Effect of Plant-Based Mouthwash (Morinda citrifolia and Ocimum sanctum) on TNF-α, IL-α, IL-β, IL-2, and IL-6 in Gingival Crevicular Fluid and Plaque Scores of Patients Undergoing Fixed Orthodontic Treatment
Abstract
:1. Introduction
2. Material and Methods
3. Preparation of 5% MC Mouthwash
4. Preparation of 4% OS Mouthwash
5. Mouthwash Rinse Instructions to Participants
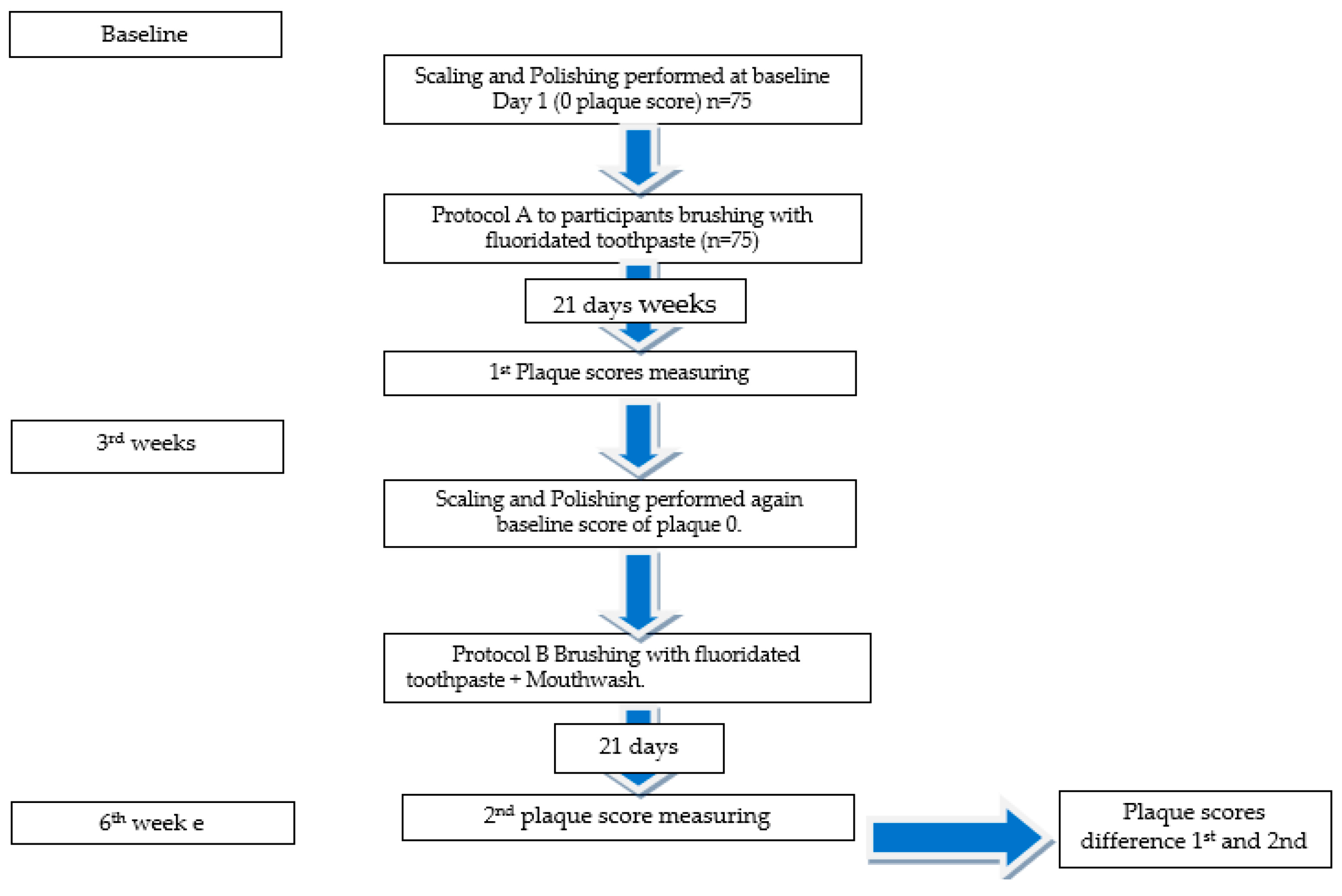
6. Evaluation of Different Mouthwash Experiences by Participants and Assessment of Plaque Scores
7. GCF Collection, Measuring, and Inflammatory Cytokine Analysis
Statistical Analysis
8. Results
9. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gudipaneni, R.K.; Aldahmeshi, R.F.; Patil, S.R.; Alam, M.K. The Prevalence of Malocclusion and the Need for Orthodontic Treatment among Adolescents in the Northern Border Region of Saudi Arabia: An Epidemiological Study. BMC Oral Health 2018, 18, 16. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Marcotty, P.; Klenke, D.; Knocks, L.; Santander, P.; Hrasky, V.; Quast, A. The Adult Orthodontic Patient over 40 Years of Age: Association between Periodontal Bone Loss, Incisor Irregularity, and Increased Orthodontic Treatment Need. Clin. Oral Investig. 2021, 25, 6357–6364. [Google Scholar] [CrossRef] [PubMed]
- Grewal, H.; Sapawat, P.; Modi, P.; Aggarwal, S. Psychological Impact of Orthodontic Treatment on Quality of Life—A Longitudinal Study. Int. Orthod. 2019, 17, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Sharma, K.; Sawhney, R. Evidence of Variable Bacterial Colonization on Coloured Elastomeric Ligatures during Orthodontic Treatment: An Intermodular Comparative Study. J. Clin. Exp. Dent. 2018, 10, e271–e278. [Google Scholar] [CrossRef]
- Akgun, O.M.; Altug, H.; Karacay, S.; Guven Polat, G.; Duyan, S.; Bedir, O. Effect of 2 Elastomeric Ligatures on Microbial Flora and Periodontal Status in Orthodontic Patients. Am. J. Orthod. Dentofac. Orthop. 2014, 145, 667–671. [Google Scholar] [CrossRef]
- Condò, R.; Casaglia, A.; Condò, S.G.; Cerroni, L. Plaque Retention on Elastomeric Ligatures. An in Vivo Study. Oral Implantol. 2012, 5, 92–99. [Google Scholar]
- Aghili, H.; Jafari Nadoushan, A.A.; Herandi, V. Antimicrobial Effect of Zataria Multiflora Extract in Comparison with Chlorhexidine Mouthwash on Experimentally Contaminated Orthodontic Elastomeric Ligatures. J. Dent. 2015, 12, 1–10. [Google Scholar]
