Horizontal and Vertical Defect Management with a Novel Degradable Pure Magnesium Guided Bone Regeneration (GBR) Membrane—A Clinical Case
Abstract
:1. Introduction
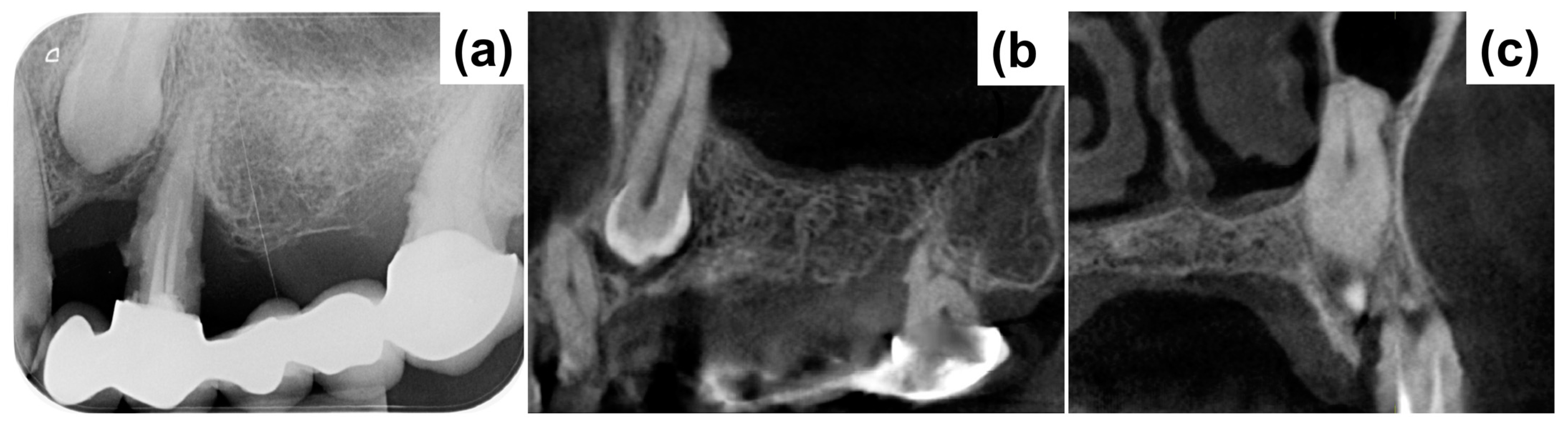
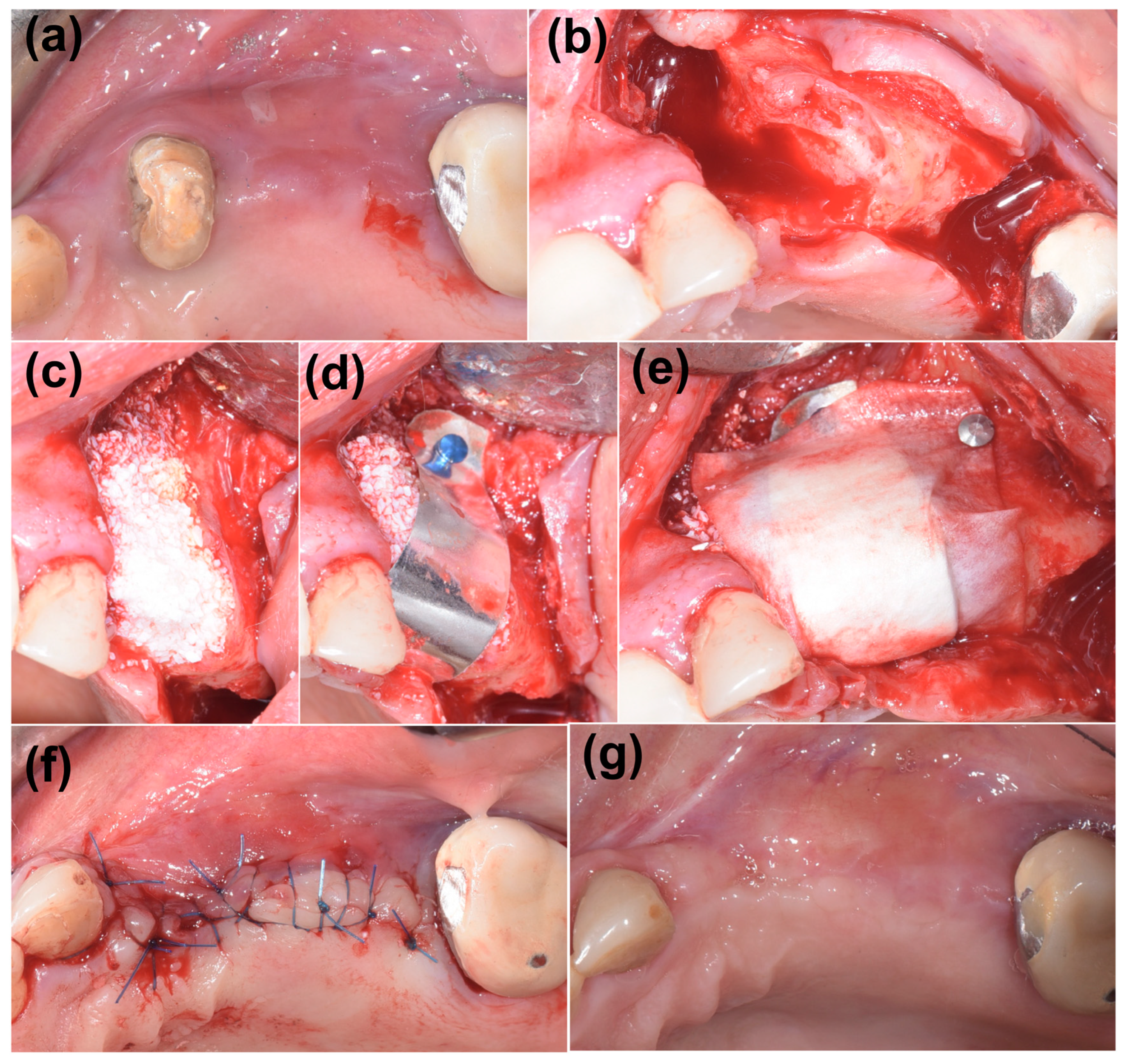
