Detroit Interventional Pain Assessment Scale: A Pain Score and Method for Measuring and Evaluating Post-Operative Pain Management—A Prospective Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Demographics
2.2. Procedure
2.3. Statistical Analysis
2.4. Ethical Considerations
3. Results
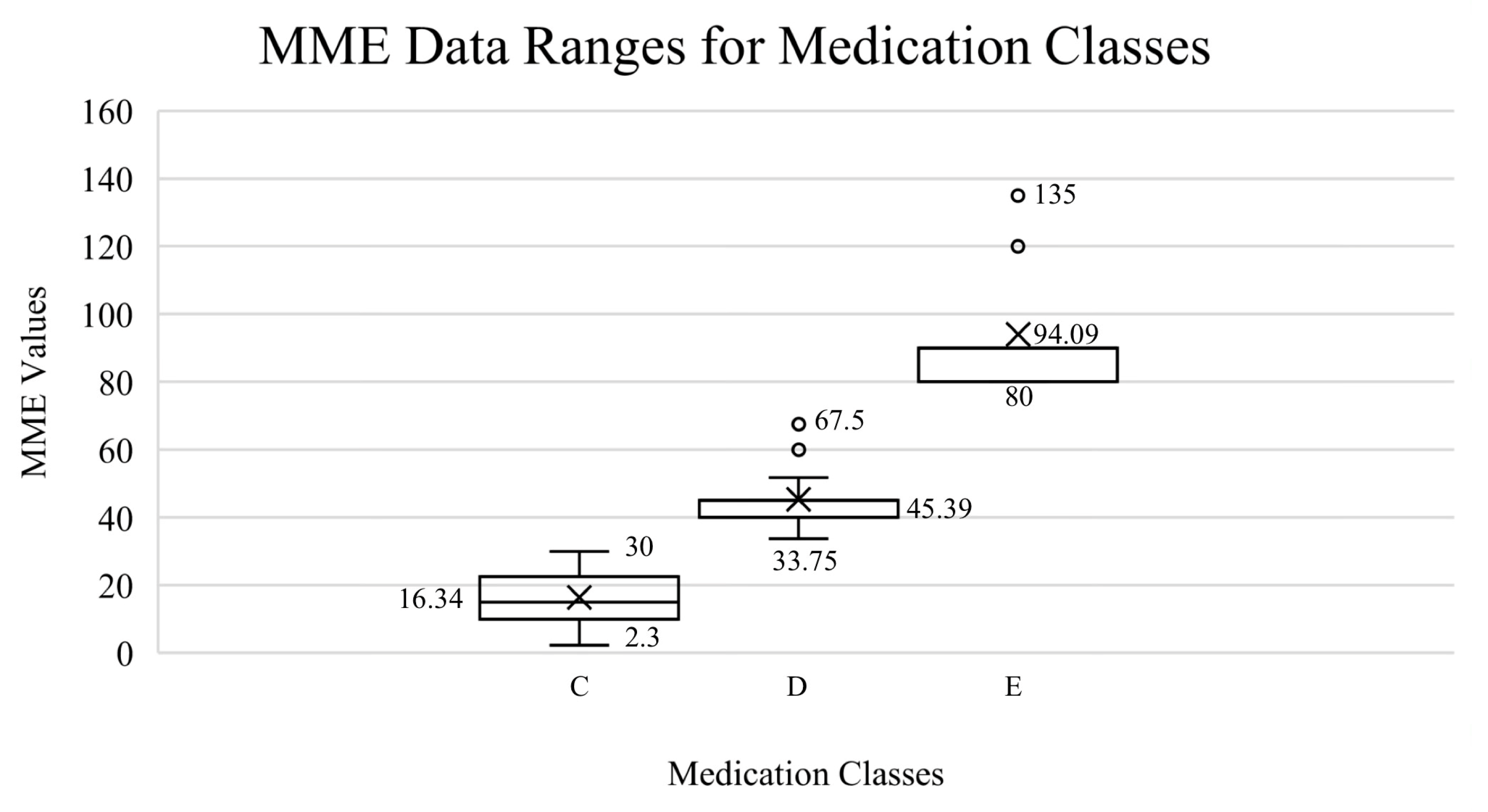