- Oshagh, M.; Dashliborun, Y.N.; Saravi, M.E.; Bazargani, A. Evaluation of Chlorhexidine and Zatariamultiflora Essential Oil in Removing Streptococcus Viridans and Candida from the Surface of Removable Orthodontic Appliances: A Randomized Clinical Trial. J. Maz. Univ. Med. Sci. 2014, 23, 191–199. [Google Scholar]
- Kharaeva, Z.F.; Mustafaev, M.S.; Khazhmetov, A.V.; Gazaev, I.H.; Blieva, L.Z.; Steiner, L.; Mayer, W.; de Luca, C.; Korkina, L.G. Anti-Bacterial and Anti-Inflammatory Effects of Toothpaste with Swiss Medicinal Herbs towards Patients Suffering from Gingivitis and Initial Stage of Periodontitis: From Clinical Efficacy to Mechanisms. Dent. J. 2020, 8, 10. [Google Scholar] [CrossRef]
- Szliszka, E.; Czuba, Z.P.; Domino, M.; Mazur, B.; Zydowicz, G.; Krol, W. Ethanolic Extract of Propolis (EEP) Enhances the Apoptosis- Inducing Potential of TRAIL in Cancer Cells. Molecules 2009, 14, 738–754. [Google Scholar] [CrossRef]
- Feres, M.; Gursky, L.C.; Faveri, M.; Tsuzuki, C.O.; Figueiredo, L.C. Clinical and Microbiological Benefits of Strict Supragingival Plaque Control as Part of the Active Phase of Periodontal Therapy. J. Clin. Periodontol. 2009, 36, 857–867. [Google Scholar] [CrossRef] [PubMed]
- Haydari, M.; Bardakci, A.G.; Koldsland, O.C.; Aass, A.M.; Sandvik, L.; Preus, H.R. Comparing the Effect of 0.06–0.12% and 0.2% Chlorhexidine on Plaque, Bleeding and Side Effects in an Experimental Gingivitis Model: A Parallel Group, Double Masked Randomized Clinical Trial. BMC Oral Health 2017, 17, 118. [Google Scholar] [CrossRef] [PubMed]
- Ajay Rao, H.; Bhat, S.; Hegde, S.; Jhamb, V. Efficacy of Garlic Extract and Chlorhexidine Mouthwash in Reduction of Oral Salivary Microorganisms, an in Vitro Study. Anc. Sci. Life 2014, 34, 85. [Google Scholar] [CrossRef]
- Cohen, M.M. Tulsi—Ocimum sanctum: A Herb for All Reasons. J. Ayurveda Integr. Med. 2014, 5, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Bhaskar, D.J.; Gupta, R.K.; Karim, B.; Jain, A.; Singh, R.; Karim, W. A Randomized Controlled Clinical Trial of Ocimum sanctum and Chlorhexidine Mouthwash on Dental Plaque and Gingival Inflammation. J. Ayurveda Integr. Med. 2014, 5, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Hosamane, M.; Acharya, A.B.; Vij, C.; Trivedi, D.; Setty, S.B.; Thakur, S.L. Evaluation of Holy Basil Mouthwash as an Adjunctive Plaque Control Agent in a Four Day Plaque Regrowth Model. J. Clin. Exp. Dent. 2014, 6, e491–e496. [Google Scholar] [CrossRef]
- Penmetsa, G.; Pitta, S. Efficacy of Ocimum sanctum, Aloe Vera and Chlorhexidine Mouthwash on Gingivitis: A Randomized Controlled Comparative Clinical Study. Ayu 2019, 40, 23. [Google Scholar] [CrossRef]
