Similarities and Differences between Three-Dimensional Speckle-Tracking Echocardiography-Derived Left and Right Atrial Volumes and Volume-Based Functional Properties in the Same Healthy Adults—A Detailed Analysis from the MAGYAR-Healthy Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Two-Dimensional Doppler Echocardiography
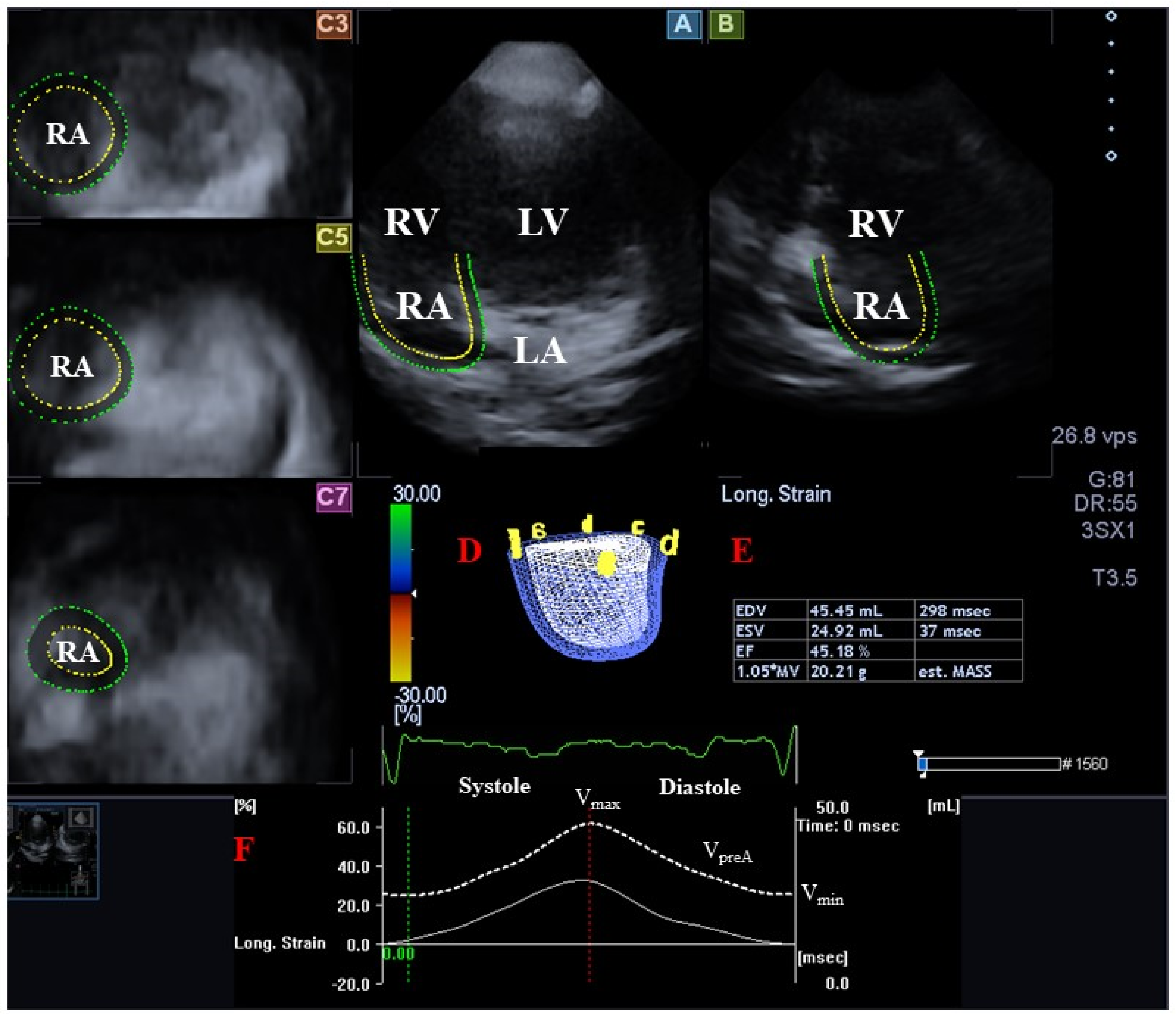
2.3. Three-Dimensional Speckle-Tracking Echocardiography
2.4. Three-Dimensional Speckle-Tracking Echocardiography-Derived Atrial Quantifications
- Vmax—maximum LA/RA volume measured at end-systole (just before mitral/tricuspid valve opening).
- VpreA—LA/RA volume before atrial contraction measured at early-diastole (at the time of the P wave on the ECG).
- Vmin—minimum LA/RA volume measured at end-diastole (just before mitral/tricuspid valve closure).