3.1. Scientific Validation of Medication and Grouping
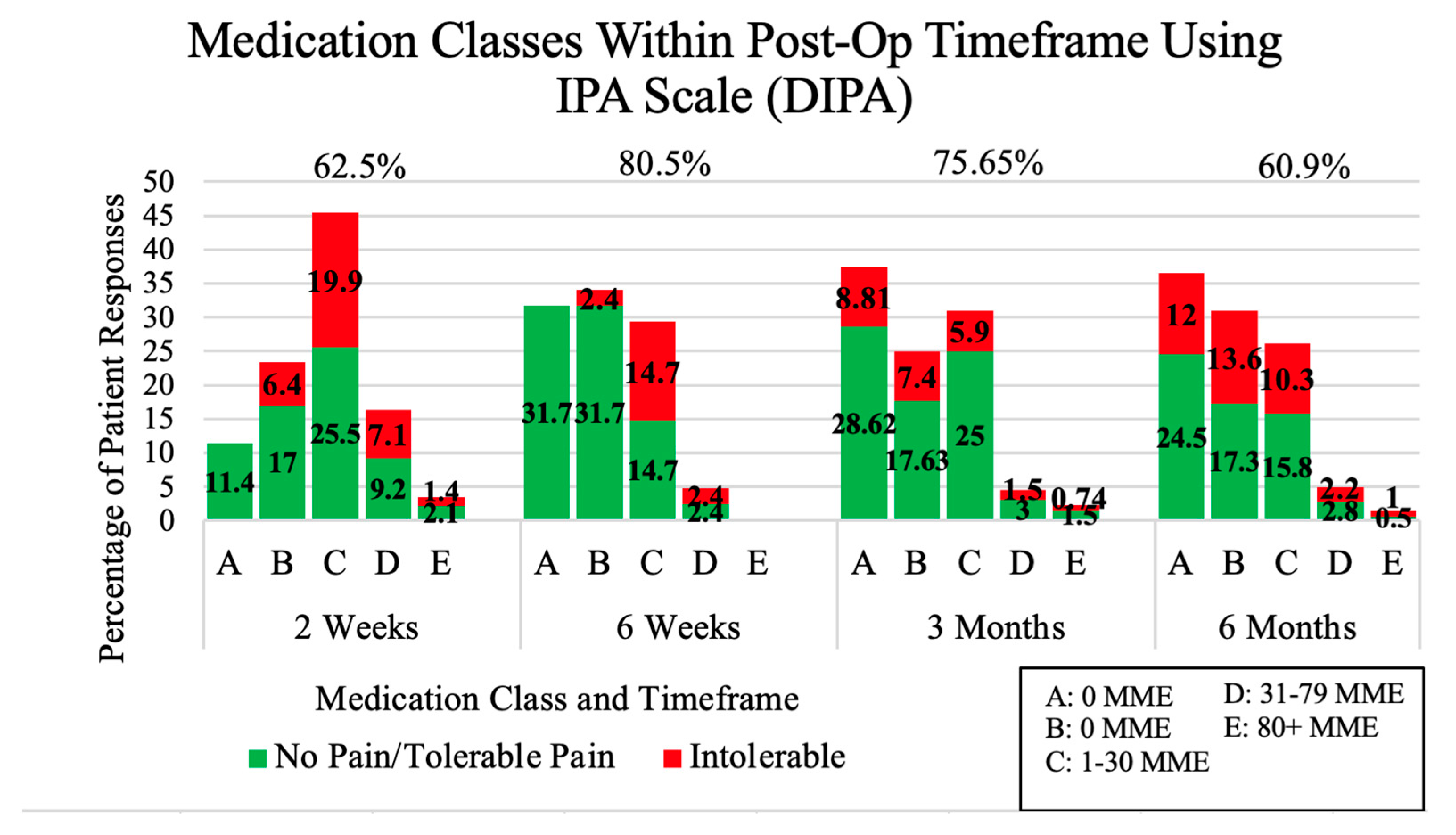
3.2. Pain Management Score
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McGranahan, D.A.; Parker, T.S. The Opioid Epidemic: A Geography in Two Phases. 2021. Available online: https://www.ers.usda.gov/webdocs/publications/100833/err-287.pdf (accessed on 7 January 2022).
