A De Novo Frameshift Mutation in RPL5 with Classical Phenotype Abnormalities and Worsening Anemia Diagnosed in a Young Adult—A Case Report and Review of the Literature
Abstract
1. Introduction
2. Case Presentation
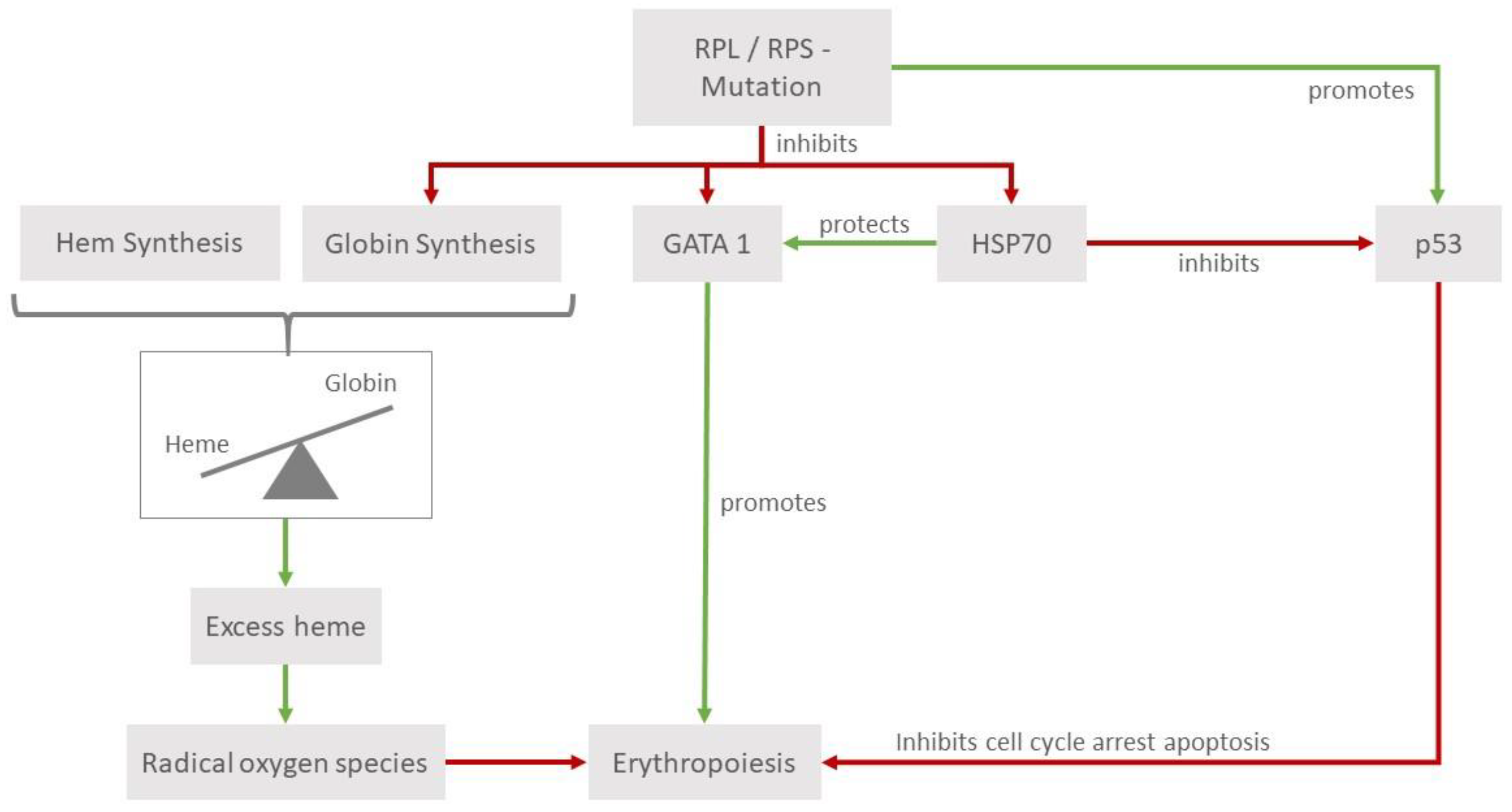
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Costa, L.M.D.; Marie, I.; Leblanc, T.M. Diamond—Blackfan anemia. Hematol. Am. Soc. Hematol. Educ. Program 2021, 2021, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Willig, T.-N.; Niemeyer, C.M.; Leblanc, T.; Tiemann, C.; Robert, A.; Budde, J.; Lambiliotte, A.; Kohne, E.; Souillet, G.; Eber, S.; et al. Identification of new prognosis factors from the clinical and epidemiologic analysis of a registry of 229 Diamond-Blackfan anemia patients. Pediatr. Res. 1999, 46, 553. [Google Scholar] [CrossRef]
- Costa, L.D.; Leblanc, T.; Mohandas, N. Diamond-Blackfan anemia. Blood 2020, 136, 1262–1273. [Google Scholar] [CrossRef]
- Sieff, C. Diamond-Blackfan Anemia. GeneReviews®. Published 25 June 2009. Available online: https://www.ncbi.nlm.nih.gov/books/NBK7047/ (accessed on 30 August 2023).
