompted to take the differentiation pathway that will lead them to present more of a Th1 phenotype [19].
- Effector phase: after their activation in the previous phase, T cells secrete a disproportionate number of cytokines that lead to migration, activation, and proliferation of other immune cells. This phenomenon, due to the high production of cytokines, is called cytokine storm [20]. Cytokine activity, however, only partially explains GvHD damage. Although these play a primary role in the morbidity and mortality of systemic GvHD, they are less important in damaging specific target organs of this disease, which are the gut, skin, and liver. Thus, the effector phase will be characterized by cytotoxic damage caused by activated donor CD8+ T cells (further invoked by the action of Th1 lymphocytes) against host cells of the target organs [19,21].
1.3.2. cGvHD Pathogenesis
- TGF-beta is the cytokine involved in the stimulation of collagen synthesis in the sclerosis phenomena characteristic of skin and mucous membranes in this disease;
- IL-4 stimulates the production of IgG and IgE by B cells, but also the proliferation of mast cells that play a primary role in the skin alterations typical of cGvHD;
- IL-5 is correlated with an increase in circulating eosinophils;
- IL-10: the association of IL-10 and high concentrations of TGF-β, is associated with immunodeficiency.
1.3.3. aGvHD Clinical Manifestations
- Skin involvement (81%) is characterized by the presence of a maculo-papular, itchy, violet-colored rash. The distribution of the rash is characteristic: at first, it involves the palms of the hands and feet soles, then it spreads to the cheeks, ears, neck, trunk, chest, and back. Histological findings show apoptotic phenomena of cells of the deep layers at the level of epithelial ridges, invaginations of epithelium that deepen in the dermis, with dermal–epidermal separation. Other typical findings are dyskeratosis, perivascular lymphocytic infiltration at the level of the dermis, and the presence of satellite lymphocytes in close proximity to dyskeratotic keratinocytes [25].
- Gastro-intestinal involvement (54%), where the most common symptom is diarrhea, but emesis, anorexia, abdominal pain, or a combination of painful cramps, occasional bleeding, and ileus (intestinal occlusion) may also occur in severe cases. Histologically present are flattening of the intestinal villi, dilation of the intestinal lumen and thickening of the walls of the small intestine, inflammation of the lamina propria, destruction of the crypts, lesions ranging from unicellular necrosis of the mucosal epithelium to complete loss of the epithelium itself and possible ulcerations surrounded by erythematous halos [22].
- Hepatic involvement (50%), manifested by cholestatic hepatopathy, with or without jaundice, and hepatomegaly. In hematochemical examinations, direct bilirubin, gamma-GT, and alkaline phosphatase levels (also individually) are altered, while transaminases (GOT and GPT) show a nonspecific increase.
1.3.4. cGvHD Clinical Manifestations
- Skin (80%), in which skin lesions are characterized by initial dry skin and ichthyosis (fish-scaled skin), then progressing to an itchy, purplish, plaque or papular rash, clinically indistinguishable from lichen planus. Another typical cutaneous manifestation is superficial or deep dermal fibrosis [27,28]. There are also some erythematous areas that precede the fibrotic lesion with plaques that mainly affect the skin of the lower limbs, joints, and bones. Skin appendages are affected with focal or diffuse alopecia and nail dystrophy [28].
- At the musculoskeletal level, fibrosis can also affect the tendons and muscles, causing fasciitis, myositis with muscle weakness, myalgia, and muscle contractures that facilitate diagnosis; in fact, muscle biopsies show phenomena of degeneration, necrosis, and regeneration of muscle fibers. Musculoskeletal system impairment seriously compromises the patient’s life quality, impacting his mobility [28].
- Respiratory patients who see lung involvement are often asymptomatic or have nonspecific symptoms at an early stage and, as a result, diagnosis is often delayed. The patient often presents exertional dyspnea, sinusitis, reduced tolerance to physical exercise, and persistent cough at the time of diagnosis. The cause of these alterations is to be traced back to obliterating bronchiolitis, which if it continues without being subjected to therapy can lead to the death of the patient from serious superinfections [29].