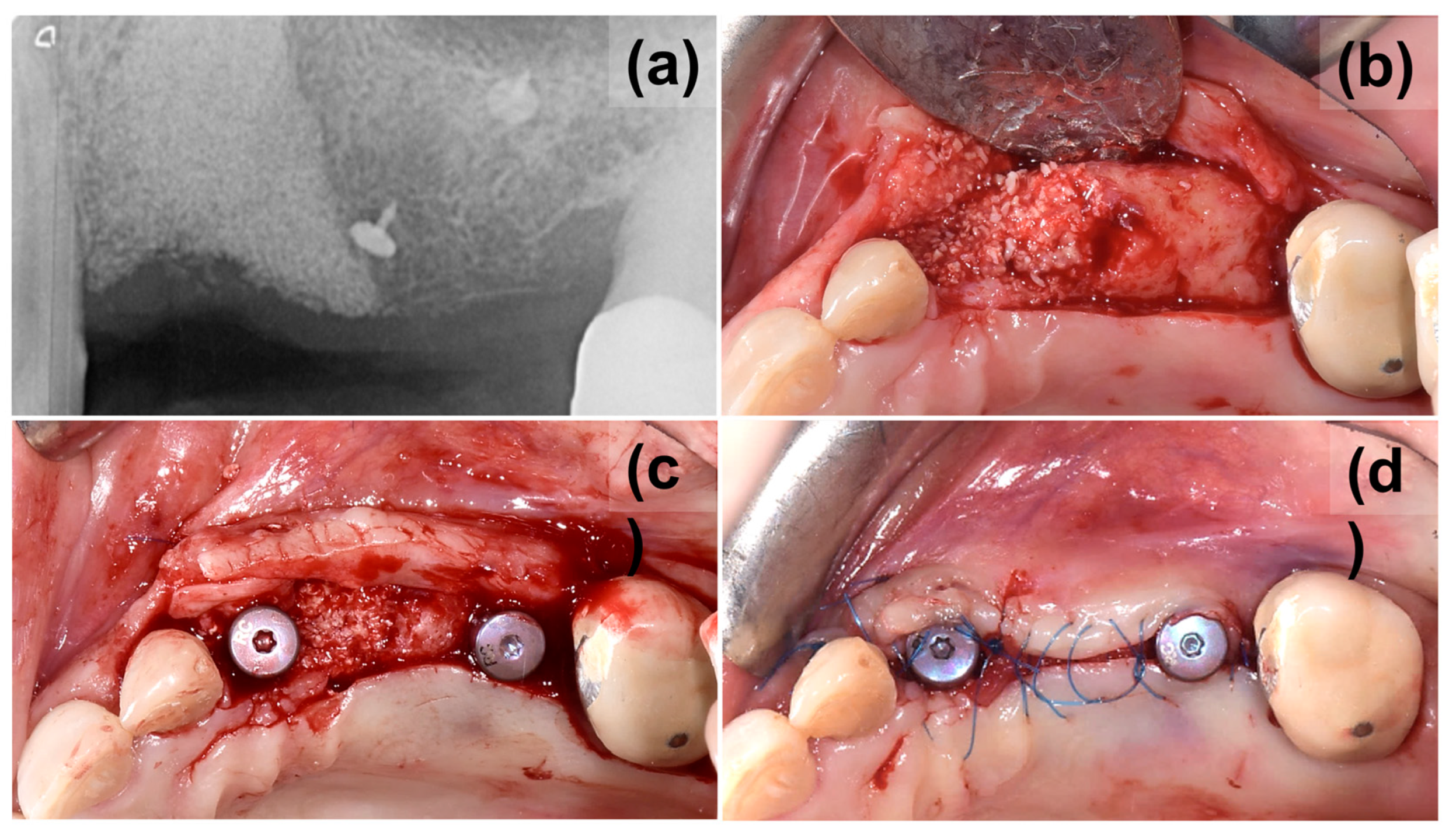
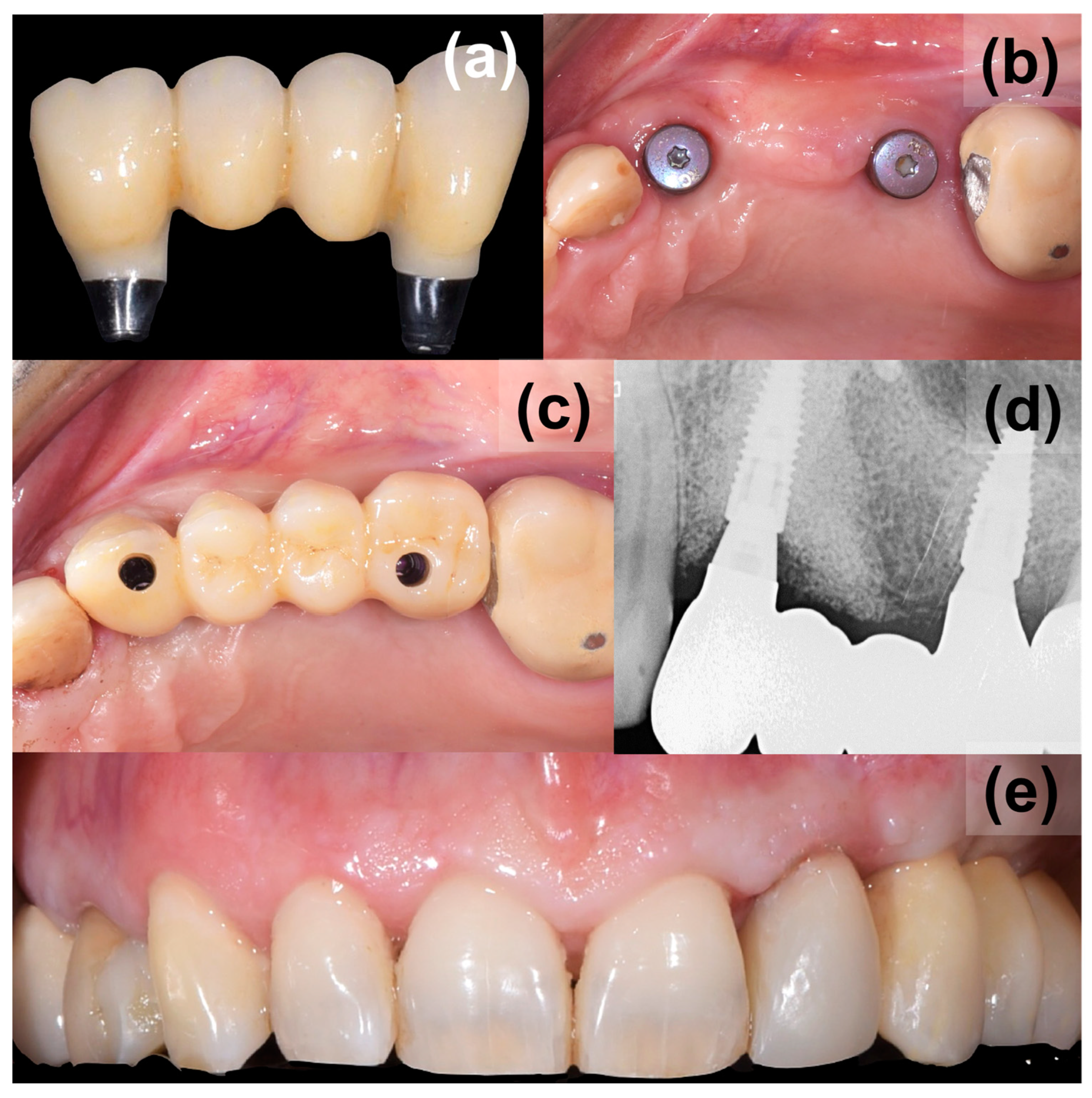
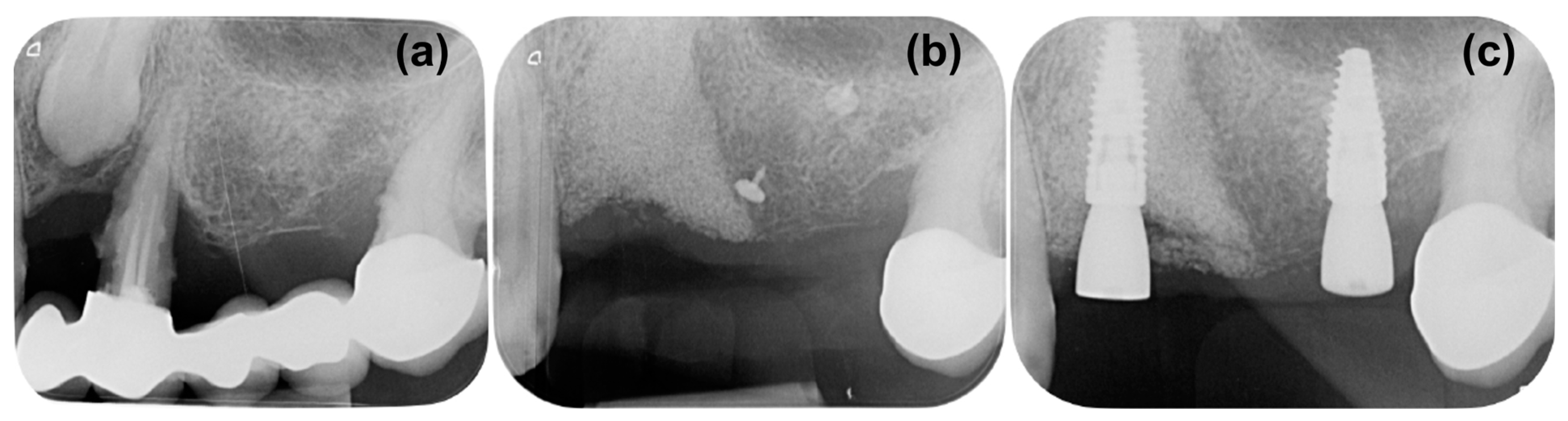
2. Case Presentation
3. Discussion
4. Conclusions
- Execution of only two surgical phases, limiting discomfort and invasiveness.