- Vlachos, A.; Ball, S.; Dahl, N.; Alter, B.P.; Sheth, S.; Ramenghi, U.; Meerpohl, J.; Karlsson, S.; Liu, J.M.; Leblanc, T.; et al. Diagnosing and treating Diamond Blackfan anaemia: Results of an international clinical consensus conference. Br. J. Haematol. 2008, 142, 859–876. [Google Scholar] [CrossRef] [PubMed]
- Gazda, H.T.; Sheen, M.R.; Vlachos, A.; Choesmel, V.; O’Donohue, M.-F.; Schneider, H.; Darras, N.; Hasman, C.; Sieff, C.A.; Newburger, P.E.; et al. Ribosomal Protein L5 and L11 Mutations Are Associated with Cleft Palate and Abnormal Thumbs in Diamond-Blackfan Anemia Patients. Am. J. Hum. Genet. 2008, 83, 769–780. [Google Scholar] [CrossRef] [PubMed]
- Ulirsch, J.C.; Verboon, J.M.; Kazerounian, S.; Guo, M.H.; Yuan, D.; Ludwig, L.S.; Handsaker, R.E.; Abdulhay, N.J.; Fiorini, C.; Genovese, G.; et al. The Genetic Landscape of Diamond-Blackfan Anemia. Am. J. Hum. Genet. 2018, 103, 930–947. [Google Scholar] [CrossRef] [PubMed]
- Khajuria, R.K.; Munschauer, M.; Ulirsch, J.C.; Fiorini, C.; Ludwig, L.S.; McFarland, S.K.; Abdulhay, N.J.; Specht, H.; Keshishian, H.; Mani, D.; et al. Ribosome Levels Selectively Regulate Translation and Lineage Commitment in Human Hematopoiesis. Cell 2018, 173, 90–103.e19. [Google Scholar] [CrossRef] [PubMed]
- Sankaran, V.G.; Ghazvinian, R.; Do, R.; Thiru, P.; Vergilio, J.-A.; Beggs, A.H.; Sieff, C.A.; Orkin, S.H.; Nathan, D.G.; Lander, E.S.; et al. Exome sequencing identifies GATA1 mutations resulting in Diamond-Blackfan anemia. J. Clin. Investig. 2012, 122, 2439–2443. [Google Scholar] [CrossRef]
- Moniz, H.; Gastou, M.; Leblanc, T.; Hurtaud, C.; Crétien, A.A.; Lécluse, Y.; Raslova, H.; Larghero, J.; Croisille, L.; Faubladier, M.; et al. Primary hematopoietic cells from DBA patients with mutations in RPL11 and RPS19 genes exhibit distinct erythroid phenotype in vitro. Cell Death Dis. 2012, 3, e356. [Google Scholar] [CrossRef] [PubMed]
- Trainor, C.D.; Mas, C.; Archambault, P.; Di Lello, P.; Omichinski, J.G. GATA-1 associates with and inhibits p53. Blood 2009, 114, 165–173. [Google Scholar] [CrossRef]
- Gastou, M.; Rio, S.; Dussiot, M.; Karboul, N.; Moniz, H.; Leblanc, T.; Sevin, M.; Gonin, P.; Larghéro, J.; Garrido, C.; et al. The severe phenotype of Diamond-Blackfan anemia is modulated by heat shock protein 70. Blood Adv. 2017, 1, 1959–1976. [Google Scholar] [CrossRef] [PubMed]
- Rio, S.; Gastou, M.; Karboul, N.; Derman, R.; Suriyun, T.; Manceau, H.; Leblanc, T.; El Benna, J.; Schmitt, C.; Azouzi, S.; et al. Regulation of globin-heme balance in Diamond-Blackfan anemia by HSP70/GATA1. Blood 2019, 133, 1358–1370. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Keel, S.B.; Shimamura, A.; Liu, L.; Gerds, A.T.; Li, H.Y.; Wood, B.L.; Scott, B.L.; Abkowitz, J.L. Delayed globin synthesis leads to excess heme and the macrocytic anemia of Diamond Blackfan anemia and del(5q) myelodysplastic syndrome. Sci. Transl. Med. 2016, 8, 338ra67. [Google Scholar] [CrossRef] [PubMed]