- In the liver, the alterations are like the acute form, with cholestasis and increased serum levels of bilirubin and alkaline phosphatase (ALP).
- Ocular symptoms such as xerophthalmia and photophobia, due to keratoconjunctivitis sicca (KCS) also known as dry eye syndrome, or other types of keratopathies.
- In women, genital involvement may be the only sign of chronic GvHD. These signs develop on average 10 months after the transplant and include dryness, erosions, thickening of the mucosa with vaginal narrowing, frequent infections, and pain during sexual activity (dyspareunia). In men, more rarely, the presence of phimosis can be highlighted [20].
1.4. Oral GVHD
1.4.1. Oral Mucositis from Pre-HSCT Conditioning
- Grade 1: presence of erythema with slight discomfort, which is the initial state of the pathology.
- Grade 2: diffuse erythema and superficial ulcers that still allow solid nutrition.
- Grade 3: painful ulcers that force the patient to switch to an exclusively liquid diet due to the high chewing pain.
- Grade 4: extensive mucositis that requires parenteral nutrition is the most severe.
1.4.2. Oral aGvHD Manifestation
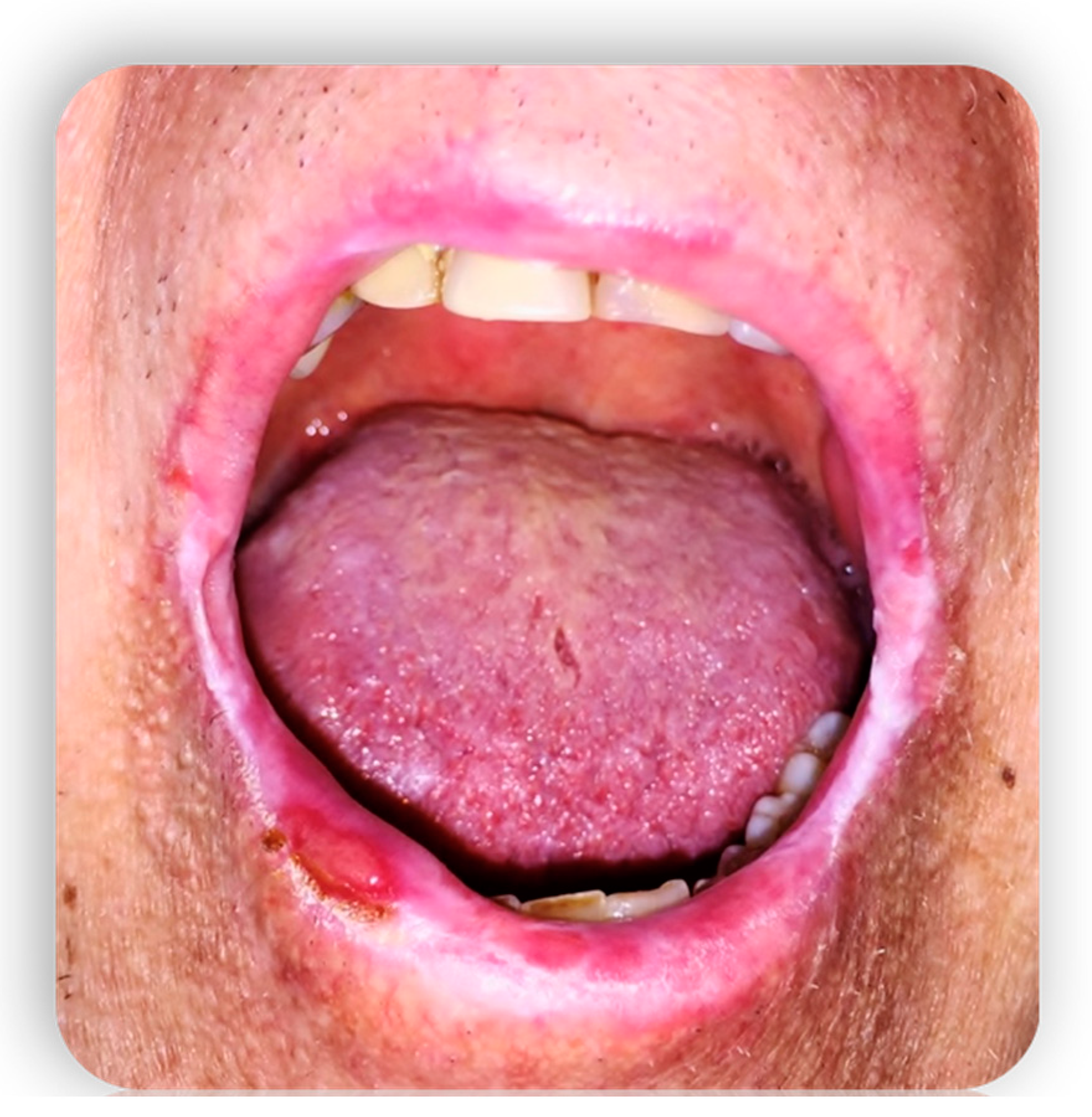
1.4.3. Oral cGvHD Manifestation
- Lesions of the oral mucosa
- ○
- Localized or diffuse erythema associated with edema or atrophy of the mucosa that reveal the underlying vascular structures [36].
- ○
- ○
- Ulcerations, interruptions in the continuity of the mucosa that emerge when the destroyed epithelium leaves the inflamed connective tissue exposed. Sometimes they can have a pseudomembrane. If they are chronic lesions, they do not heal spontaneously and the reason is that, despite the removal of the traumatic factor that predisposed the formation, there is a low percentage of local growth factors which is also associated with a reduced proliferative capacity of the cells resulting from the disease [31].
- ○
- Mucoceles mainly localized on the palate, caused by extravasation of saliva secondary to the sclerotic process of the ductal walls, which obliterates the lumen of the minor salivary glands. The inflammation of the salivary glands, in addition to this stenosis process, is aggravated by the decrease in secretion and the increase in saliva viscosity. The most affected areas are the hard palate and the lower lip [37].
- Limitation of mouth opening
- 3.
- Salivary gland dysfunction
2. Systemic Treatment of Oral Lesions
3. Topical Treatment of Oral Lesions
4. GvHD and Implantology: Case Reports in the Literature
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, S.J. Classification systems for chronic graft-versus-host disease. Blood 2017, 129, 30–37. [Google Scholar] [CrossRef]