- Deepika, B.A.; Ramamurthy, J.; Girija, S.; Jayakumar, N.D. Evaluation of the Antimicrobial Effect of Ocimum sanctum L. Oral Gel against Anaerobic Oral Microbes: An In Vitro Study. World J. Dent. 2022, 13, S23–S27. [Google Scholar] [CrossRef]
- Glang, J.; Falk, W.; Westendorf, J. Effect of Morinda citrifolia L. Fruit Juice on Gingivitis/Periodontitis. Mod. Res. Inflamm. 2013, 2, 21–27. [Google Scholar] [CrossRef]
- Semeniuc, C.A.; Rotar, M.A.; Suharoschi, R.; Tofana, M.; Muste, S. Overview of the EU Legislation on Novel Foods and Novel Food Ingredients. Agriculture 2011, 68, 68–73. [Google Scholar] [CrossRef]
- Goodyear, M.D.E.; Krleza-Jeric, K.; Lemmens, T. The Declaration of Helsinki. Br. Med. J. 2007, 335, 624–625. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.F.; Altman, D.G.; Moher, D. CONSORT 2010 Statement: Updated Guidelines for Reporting Parallel Group Randomised Trials. BMC Med. 2010, 8, 18. [Google Scholar] [CrossRef]
- Niazi, F.H.; Kamran, M.A.; Naseem, M.; AlShahrani, I.; Fraz, T.R.; Hosein, M. Anti-Plaque Efficacy of Herbal Mouthwashes Compared to Synthetic Mouthwashes in Patients Undergoing Orthodontic Treatment: A Randomised Controlled Trial. Oral Health Prev. Dent. 2018, 16, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.L.; Liu, C.T.; Chou, M.C.; Ko, C.H.; Wang, C.K. Noni (Morinda citrifolia L.) Fruit Extracts Improve Colon Microflora and Exert Anti-Inflammatory Activities in Caco-2 Cells. J. Med. Food 2015, 18, 663–676. [Google Scholar] [CrossRef]
- Bhat, S.S.; Kochikar Pai, R.; Salman, A.; Chandra, J. Use of an Extract of Indian Sacred Plant Ocimum sanctum as an Anticariogenic Agent: An in Vitro Study. Int. J. Clin. Pediatr. Dent. 2015, 8, 99–101. [Google Scholar] [CrossRef]
- Agarwal, P.; Nagesh, L. Murlikrishnan Evaluation of the Antimicrobial Activity of Various Concentrations of Tulsi (Ocimum sanctum) Extract against Streptococcus Mutans: An in Vitro Study. Indian J. Dent. Res. 2010, 21, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Kamran, M.A.; Alnazeh, A.A.; Almagbol, M.; Almoammar, S.; Alhaizaey, A.H.A.; Alshahrani, I. Role of Six Cytokines and Bone Metabolism Biomarkers in Gingival Crevicular Fluid in Patients Undergoing Fixed Orthodontic Appliance Treatment in Comparison with Aligners: A Clinical Study. Angle Orthod. 2023, 93, 335–340. [Google Scholar] [CrossRef]