- Use of materials that are completely resorbable and are capable of handling complex bone defects with a vertical component.
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khojasteh, A.; Kheiri, L.; Motamedian, S.; Khoshkam, V. Guided bone regeneration for the reconstruction of alveolar bone defects. Ann. Maxillofac. Surg. 2017, 7, 263. [Google Scholar] [CrossRef]
- Perić Kačarević, Ž.; Rider, P.; Alkildani, S.; Retnasingh, S.; Pejakić, M.; Schnettler, R.; Gosau, M.; Smeets, R.; Jung, O.; Barbeck, M. An introduction to bone tissue engineering. Int. J. Artif. Organs 2020, 43, 69–86. [Google Scholar] [CrossRef]
- Scantlebury, T.; Ambruster, J. The development of guided regeneration: Making the impossible possible and the unpredictable predictable. J. Evid. Based Dent. Pract. 2012, 12, 101–117. [Google Scholar] [CrossRef]
- Elnayef, B.; Monje, A.; Albiol, G.; Galindo-Moreno, P.; Wang, H.-L.; Hernández-Alfaro, F. Vertical Ridge Augmentation in the Atrophic Mandible: A Systematic Review and Meta-Analysis. Int. J. Oral Maxillofac. Implant. 2017, 32, 291–312. [Google Scholar] [CrossRef]
- Plonka, A.; Urban, I.; Wang, H.-L. Decision Tree for Vertical Ridge Augmentation. Int. J. Periodontics Restor. Dent. 2018, 38, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Ricci, L.; Perrotti, V.; Ravera, L.; Scarano, A.; Piattelli, A.; Iezzi, G. Rehabilitation of Deficient Alveolar Ridges Using Titanium Grids before and Simultaneously with Implant Placement: A Systematic Review. J. Periodontol. 2013, 84, 1234–1242. [Google Scholar] [CrossRef] [PubMed]
- Urban, I.A.; Montero, E.; Monje, A.; Sanz-Sánchez, I. Effectiveness of vertical ridge augmentation interventions: A systematic review and meta-analysis. J. Clin. Periodontol. 2019, 46, 319–339. [Google Scholar] [CrossRef] [PubMed]
- Bunyaratavej, P.; Wang, H.-L. Collagen Membranes: A Review. J. Periodontol. 2001, 72, 215–229. [Google Scholar] [CrossRef]
- Naenni, N.; Sapata, V.; Bienz, S.P.; Leventis, M.; Jung, R.E.; Hämmerle, C.H.F.; Thoma, D.S. Effect of flapless ridge preservation with two different alloplastic materials in sockets with buccal dehiscence defects—Volumetric and linear changes. Clin. Oral Investig. 2018, 22, 2187–2197. [Google Scholar] [CrossRef] [PubMed]
- Mir-Mari, J.; Wui, H.; Jung, R.E.; Hämmerle, C.H.F.F.; Benic, G.I.; Wui, H.; Jung, R.E.; Hämmerle, C.H.F.F.; Benic, G.I. Influence of blinded wound closure on the volume stability of different GBR materials: An in vitro cone-beam computed tomographic examination. Clin. Oral Implant. Res. 2016, 27, 258–265. [Google Scholar] [CrossRef]
- Hameed, M.H.; Gul, M.; Ghafoor, R.; Khan, F.R. Vertical Ridge Gain with Various Bone Augmentation Techniques: A Systematic Review and Meta-Analysis. J. Prosthodont. 2019, 28, 421–427. [Google Scholar] [CrossRef]