- Arora, M.; Nagaraj, S.; Witte, J.; DeFor, T.E.; MacMillan, M.; Burns, L.J.; Weisdorf, D.J. New classification of chronic GVHD: Added clarity from the consensus diagnoses. Bone Marrow Transpl. 2009, 43, 149–153. [Google Scholar] [CrossRef]
- Flowers, M.E.D.; Inamoto, Y.; Carpenter, P.A.; Lee, S.J.; Kiem, H.-P.; Petersdorf, E.W.; Pereira, S.E.; Nash, R.A.; Mielcarek, M.; Fero, M.L.; et al. Comparative analysis of risk factors for acute graft-versus-host disease and for chronic graft-versus-host disease according to National Institutes of Health consensus criteria. Blood 2011, 117, 3214–3219. [Google Scholar] [CrossRef]
- Pavletic, S.Z.; Martin, P.; Lee, S.J.; Mitchell, S.; Jacobsohn, D.; Cowen, E.W.; Turner, M.L.; Akpek, G.; Gilman, A.; McDonald, G.; et al. Measuring therapeutic response in chronic graft-versus-host disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: IV. Response Criteria Working Group report. Biol. Blood Marrow Transplant. 2006, 12, 252–266. [Google Scholar] [CrossRef]
- Jagasia, M.H.; Greinix, H.T.; Arora, M.; Williams, K.M.; Wolff, D.; Cowen, E.W.; Palmer, J.; Weisdorf, D.; Treister, N.S.; Cheng, G.S.; et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol. Blood Marrow Transplant. 2015, 21, 389–401.e1. [Google Scholar] [CrossRef]
- Ion, D.; Stevenson, K.; Woo, S.-B.; Ho, V.T.; Soiffer, R.; Antin, J.H.; Treister, N.S. Characterization of oral involvement in acute graft-versus-host disease. Biol. Blood Marrow Transplant. 2014, 20, 1717–1721. [Google Scholar] [CrossRef]
- Zeiser, R.; Blazar, B.R. Pathophysiology of Chronic Graft-versus-Host Disease and Therapeutic Targets. N. Engl. J. Med. 2017, 377, 2565–2579. [Google Scholar] [CrossRef] [PubMed]
- Elad, S.; Cheng, K.K.F.; Lalla, R.V.; Yarom, N.; Hong, C.; Logan, R.M.; Bowen, J.; Gibson, R.; Saunders, D.P.; Zadik, Y.; et al. MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 2020, 126, 4423–4431. [Google Scholar] [CrossRef] [PubMed]
- Yuan, A.; Chai, X.; Martins, F.; Arai, S.; Arora, M.; Correa, M.E.; Pidala, J.; Cutler, C.S.; Lee, S.J.; Treister, N.S. Oral chronic GVHD outcomes and resource utilization: A subanalysis from the chronic GVHD consortium. Oral. Dis. 2016, 22, 235–240. [Google Scholar] [CrossRef]
- Brunstein, C.G.; Fuchs, E.J.; Carter, S.L.; Karanes, C.; Costa, L.J.; Wu, J.; Devine, S.M.; Wingard, J.R.; Aljitawi, O.S.; Cutler, C.S.; et al. Alternative donor transplantation after reduced intensity conditioning: Results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood 2011, 118, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Al-Hashimi, I.; Schifter, M.; Lockhart, P.B.; Wray, D.; Brennan, M.; Migliorati, C.A.; Axéll, T.; Bruce, A.J.; Carpenter, W.; Eisenberg, E.; et al. Oral lichen planus and oral lichenoid lesions: Diagnostic and therapeutic considerations. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2007, 103 (Suppl. S25), e1–e12. [Google Scholar] [CrossRef] [PubMed]