- Ringwalt, C.; Gugelmann, H.; Garrettson, M.; Dasgupta, N.; Chung, A.E.; Proescholdbell, S.K.; Skinner, A.C. Differential prescribing of opioid analgesics according to physician specialty for Medicaid patients with chronic noncancer pain diagnoses. Pain Res. Manag. 2014, 19, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Delaney, L.D.; Clauw, D.J.; Waljee, J.F. The Management of Acute Pain for Musculoskeletal Conditions: The Challenges of Opioids and Opportunities for the Future. J. Bone Jt. Surg. Am. 2020, 102 (Suppl. S1), 3–9. [Google Scholar] [CrossRef] [PubMed]
- Taipale, H.; Särkilä, H.; Tanskanen, A.; Kurko, T.; Taiminen, T.; Tiihonen, J.; Sund, R.; Tuulio-Henriksson, A.; Saastamoinen, L.; Hietala, J. Incidence of and Characteristics Associated With Long-term Benzodiazepine Use in Finland. JAMA Netw. Open. 2020, 3, e2019029. [Google Scholar] [CrossRef] [PubMed]
- Tompkins, D.A.; Hobelmann, J.G.; Compton, P. Providing chronic pain management in the “Fifth Vital Sign” Era: Historical and treatment perspectives on a modern-day medical dilemma. Drug Alcohol Depend. 2017, 173 (Suppl. S1), S11–S21. [Google Scholar] [CrossRef] [PubMed]
- Finnerup, N.B. Nonnarcotic Methods of Pain Management. N. Engl. J. Med. 2019, 380, 2440–2448. [Google Scholar] [CrossRef] [PubMed]
- George, S.Z.; Goode, A.P. Physical therapy and opioid use for musculoskeletal pain management: Competitors or companions? Pain Rep. 2020, 5, e827. [Google Scholar] [CrossRef] [PubMed]
- McPherson, A.L. Safety in numbers, or lack thereof: Opioid conversion calculations. Pharm. Today. 2017, 23, 44. [Google Scholar] [CrossRef]
- Vaidya, R.; Washington, A.; Stine, S.; Geamanu, A.; Hudson, I. The IPA, a Modified Numerical System for Pain Assessment and Intervention. J. Am. Acad. Orthop. Surg. Glob. Res. Rev. 2021, 5, e21.00174. [Google Scholar] [CrossRef] [PubMed]
- Arefayne, N.R.; Seid Tegegne, S.; Gebregzi, A.H.; Mustofa, S.Y. Incidence and associated factors of post-operative pain after emergency orthopedic surgery: A multi-centered prospective observational cohort study. Int. J. Surg. Open 2020, 27, 103–113. [Google Scholar] [CrossRef]
- Eriksson, K.; Wikström, L.; Årestedt, K.; Fridlund, B.; Broström, A. Numeric rating scale: Patients’ perceptions of its use in postoperative pain assessments. Appl. Nurs. Res. 2014, 27, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Pisansky, A.; Berna, C.; Rathmell, J. Opioid Tapering for Patients with Chronic Pain. Available online: https://www.uptodate.com/contents/opioid-tapering-for-patients-with-chronic-pain (accessed on 20 February 2022).