- Jung, R.E.; Brügger, L.V.; Bienz, S.P.; Hüsler, J.; Hämmerle, C.H.F.; Zitzmann, N.U. Clinical and radiographical performance of implants placed with simultaneous guided bone regeneration using resorbable and nonresorbable membranes after 22–24 years, a prospective, controlled clinical trial. Clin. Oral Implant. Res. 2021, 32, 1455–1465. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhou, Z.; Yun, J.; Liu, R.; Li, J.; Chen, Y.; Cai, H.; Jiang, H.B.; Lee, E.-S.; Han, J.; et al. Effect of Different Membranes on Vertical Bone Regeneration: A Systematic Review and Network Meta-Analysis. Biomed. Res. Int. 2022, 2022, 7742687. [Google Scholar] [CrossRef] [PubMed]
- Rider, P.; Kačarević, Ž.P.; Elad, A.; Tadic, D.; Rothamel, D.; Sauer, G.; Bornert, F.; Windisch, P.; Hangyási, D.B.; Molnar, B.; et al. Biodegradable magnesium barrier membrane used for guided bone regeneration in dental surgery. Bioact. Mater. 2022, 14, 152–168. [Google Scholar] [CrossRef]
- Rossi, R.; Ghezzi, C.; Tomecek, M. Cortical lamina: A new device for the treatment of moderate and severe tridimensional bone and soft tissue defects. Int. J. Esthet. Dent. 2020, 15, 454–473. [Google Scholar] [PubMed]
- Rider, P.; Kačarević, Ž.P.; Elad, A.; Rothamel, D.; Sauer, G.; Bornert, F.; Windisch, P.; Hangyási, D.; Molnar, B.; Hesse, B.; et al. Analysis of a Pure Magnesium Membrane Degradation Process and Its Functionality When Used in a Guided Bone Regeneration Model in Beagle Dogs. Materials 2022, 15, 3106. [Google Scholar] [CrossRef] [PubMed]
- May, H.; Alper Kati, Y.; Gumussuyu, G.; Yunus Emre, T.; Unal, M.; Kose, O. Bioabsorbable magnesium screw versus conventional titanium screw fixation for medial malleolar fractures. J. Orthop. Traumatol. 2020, 21, 9. [Google Scholar] [CrossRef] [PubMed]
- Cerrato, E.; Barbero, U.; Gil Romero, J.A.; Quadri, G.; Mejia-Renteria, H.; Tomassini, F.; Ferrari, F.; Varbella, F.; Gonzalo, N.; Escaned, J. Magmaris™ resorbable magnesium scaffold: State-of-art review. Future Cardiol. 2019, 15, 267–279. [Google Scholar] [CrossRef]
- Zan, R.; Shen, S.; Huang, Y.; Yu, H.; Liu, Y.; Yang, S.; Zheng, B.; Gong, Z.; Wang, W.; Zhang, X.; et al. Research hotspots and trends of biodegradable magnesium and its alloys. Smart Mater. Med. 2023, 4, 468–479. [Google Scholar] [CrossRef]
- Zheng, Y.F.; Gu, X.N.; Witte, F. Biodegradable metals. Mater. Sci. Eng. R Rep. 2014, 77, 1–34. [Google Scholar] [CrossRef]
- Gröber, U.; Schmidt, J.; Kisters, K. Magnesium in prevention and therapy. Nutrients 2015, 7, 8199–8226. [Google Scholar] [CrossRef] [PubMed]
- Kačarević, Ž.P.; Rider, P.; Elad, A.; Tadic, D.; Rothamel, D.; Sauer, G.; Bornert, F.; Windisch, P.; Hangyási, D.B.; Molnar, B.; et al. Biodegradable magnesium fixation screw for barrier membranes used in guided bone regeneration. Bioact. Mater. 2022, 14, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.Y.; Zhu, J.H.; Liu, G.Q.; Liu, Z.C.; Guo, C.B.; Cui, N.H.; Han, J.M. Feasibility and Efficacy of a Degradable Magnesium-Alloy GBR Membrane for Bone Augmentation in a Distal Bone-Defect Model in Beagle Dogs. Bioinorg. Chem. Appl. 2022, 2022, 4941635. [Google Scholar] [CrossRef] [PubMed]
- Blašković, M.; Butorac Prpić, I.; Blašković, D.; Rider, P.; Tomas, M.; Čandrlić, S.; Botond Hangyasi, D.; Čandrlić, M.; Perić Kačarević, Ž. Guided Bone Regeneration Using a Novel Magnesium Membrane: A Literature Review and a Report of Two Cases in Humans. J. Funct. Biomater. 2023, 14, 307. [Google Scholar] [CrossRef]