- Elad, S.; Zadik, Y.; Yarom, N. Oral Complications of Nonsurgical Cancer Therapies. Atlas Oral Maxillofac. Surg. Clin. N. Am. 2017, 25, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Zadik, Y. Restricted mouth opening in chronic graft-versus-host disease. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 121, 201–202. [Google Scholar] [CrossRef]
- Bertelli, L.; Di Nardo, G.; Zama, D.; Bardasi, G.; Morello, W.; Masetti, R.; Belotti, T.; Forchielli, M.L.; Prete, A.; Pession, A. A New Formulation of an Old Drug: A Potential New Therapy in the Management of Oral cGvHD. J. Pediatr. Hematol. Oncol. 2016, 38, e295–e297. [Google Scholar] [CrossRef]
- Gomes, C.B.F.; Treister, N.S.; Miller, B.; Armand, P.; Friedland, B. Pulp Obliteration in a Patient with Sclerodermatous Chronic Graft-versus-Host Disease. J. Endod. 2016, 42, 678–680. [Google Scholar] [CrossRef] [PubMed]
- Zadik, Y.; Elad, S.; Shapira, A.; Shapira, M.Y. Treatment of oral mucosal manifestations of chronic graft-versus-host disease: Dexamethasone vs. budesonide. Expert. Opin. Pharmacother. 2017, 18, 235–242. [Google Scholar] [CrossRef]
- Park, A.R.; La, H.O.; Cho, B.S.; Kim, S.J.; Lee, B.K.; Rhie, J.Y.; Gwak, H.S. Comparison of budesonide and dexamethasone for local treatment of oral chronic graft-versus-host disease. Am. J. Health Syst. Pharm. 2013, 70, 1383–1391. [Google Scholar] [CrossRef] [PubMed]
- Bleakley, M.; Sehgal, A.; Seropian, S.; Biernacki, M.A.; Krakow, E.F.; Dahlberg, A.; Persinger, H.; Hilzinger, B.; Martin, P.J.; Carpenter, P.A.; et al. Naive T-Cell Depletion to Prevent Chronic Graft-Versus-Host Disease. J. Clin. Oncol. 2022, 40, 1174–1185. [Google Scholar] [CrossRef]
- Jiang, H.; Fu, D.; Bidgoli, A.; Paczesny, S. T Cell Subsets in Graft Versus Host Disease and Graft Versus Tumor. Front. Immunol. 2021, 12, 761448. [Google Scholar] [CrossRef]
- Cantile, T.; Coppola, N.; Canfora, F.; Adamo, D.; Ruoppo, E.; Mignogna, M.D.; Leuci, S. Oral Cancer in HSCT Pediatric Patients Arising on GVHD: A Comprehensive Review. Cancers 2022, 14, 5775. [Google Scholar] [CrossRef]
- Janowiak-Majeranowska, A.; Osowski, J.; Mikaszewski, B.; Majeranowski, A. Secondary Oral Cancer after Systemic Treatment of Hematological Malignancies and Oral GVHD: A Systematic Review. Cancers 2022, 14, 2175. [Google Scholar] [CrossRef]
- Greco, R.; Lorentino, F.; Morelli, M.; Giglio, F.; Mannina, D.; Assanelli, A.; Mastaglio, S.; Dalto, S.; Perini, T.; Lazzari, L.; et al. Posttransplantation cyclophosphamide and sirolimus for prevention of GVHD after HLA-matched PBSC transplantation. Blood 2016, 128, 1528–1531. [Google Scholar] [CrossRef] [PubMed]