- Iskander, D.; Roy, N.B.; Payne, E.; Drasar, E.; Hennessy, K.; Harrington, Y.; Christodoulidou, C.; Karadimitris, A.; Batkin, L.; de la Fuente, J. Diamond-Blackfan anemia in adults: In pursuit of a common approach for a rare disease. Blood Rev. 2023, 61, 101097. [Google Scholar] [CrossRef] [PubMed]
- Farruggia, P.; Quarello, P.; Garelli, E.; Paolicchi, O.; Ruffo, G.B.; Cuccia, L.; Cannella, S.; Bruno, G.; D’Angelo, P. The spectrum of non-classical Diamond-Blackfan anemia: A case of late beginning transfusion dependency associated to a new RPL5 mutation. Pediatr. Rep. 2012, 4, 91–93. [Google Scholar] [CrossRef]
- Ballester, E.F.; Gil-Fernández, J.J.; Blanco, M.V.; Mesa, J.M.; García, J.D.; Tamayo, A.T.; Burgaleta, C. Adult-onset Diamond-Blackfan anemia with a novel mutation in the exon 5 of RPL 11: Too late and too rare. Clin. Case Rep. 2015, 3, 392–395. [Google Scholar] [CrossRef]
- Tamefusa, K.; Muraoka, M.; Washio, K.; Wakamatsu, M.; Shimada, A. Late-onset familial Diamond–Blackfan anemia with neutropenia caused by RPL35A variant. Pediatr. Int. 2022, 64, e15275. [Google Scholar] [CrossRef]
- Vlachos, A.; Rosenberg, P.S.; Atsidaftos, E.; Kang, J.; Onel, K.; Sharaf, R.N.; Alter, B.P.; Lipton, J.M. Increased risk of colon cancer and osteogenic sarcoma in diamond-Blackfan anemia. Blood 2018, 132, 2205–2208. [Google Scholar] [CrossRef]
- Strahm, B.; Loewecke, F.; Niemeyer, C.M.; Albert, M.; Ansari, M.; Bader, P.; Bertrand, Y.; Burkhardt, B.; Da Costa, L.M.; Ferster, A.; et al. Favorable outcomes of hematopoietic stem cell transplantation in children and adolescents with Diamond-Blackfan anemia. Blood Adv. 2020, 4, 1760–1769. [Google Scholar] [CrossRef]
- Fagioli, F.; Quarello, P.; Zecca, M.; Lanino, E.; Corti, P.; Favre, C.; Ripaldi, M.; Ramenghi, U.; Locatelli, F.; Prete, A. Haematopoietic stem cell transplantation for Diamond Blackfan anaemia: A report from the Italian Association of Paediatric Haematology and Oncology Registry. Br. J. Haematol. 2014, 165, 673–681. [Google Scholar] [CrossRef]
- Cheng, G.; Saleh, M.N.; Marcher, C.; Vasey, S.; Mayer, B.; Aivado, M.; Arning, M.; Stone, N.L.; Bussel, J.B. Eltrombopag for management of chronic immune thrombocytopenia (RAISE): A 6-month, randomised, phase 3 study. Lancet 2011, 377, 393–402. [Google Scholar] [CrossRef]
- Afdhal, N.H.; Dusheiko, G.M.; Giannini, E.G.; Chen, P.; Han, K.; Mohsin, A.; Rodriguez–Torres, M.; Rugina, S.; Bakulin, I.; Lawitz, E.; et al. Eltrombopag increases platelet numbers in thrombocytopenic patients with hcv infection and cirrhosis, allowing for effective antiviral therapy. Gastroenterology 2014, 146, 442–452.e1. [Google Scholar] [CrossRef]
- Townsley, D.M.; Scheinberg, P.; Winkler, T.; Desmond, R.; Dumitriu, B.; Rios, O.; Weinstein, B.; Valdez, J.; Lotter, J.; Feng, X.; et al. Eltrombopag Added to Standard Immunosuppression for Aplastic Anemia. N. Engl. J. Med. 2017, 376, 1540–1550. [Google Scholar] [CrossRef]
- McHutchison, J.G.; Dusheiko, G.; Shiffman, M.L.; Rodriguez-Torres, M.; Sigal, S.; Bourliere, M.; Berg, T.; Gordon, S.C.; Campbell, F.M.; Theodore, D.; et al. Eltrombopag for Thrombocytopenia in Patients with Cirrhosis Associated with Hepatitis C. N. Engl. J. Med. 2007, 357, 2227–2236. [Google Scholar] [CrossRef]