- Sharma, R.; Hebbal, M.; Ankola, A.; Murugaboopathy, V.; Shetty, S. Effect of Two Herbal Mouthwashes on Gingival Health of School Children. J. Tradit. Complement. Med. 2014, 4, 272–278. [Google Scholar] [CrossRef]
- Talattof, Z.; Azad, A.; Zahed, M.; Shahradnia, N. Antifungal Activity of Xylitol against Candida Albicans: An in Vitro Study. J. Contemp. Dent. Pract. 2018, 19, 125–129. [Google Scholar] [CrossRef]
- Zaini, W.S. Antibacterial Effectiveness of Morinda citrifolia L. Extract on Salmonella Typhi Bacteria Using Serial Dilution Method with 15–60 Minutes Contact Time. Pharmacogn. J. 2021, 13, 839–843. [Google Scholar] [CrossRef]
- Singh, A.; Daing, A.; Dixit, J. The Effect of Herbal, Essential Oil and Chlorhexidine Mouthrinse on de Novo Plaque Formation. Int. J. Dent. Hyg. 2013, 11, 48–52. [Google Scholar] [CrossRef]
- Quintas, V.; Prada-López, I.; Donos, N.; Suárez-Quintanilla, D.; Tomás, I. Antiplaque Effect of Essential Oils and 0.2% Chlorhexidine on an in Situ Model of Oral Biofilm Growth: A Randomised Clinical Trial. PLoS ONE 2015, 10, e0117177. [Google Scholar] [CrossRef]
- Ren, Y.; Hazemeijer, H.; de Haan, B.; Qu, N.; de Vos, P. Cytokine Profiles in Crevicular Fluid During Orthodontic Tooth Movement of Short and Long Durations. J. Periodontol. 2007, 78, 453–458. [Google Scholar] [CrossRef]
- Tatullo, M.; Codispoti, B.; Paduano, F.; Nuzzolese, M.; Makeeva, I. Strategic Tools in Regenerative and Translational Dentistry. Int. J. Mol. Sci. 2019, 20, 1879. [Google Scholar] [CrossRef] [PubMed]
- Bonato, R.C.S.; Mapengo, M.A.A.; De Azevedo-Silva, L.J.; Janson, G.; De Carvalho Sales-Peres, S.H. Tooth Movement, Orofacial Pain, and Leptin, Interleukin-1β, and Tumor Necrosis Factor-α Levels in Obese Adolescents. Angle Orthod. 2022, 92, 95–100. [Google Scholar] [CrossRef]
- Graves, D.T.; Cochran, D. The Contribution of Interleukin-1 and Tumor Necrosis Factor to Periodontal Tissue Destruction. J. Periodontol. 2003, 74, 391–401. [Google Scholar] [CrossRef]
- Mohammed, A.; Saidath, K.; Mohindroo, A.; Shetty, A.; Shetty, V.; Rao, S.; Shetty, P. Assessment and Measurement of Interleukin 6 in Periodontal Ligament Tissues during Orthodontic Tooth Movement. World J. Dent. 2019, 10, 88–92. [Google Scholar] [CrossRef]
- Başaran, G.; Özer, T.; Kaya, F.A.; Hamamci, O. Interleukins 2, 6, and 8 Levels in Human Gingival Sulcus during Orthodontic Treatment. Am. J. Orthod. Dentofac. Orthop. 2006, 130, e1–e7. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, F.; Tatullo, M.; Marrelli, M.; Inchingolo, A.M.; Tarullo, A.; Inchingolo, A.D.; Dipalma, G.; Brunetti, S.P.; Tarullo, A.; Cagiano, R. Combined Occlusal and Pharmacological Therapy in the Treatment of Temporo-Mandibular Disorders. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 1296–1300. [Google Scholar] [PubMed]