- Dasgupta, N.; Wang, Y.; Bae, J.; Kinlaw, A.C.; Chidgey, B.A.; Cooper, T.; Delcher, C. Inches, Centimeters, and Yards: Overlooked Definition Choices Inhibit Interpretation of Morphine Equivalence. Clin. J. Pain 2021, 37, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Traven, S.A.; Brinton, D.L.; Woolf, S.K.; Leddy, L.R.; Gottschalk, M.B.; Slone, H.S. Notable Variability in Opioid-prescribing Practices After Common Orthopaedic Procedures. J. Am. Acad. Orthop. Surg. 2021, 29, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Treede, R.D.; Rief, W.; Barke, A.; Aziz, Q.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Evers, S.; Finnerup, N.B.; First, M.B.; et al. Chronic pain as a symptom or a disease: The IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain 2019, 160, 19–27. [Google Scholar] [CrossRef] [PubMed]
- LARA—MI Automated Prescription System (MAPS). Available online: https://www.michigan.gov/lara/0,4601,7-154-89334_72600_72603_55478---,00.html (accessed on 10 December 2021).
- Fudin, J.; Raouf, M.; Wegrzyn, E.L.; Schatman, M.E. Safety concerns with the Centers for Disease Control opioid calculator. J. Pain Res. 2017, 11, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.M. JCAHO pain management standards are unveiled. Joint Commission on Accreditation of Healthcare Organizations. JAMA 2000, 284, 428–429. [Google Scholar] [CrossRef] [PubMed]
- Horn, R.; Kramer, J. Postoperative Pain Control. In StatPearls; StatPearls Publishing: Tampa, FL, USA, 2022. Available online: http://www.ncbi.nlm.nih.gov/books/NBK544298/ (accessed on 2 November 2022).
- Fulton-Kehoe, D.; Von Korff, M.; Mai, J.; Weir, V.; Lofy, K.H.; Sabel, J.; Tauben, D.; Franklin, G. Surveillance of Opioid Prescribing as a Public Health Intervention: Washington State Bree Collaborative Opioid Metrics. J. Public Health Manag. Pract. 2020, 26, 206–213. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boggs, L.J.; Stine, S.A.; Boggs-Hughey, B.J.; Geamanu, A.; Little, B.E.; Darwiche, H.F.; Vaidya, R. Detroit Interventional Pain Assessment Scale: A Pain Score and Method for Measuring and Evaluating Post-Operative Pain Management—A Prospective Study. Medicina 2023, 59, 1976. https://doi.org/10.3390/medicina59111976
Boggs LJ, Stine SA, Boggs-Hughey BJ, Geamanu A, Little BE, Darwiche HF, Vaidya R. Detroit Interventional Pain Assessment Scale: A Pain Score and Method for Measuring and Evaluating Post-Operative Pain Management—A Prospective Study. Medicina. 2023; 59(11):1976. https://doi.org/10.3390/medicina59111976
Chicago/Turabian StyleBoggs, Lauryn J., Sasha A. Stine, Barbara J. Boggs-Hughey, Andreea Geamanu, Bryan E. Little, Hussein F. Darwiche, and Rahul Vaidya. 2023. "Detroit Interventional Pain Assessment Scale: A Pain Score and Method for Measuring and Evaluating Post-Operative Pain Management—A Prospective Study" Medicina 59, no. 11: 1976. https://doi.org/10.3390/medicina59111976
APA StyleBoggs, L. J., Stine, S. A., Boggs-Hughey, B. J., Geamanu, A., Little, B. E., Darwiche, H. F., & Vaidya, R. (2023). Detroit Interventional Pain Assessment Scale: A Pain Score and Method for Measuring and Evaluating Post-Operative Pain Management—A Prospective Study. Medicina, 59(11), 1976. https://doi.org/10.3390/medicina59111976