- Blašković, M.; Blašković, D.; Hangyasi, D.B.; Peloza, O.C.; Tomas, M.; Čandrlić, M.; Rider, P.; Mang, B.; Kačarević, Ž.P.; Trajkovski, B. Evaluation between Biodegradable Magnesium Metal GBR Membrane and Bovine Graft with or without Hyaluronate. Membranes 2023, 13, 691. [Google Scholar] [CrossRef]
- Elad, A.; Rider, P.; Rogge, S.; Witte, F.; Tadić, D.; Kačarević, Ž.P.; Steigmann, L. Application of Biodegradable Magnesium Membrane Shield Technique for Immediate Dentoalveolar Bone Regeneration. Biomedicines 2023, 11, 744. [Google Scholar] [CrossRef]
- Palkovics, D.; Rider, P.; Rogge, S.; Kačarević, Ž.P.; Windisch, P. Possible Applications for a Biodegradable Magnesium Membrane in Alveolar Ridge Augmentation—Retrospective Case Report with Two Years of Follow-Up. Medicina 2023, 59, 1698. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Periodontol. 2018, 89, S159–S172. [Google Scholar] [CrossRef]
- Tawil, G.; Barbeck, M.; Unger, R.; Tawil, P.; Witte, F. Sinus Floor Elevation Using the Lateral Approach and Window Repositioning and a Xenogeneic Bone Substitute as a Grafting Material: A Histologic, Histomorphometric, and Radiographic Analysis. Int. J. Oral Maxillofac. Implant. 2018, 33, 1089–1096. [Google Scholar] [CrossRef]
- Trajkovski, B.; Jaunich, M.; Müller, W.-D.; Beuer, F.; Zafiropoulos, G.-G.; Houshmand, A. Hydrophilicity, Viscoelastic, and Physicochemical Properties Variations in Dental Bone Grafting Substitutes. Materials 2018, 11, 215. [Google Scholar] [CrossRef]
- Barbeck, M.; Udeabor, S.; Lorenz, J.; Schlee, M.; Holthaus, M.G.; Raetscho, N.; Choukroun, J.; Sader, R.; Kirkpatrick, C.J.; Ghanaati, S. High-Temperature Sintering of Xenogeneic Bone Substitutes Leads to Increased Multinucleated Giant Cell Formation: In Vivo and Preliminary Clinical Results. J. Oral Implantol. 2015, 41, e212–e222. [Google Scholar] [CrossRef] [PubMed]
- Khojasteh, A. Polymeric vs hydroxyapatite-based scaffolds on dental pulp stem cell proliferation and differentiation. World J. Stem Cells 2015, 7, 1215. [Google Scholar] [CrossRef] [PubMed]
- Panagiotou, D.; Özkan Karaca, E.; Dirikan İpçi, Ş.; Çakar, G.; Olgaç, V.; Yılmaz, S. Comparison of two different xenografts in bilateral sinus augmentation: Radiographic and histologic findings. Quintessence Int. 2015, 46, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Soldatos, N.K.; Stylianou, P.; Koidou, V.P.; Angelov, N.; Yukna, R.; Romanos, G.E. Limitations and options using resorbable versus nonresorbable membranes for successful guided bone regeneration. Quintessence Int. 2017, 48, 131–147. [Google Scholar] [CrossRef]
- Briguglio, F.; Falcomatà, D.; Marconcini, S.; Fiorillo, L.; Briguglio, R.; Farronato, D. The use of titanium mesh in guided bone regeneration: A systematic review. Int. J. Dent. 2019, 2019, 9065423. [Google Scholar] [CrossRef]