- Gürgan, C.A.; Zaim, E.; Bakirsoy, I.; Soykan, E. Short-Term Side Effects of 0.2% Alcohol-Free Chlorhexidine Mouthrinse Used as an Adjunct to Non-Surgical Periodontal Treatment: A Double-Blind Clinical Study. J. Periodontol. 2006, 77, 370–384. [Google Scholar] [CrossRef]
- Marinone, M.G.; Savoldi, E. Chlorhexidine and Taste. Influence of Mouthwashes Concentration and of Rinsing Time. Minerva Stomatol. 2000, 49, 221–226. [Google Scholar] [PubMed]
- Asiri, F.Y.I.; Alomri, O.M.H.; Alghmlas, A.S.; Gufran, K.; Sheehan, S.A.; Shah, A.H. Evaluation of Efficacy of a Commercially Available Herbal Mouthwash on Dental Plaque and Gingivitis: A Double-Blinded, Parallel, Randomized, Controlled Trial. J. Int. Oral Health 2016, 8, 224–226. [Google Scholar] [CrossRef]

| Characteristics | Interventional Group | |||
|---|---|---|---|---|
| Gender | Group 1: 0.2% CHX | Group 2: 5% MC | Group 3: 4% OS | Total |
| Male | 6 | 8 | 4 | 18 |
| Female | 16 | 14 | 18 | 48 |
| Age (mean ± SD) | 19.25 ± 1.58 | 20.66 ± 1.29 | 19.98 ± 1.36 | |
| Education | ||||
| Basic school | 14 | 18 | 15 | 47 |
| High school | 6 | 2 | 4 | 12 |
| Bachelor’s | 1 | 1 | 2 | 4 |
| Masters | 1 | 1 | 1 | 3 |
| Plaque Scores (Mean ± SD) | Group 1: 0.2% CHX | Group 2: 5% MC | Group 3: 4% OS | p-Value |
|---|---|---|---|---|
| First score (Protocol A) | 33.25 ± 4.26 | 32.29 ± 3.97 | 31.59 ± 4.01 | |
| Second Score (Protocol B) | 19.26 ± 2.11 | 15.71 ± 2.27 | 16.33 ± 1.98 | |
| Tukey Post Hoc | ||||
| Group 1: 0.12% CHX | - | - | - | 0.007 |
| Group 2: 5% MC | 0.011 * | - | - | |
| Group 3: 4% OS | 0.013 * | 0.289 | - |
| Symptoms | Intervention Groups | |||
|---|---|---|---|---|
| Group 1: 0.2% CHX | Group 2: 5% MC | Group 3: 4% OS | Total | |
| Taste Changes | ||||
| Yes | 18 | 2 | 1 | 21 |
| No | 4 | 20 | 21 | 45 |
| Mouth Burning | ||||
| Yes | 16 | - | 4 | 20 |
| No | 6 | 22 | 18 | 46 |
| Mouth Dryness | ||||
| Yes | 14 | 1 | 5 | 20 |
| No | 8 | 21 | 17 | 46 |
| Taste | ||||
| Pleasant | 2 | 15 | 16 | 33 |
| Unpleasant | 20 | 7 | 6 | 33 |
| Feeling of Freshness | ||||
| Yes | 10 | 17 | 18 | 45 |
| No | 12 | 5 | 4 | 21 |
| Inflammatory Biomarkers in GCF | Mean ± SD Baseline | Mean ± SD 3 Weeks (Protocol A) Brushing and Fluoridated Toothpaste | Groups | Mean ± SD 6 Weeks (Protocol B) Brushing and Fluoridated Toothpaste | p-Value |