- Zadik, Y.; Nakdimon, I.; Meyerowitz, C.; Shapira, M.Y.; Elad, S. Topical budesonide for severe oral chronic graft-versus-host disease. Am. J. Health Syst. Pharm. 2014, 71, 181–182. [Google Scholar] [CrossRef] [PubMed]
- Wolff, D.; Anders, V.; Corio, R.; Horn, T.; Morison, W.L.; Farmer, E.; Vogelsang, G.B. Oral PUVA and topical steroids for treatment of oral manifestations of chronic graft-vs.-host disease. Photodermatol. Photoimmunol. Photomed. 2004, 20, 184–190. [Google Scholar] [CrossRef]
- Schubert, M.M. Correa MEP Oral graft-versus-host disease. Dent. Clin. N. Am. 2008, 52, 79–109. [Google Scholar] [CrossRef] [PubMed]
- Fall-Dickson, J.M.; Pavletic, S.Z.; Mays, J.W.; Schubert, M.M. Oral Complications of Chronic Graft-Versus-Host Disease. J. Natl. Cancer Inst. Monogr. 2019, 2019, lgz007. [Google Scholar] [CrossRef] [PubMed]
- França, C.M.; Domingues-Martins, M.; Volpe, A.; Pallotta Filho, R.S.; Soares de Araújo, N. Severe oral manifestations of chronic graft-vs. -host disease. J. Am. Dent. Assoc. 2019, 132, 1124–1127. [Google Scholar] [CrossRef]
- Couriel, D.; Carpenter, P.A.; Cutler, C.; Bolaños-Meade, J.; Treister, N.S.; Gea-Banacloche, J.; Shaughnessy, P.; Hymes, S.; Kim, S.; Wayne, A.S.; et al. Ancillary therapy and supportive care of chronic graft-versus-host disease: National institutes of health consensus development project on criteria for clinical trials in chronic Graft-versus-host disease: V. Ancillary Therapy and Supportive Care Working Group Report. Biol. Blood Marrow Transplant. 2006, 12, 375–396. [Google Scholar]
- Treister, N.; Duncan, C.; Cutler, C.; Lehmann, L. How we treat oral chronic graft-versus-host disease. Blood 2012, 120, 3407–3418. [Google Scholar] [CrossRef]
- Hashmi, S.K. Ocular and Oral Complications. In The EBMT Handbook: Hematopoietic Stem Cell Transplantation and Cellular Therapies; Springer: Cham, Switzerland, 2019; pp. 367–371. [Google Scholar]
- Emperumal, C.P.; Weller, B.; Okane, S.; Joseph, R.; Kharbanda, S.; Ling, Z.; Villa, A. Oral complications in a paediatric graft versus host disease (GVHD) clinic: A retrospective study. Oral Dis. 2023. [Google Scholar] [CrossRef]
- Mays, J.W.; Fassil, H.; Edwards, D.A.; Pavletic, S.Z.; Bassim, C.W. Oral chronic graft-versus-host disease: Current pathogenesis, therapy, and research. Oral Dis. 2013, 19, 327–346. [Google Scholar] [CrossRef] [PubMed]
- Stoopler, E.T. Management of oral chronic graft-versus-host disease. J. Can. Dent. Assoc. 2013, 79, d37. [Google Scholar] [PubMed]
- Alhussain, A.; Alkhayal, Z.; Ayas, M.; Abed, H. Prevalence and risk factors of oral mucositis in paediatric patients undergoing haematopoietic stem cell transplantation. Oral. Dis. 2022, 28, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Elad, S.; Zinchuk, K.; Li, S.; Cutler, C.; Liesveld, J.; Treister, N.S. Economic and Practical Considerations in the Treatment of Oral Mucosal Chronic Graft-Versus-Host Disease. Biol. Blood Marrow Transplant. 2018, 24, 1748–1753. [Google Scholar] [CrossRef] [PubMed]