- Bussel, J.; Kulasekararaj, A.; Cooper, N.; Verma, A.; Steidl, U.; Semple, J.W.; Will, B. Mechanisms and therapeutic prospects of thrombopoietin receptor agonists. Semin. Hematol. 2019, 56, 262–278. [Google Scholar] [CrossRef]
- Qanash, H.; Li, Y.; Smith, R.H.; Linask, K.; Young-Baird, S.; Hakami, W.; Keyvanfar, K.; Choy, J.S.; Zou, J.; Larochelle, A. Eltrombopag improves erythroid differentiation in a human induced pluripotent stem cell model of diamond blackfan anemia. Cells 2021, 10, 734. [Google Scholar] [CrossRef] [PubMed]
- Hoffbrand, A.V.; Taher, A.; Cappellini, M.D. How I treat transfusional iron overload. Blood 2012, 120, 3657–3669. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fan, X.; Desmond, R.; Winkler, T.; Young, D.J.; Dumitriu, B.; Townsley, D.M.; Gutierrez-Rodrigues, F.; Lotter, J.; Valdez, J.; Sellers, S.E.; et al. Eltrombopag for patients with moderate aplastic anemia or uni-lineage cytopenias. Blood Adv. 2020, 4, 1700–1710. [Google Scholar] [CrossRef] [PubMed]



| Feature | Patient Female, Born 2001 |
|---|---|
| Clinical onset of anemia | At ten weeks of age |
| Heart anomalies | Ventricular septal defect, spontaneous closure at 5 years of age |
| Facial anomalies | Hypertelorism, relative macrocephaly, lateral cleft lip |
| Growth anomalies | 145 cm after prepubescent growth hormone treatment without corticosteroids |
| Limb anomalies | Polysyndactyly of the thumb |
| Adenosine deaminase | Elevated |
| Steroid responsive | Yes (first trial at 21, second trial at 22 years) |
| Bone marrow histology | (Atypical for DBA)—normal cellularity according to age but CD4-lymphocytosis (60% of cellularity), dysplastic megakaryopoiesis, reduced erythropoiesis and myeloid line |
| Genetics | RPL5 c.392dup, p.(Asn131Lysfs*6) No somatic myeloid mutations * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dorenkamp, M.; Porret, N.; Diepold, M.; Rovó, A. A De Novo Frameshift Mutation in RPL5 with Classical Phenotype Abnormalities and Worsening Anemia Diagnosed in a Young Adult—A Case Report and Review of the Literature. Medicina 2023, 59, 1953. https://doi.org/10.3390/medicina59111953
Dorenkamp M, Porret N, Diepold M, Rovó A. A De Novo Frameshift Mutation in RPL5 with Classical Phenotype Abnormalities and Worsening Anemia Diagnosed in a Young Adult—A Case Report and Review of the Literature. Medicina. 2023; 59(11):1953. https://doi.org/10.3390/medicina59111953
Chicago/Turabian StyleDorenkamp, Moritz, Naomi Porret, Miriam Diepold, and Alicia Rovó. 2023. "A De Novo Frameshift Mutation in RPL5 with Classical Phenotype Abnormalities and Worsening Anemia Diagnosed in a Young Adult—A Case Report and Review of the Literature" Medicina 59, no. 11: 1953. https://doi.org/10.3390/medicina59111953
APA StyleDorenkamp, M., Porret, N., Diepold, M., & Rovó, A. (2023). A De Novo Frameshift Mutation in RPL5 with Classical Phenotype Abnormalities and Worsening Anemia Diagnosed in a Young Adult—A Case Report and Review of the Literature. Medicina, 59(11), 1953. https://doi.org/10.3390/medicina59111953