|---|---|---|---|---|---|
| TNF-α (pg/mL) | 6.32 ± 0.65 | 5.27 ± 0.27 | 0.12% CHX | 4.25 ± 0.63 | 0.030 |
| 5% MC | 2.99 ± 0.75 | 0.010 * | |||
| 4% OS | 2.79 ± 1.01 | 0.020 * | |||
| IL-α (pg/mL) | 0.084 ± 0.06 | 0.077 ± 0.03 | 0.12% CHX | 0.058 ± 0.002 | 0.270 |
| 5% MC | 0.021 ± 0.001 | 0.020 * | |||
| 4% OS | 0.020 ± 0.005 | 0.030 * | |||
| IL-β (pg/mL) | 8.69 ± 0.25 | 7.14 ± 0.33 | 0.12% CHX | 7.02 ± 0.35 | 0.450 |
| 5% MC | 6.15 ± 0.15 | 0.040 * | |||
| 4% OS | 6.10 ± 0.22 | 0.010 * | |||
| IL-2 (pg/mL) | 3.5 ± 1.21 | 3.0 ± 1.01 | 0.12% CHX | 2.79 ± 0.87 | 0.660 |
| 5% MC | 2.23 ± 0.65 | 0.740 | |||
| 4% OS | 2.10 ± 0.89 | 0.890 | |||
| IL-6 (pg/mL) | 3.85 ± 1.01 | 3.26 ± 1.30 | 0.12% CHX | 2.95 ± 1.36 | 0.550 |
| 5% MC | 2.98 ± 0.98 | 0.980 | |||
| 4% OS | 2.85 ± 0.33 | 0.780 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamran, M.A.; Alnazeh, A.A.; Almoammar, S.; Almagbol, M.; Baig, E.A.; Alrwuili, M.R.; Aljabab, M.A.; Alshahrani, I. Effect of Plant-Based Mouthwash (Morinda citrifolia and Ocimum sanctum) on TNF-α, IL-α, IL-β, IL-2, and IL-6 in Gingival Crevicular Fluid and Plaque Scores of Patients Undergoing Fixed Orthodontic Treatment. Medicina 2023, 59, 1968. https://doi.org/10.3390/medicina59111968
Kamran MA, Alnazeh AA, Almoammar S, Almagbol M, Baig EA, Alrwuili MR, Aljabab MA, Alshahrani I. Effect of Plant-Based Mouthwash (Morinda citrifolia and Ocimum sanctum) on TNF-α, IL-α, IL-β, IL-2, and IL-6 in Gingival Crevicular Fluid and Plaque Scores of Patients Undergoing Fixed Orthodontic Treatment. Medicina. 2023; 59(11):1968. https://doi.org/10.3390/medicina59111968
Chicago/Turabian StyleKamran, Muhammad Abdullah, Abdullah A. Alnazeh, Salem Almoammar, Mohammad Almagbol, Eisha Abrar Baig, Mohammad Raji Alrwuili, Mohammed Ahmed Aljabab, and Ibrahim Alshahrani. 2023. "Effect of Plant-Based Mouthwash (Morinda citrifolia and Ocimum sanctum) on TNF-α, IL-α, IL-β, IL-2, and IL-6 in Gingival Crevicular Fluid and Plaque Scores of Patients Undergoing Fixed Orthodontic Treatment" Medicina 59, no. 11: 1968. https://doi.org/10.3390/medicina59111968
APA StyleKamran, M. A., Alnazeh, A. A., Almoammar, S., Almagbol, M., Baig, E. A., Alrwuili, M. R., Aljabab, M. A., & Alshahrani, I. (2023). Effect of Plant-Based Mouthwash (Morinda citrifolia and Ocimum sanctum) on TNF-α, IL-α, IL-β, IL-2, and IL-6 in Gingival Crevicular Fluid and Plaque Scores of Patients Undergoing Fixed Orthodontic Treatment. Medicina, 59(11), 1968. https://doi.org/10.3390/medicina59111968