- Rosa, A.; Miranda, M.; Franco, R.; Guarino, M.G.; Barlattani, A.; Bollero, P. Experimental protocol of dental procedures in patients with hereditary angioedema: The role of anxiety and the use of nitrogen oxide. Oral Implantol. 2016, 9, 49–53. [Google Scholar]
- Piccin, A.; Tagnin, M.; Vecchiato, C.; Al-Khaffaf, A.; Beqiri, L.; Kaiser, C.; Agreiter, I.; Negri, G.; Kob, M.; Di Pierro, A.; et al. Graft-versus-host disease (GvHD) of the tongue and of the oral cavity: A large retrospective study. Int. J. Hematol. 2018, 108, 615–621. [Google Scholar] [CrossRef]
- Ramachandran, V.; Kolli, S.S.; Strowd, L.C. Review of Graft-Versus-Host Disease. Dermatol. Clin. 2019, 37, 569–582. [Google Scholar] [CrossRef]
- Bazinet, A.; Popradi, G. A General Practitioner’s Guide to Hematopoietic Stem-cell Transplantation. Curr. Oncol. 2019, 26, 187–191. [Google Scholar] [CrossRef]
- Treister, N.; Li, S.; Lerman, M.A.; Lee, S.; Soiffer, R. Narrow-band UVB phototherapy for management of oral chronic graft-versus-host disease. Photodermatol. Photoimmunol. Photomed. 2015, 31, 75–82. [Google Scholar] [CrossRef]
- Miranda, M.; Gianfreda, F.; Rosa, A.; Fiorillo, L.; Cervino, G.; Cicciù, M.; Bollero, P. Treatment of Oral Mucositis Using Platelet-Rich-Fibrin: A Retrospective Study on Oncological Patients. J. Craniofac. Surg. 2023, 34, 1527–1529. [Google Scholar] [CrossRef]
- Sánchez, A.R.; Sheridan, P.J.; Rogers, R.S. Successful treatment of oral lichen planus-like chronic graft-versus-host disease with topical tacrolimus: A case report. J. Periodontol. 2004, 75, 613–619. [Google Scholar] [CrossRef]
- Dean, D.; Sroussi, H. Oral Chronic Graft-Versus-Host Disease. Front. Oral Health 2022, 3, 903154. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.S.; Edwards, D.; Walsh-Chocolaad, T.; Childs, R.W. Topical tacrolimus with custom trays in the treatment of severe oral chronic graft-versus-host disease refractory to a potent topical steroid therapy: A case report. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 115, e26–e30. [Google Scholar] [CrossRef] [PubMed]
- Vogelsang, G.B.; Wolff, D.; Altomonte, V.; Farmer, E.; Morison, W.L.; Corio, R.; Horn, T. Treatment of chronic graft-versus-host disease with ultraviolet irradiation and psoralen (PUVA). Bone Marrow Transplant. 1996, 17, 1061–1067. [Google Scholar] [PubMed]
- Curtis, J.R.; Yamaoka, K.; Chen, Y.-H.; Bhatt, D.L.; Gunay, L.M.; Sugiyama, N.; Connell, C.A.; Wang, C.; Wu, J.; Menon, S.; et al. Malignancy risk with tofacitinib versus TNF inhibitors in rheumatoid arthritis: Results from the open-label, randomised controlled ORAL Surveillance trial. Ann. Rheum. Dis. 2023, 82, 331–343. [Google Scholar] [CrossRef]