- Watzinger, F.; Luksch, J.; Millesi, W.; Schopper, C.; Neugebauer, J.; Moser, D.; Ewers, R. Guided bone regeneration with titanium membranes: A clinical study. Br. J. Oral Maxillofac. Surg. 2000, 38, 312–315. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.-C.; Lee, J.-S.; Choi, S.-H.; Jung, U.-W. The effect of overlaying titanium mesh with collagen membrane for ridge preservation. J. Periodontal Implant. Sci. 2015, 45, 128. [Google Scholar] [CrossRef]
- Konstantinidis, I.; Kumar, T.; Kher, U.; Stanitsas, P.D.; Hinrichs, J.E.; Kotsakis, G.A. Clinical results of implant placement in resorbed ridges using simultaneous guided bone regeneration: A multicenter case series. Clin. Oral Investig. 2015, 19, 553–559. [Google Scholar] [CrossRef]
- Cucchi, A.; Vignudelli, E.; Napolitano, A.; Marchetti, C.; Corinaldesi, G. Evaluation of complication rates and vertical bone gain after guided bone regeneration with non-resorbable membranes versus titanium meshes and resorbable membranes. A randomized clinical trial. Clin. Implant. Dent. Relat. Res. 2017, 19, 821–832. [Google Scholar] [CrossRef]
- Gu, C.; Xu, L.; Shi, A.; Guo, L.; Chen, H.; Qin, H. Titanium Mesh Exposure in Guided Bone Regeneration Procedures: A Systematic Review and Meta-analysis. Int. J. Oral Maxillofac. Implant. 2022, 37, e29–e40. [Google Scholar] [CrossRef]
- Cucchi, A.; Vignudelli, E.; Franceschi, D.; Randellini, E.; Lizio, G.; Fiorino, A.; Corinaldesi, G. Vertical and horizontal ridge augmentation using customized CAD/CAM titanium mesh with versus without resorbable membranes. A randomized clinical trial. Clin. Oral Implant. Res. 2021, 32, 1411–1424. [Google Scholar] [CrossRef] [PubMed]
- Elgali, I.; Omar, O.; Dahlin, C.; Thomsen, P. Guided bone regeneration: Materials and biological mechanisms revisited. Eur. J. Oral Sci. 2017, 125, 315–337. [Google Scholar] [CrossRef]
- Atrens, A.; Song, G.L.; Liu, M.; Shi, Z.; Cao, F.; Dargusch, M.S. Review of recent developments in the field of magnesium corrosion. Adv. Eng. Mater. 2015, 17, 400–453. [Google Scholar] [CrossRef]
- Witte, F.; Hort, N.; Vogt, C.; Cohen, S.; Kainer, K.U.; Willumeit, R.; Feyerabend, F. Degradable biomaterials based on magnesium corrosion. Curr. Opin. Solid. State Mater. Sci. 2008, 12, 63–72. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frosecchi, M. Horizontal and Vertical Defect Management with a Novel Degradable Pure Magnesium Guided Bone Regeneration (GBR) Membrane—A Clinical Case. Medicina 2023, 59, 2009. https://doi.org/10.3390/medicina59112009
Frosecchi M. Horizontal and Vertical Defect Management with a Novel Degradable Pure Magnesium Guided Bone Regeneration (GBR) Membrane—A Clinical Case. Medicina. 2023; 59(11):2009. https://doi.org/10.3390/medicina59112009
Chicago/Turabian StyleFrosecchi, Massimo. 2023. "Horizontal and Vertical Defect Management with a Novel Degradable Pure Magnesium Guided Bone Regeneration (GBR) Membrane—A Clinical Case" Medicina 59, no. 11: 2009. https://doi.org/10.3390/medicina59112009
APA StyleFrosecchi, M. (2023). Horizontal and Vertical Defect Management with a Novel Degradable Pure Magnesium Guided Bone Regeneration (GBR) Membrane—A Clinical Case. Medicina, 59(11), 2009. https://doi.org/10.3390/medicina59112009