- Etebarian, A.; Mirshamsi, H.; Sadeghi, H.S.; Hemmati, F. Oral rehabilitation in a patient with sclerotic-phenotype chronic graft versus host disease: A case report. Quintessence Int. 2019, 50, 208–213. [Google Scholar]
- Castellarin, P.; Stevenson, K.; Biasotto, M.; Yuan, A.; Woo, S.-B.; Treister, N.S. Extensive dental caries in patients with oral chronic graft-versus-host disease. Biol. Blood Marrow Transplant. 2012, 18, 1573–1579. [Google Scholar] [CrossRef]
- Bollero, P.; Franco, R.; Gianfreda, F.; Gualtieri, P.; Miranda, M.; Barlattani, A. Epidemiology, Etiopathogenesis, Treatment and Prognosis of Oral Thermal Burns from Food and Drinks. Dent. Hypotheses 2019, 10, 80. [Google Scholar]
- Thi, H.N.; Manh, C.P.; Tuan, L.T.; Le Thi, L.A.; Thanh, N.N.; Vilaiyuk, S. Tumor-Induced Osteomalacia Associated with a Maxillary Tumor in Children: A Case Report and Review of the Literature. J. Clin. Res. Pediatr. Endocrinol. 2022. [Google Scholar] [CrossRef]







| Pathology | Pathological State | Treatment Options |
|---|---|---|
| Carious pathology |
| Restorative (if sufficient timing or no treatment), Pulpectomy |
| Apical paradentitis |
| Extraction or root canal therapy (depending on available time frame) Extraction or root canal therapy if radiotransparency > 5 mm No treatment for radiotransparency < 5 mm |
| Periodontal disease |
| Extraction Extraction Renew dental hygiene instructions and perform scaling maneuvers |
| Partially erupted third molars |
| Extraction No treatment |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miranda, M.; Gianfreda, F.; Carlotta, D.; Armati, S.; Barlattani, A.; Bollero, P. Oral Manifestations of Graft vs. Host Disease: A Comprehensive Review for Best Practice in Dentistry. Medicina 2023, 59, 1937. https://doi.org/10.3390/medicina59111937
Miranda M, Gianfreda F, Carlotta D, Armati S, Barlattani A, Bollero P. Oral Manifestations of Graft vs. Host Disease: A Comprehensive Review for Best Practice in Dentistry. Medicina. 2023; 59(11):1937. https://doi.org/10.3390/medicina59111937
Chicago/Turabian StyleMiranda, Michele, Francesco Gianfreda, Danesi Carlotta, Sofia Armati, Alberta Barlattani, and Patrizio Bollero. 2023. "Oral Manifestations of Graft vs. Host Disease: A Comprehensive Review for Best Practice in Dentistry" Medicina 59, no. 11: 1937. https://doi.org/10.3390/medicina59111937
APA StyleMiranda, M., Gianfreda, F., Carlotta, D., Armati, S., Barlattani, A., & Bollero, P. (2023). Oral Manifestations of Graft vs. Host Disease: A Comprehensive Review for Best Practice in Dentistry. Medicina, 59(11), 1937. https://doi.org/10.3390/medicina59111937