- For characterization of LA/RA reservoir function:
- ∘
- Total atrial stroke volume (TASV): Vmax − Vmin.
- ∘
- Total atrial emptying fraction (TAEF): TASV/Vmax × 100.
- For characterization of LA/RA conduit function:
- ∘
- Passive atrial stroke volume (PASV): Vmax − VpreA.
- ∘
- Passive atrial emptying fraction (PAEF): PASV/Vmax × 100.
- For characterization of LA/RA active contraction:
- ∘
- Active atrial stroke volume (AASV): VpreA − Vmin.
- ∘
- Active atrial emptying fraction (AAEF): AASV/VpreA × 100.
2.5. Statistical Analysis
3. Results
3.1. Clinical and Two-Dimensional Doppler Echocardiographic Data
3.2. Classification of Subjects
3.3. Atrial Volumes in Different Left Atrial Volume Groups
3.4. Atrial Volumes in Different Right Atrial Volume Groups
3.5. Atrial Stroke Volumes in Different Left Atrial Volume Groups
3.6. Atrial Stroke Volumes in Different Right Atrial Volume Groups
3.7. Atrial Emptying Fractions in Different Left Atrial Volume Groups
3.8. Atrial Emptying Fractions in Different Right Atrial Volume Groups
3.9. Reproducibility of Three-Dimensional Speckle-Tracking Echocardiography-Derived Left Atrial/Right Atrial Assessments
4. Discussion
Limitations
- Three-dimensional speckle-tracking echocardiography is suitable for measuring so-called strain parameters simultaneously with volumetric measurements of both LA and RA, using the 3D models of the atria. However, this study did not aim to perform such assessments.
- Moreover, it did not aim to assess 3DSTE-derived LV/RV parameters either.
- The atrial septum was part of the 3D model during the analysis of both atria, which can be considered a serious limitation.
- Calculation of body mass indexed atrial volumes would have been performed. However, the purpose of the present study was to examine hearts of different sizes and volumes, not individuals. Accordingly, body mass indexation has no relevance.
- We could not absolutely exclude subjects who had any latent non-diagnosed disorders, which could affect results.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ammar, K.A.; Paterick, T.E.; Khandheria, B.K.; Jan, M.F.; Kramer, C.; Umland, M.M.; Tercius, A.J.; Baratta, L.; Tajik, A.J. Myocardial mechanics: Understanding and applying three-dimensional speckle tracking echocardiography in clinical practice. Echocardiography 2012, 29, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Urbano-Moral, J.A.; Patel, A.R.; Maron, M.S.; Arias-Godinez, J.A.; Pandian, N.G. Three-dimensional speckle-tracking echocardiography: Methodological aspects and clinical potential. Echocardiography 2012, 29, 997–1010. [Google Scholar] [CrossRef] [PubMed]
- Muraru, D.; Niero, A.; Rodriguez-Zanella, H.; Cherata, D.; Badano, L. Three-dimensional speckle-tracking echocardiography: Benefits and limitations of integrating myocardial mechanics with three-dimensional imaging. Cardiovasc. Diagn. Ther. 2018, 8, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Lin, Y.; Ji, M.; Wu, W.; Li, H.; Qian, M.; Zhang, L.; Xie, M.; Li, Y. Clinical Utility of Three-Dimensional Speckle-Tracking Echocardiography in Heart Failure. J. Clin. Med. 2022, 11, 6307. [Google Scholar] [CrossRef] [PubMed]
- Hoit, B.D. Left atrial size and function: Role in prognosis. J. Am. Coll. Cardiol. 2014, 63, 493–505. [Google Scholar] [CrossRef]
- Tadic, M. The right atrium, a forgotten cardiac chamber: An updated review of multimodality imaging. J. Clin. Ultrasound 2015, 43, 335–345. [Google Scholar] [CrossRef]
- Nemes, A.; Kormányos, Á.; Domsik, P.; Kalapos, A.; Ambrus, N.; Lengyel, C. Normal reference values of left atrial volumes and volume-based functional properties using three-dimensional speckle-tracking echocardiography in healthy adults (Insights from the MAGYAR-Healthy Study). J. Clin. Ultrasound 2021, 49, 49–55. [Google Scholar] [CrossRef]
- Nemes, A.; Kormányos, Á.; Domsik, P.; Kalapos, A.; Ambrus, N.; Lengyel, C. Normal reference values of three-dimensional speckle-tracking echocardiography-derived right atrial volumes and volume-based functional properties in healthy adults (Insights from the MAGYAR-Healthy Study). J. Clin. Ultrasound 2020, 48, 263–268. [Google Scholar] [CrossRef]
- Aune, E.; Baekkevar, M.; Roislien, J.; Rodevand, O.; Otterstad, J.E. Normal reference ranges for left and right atrial volume indexes and ejection fractions obtained with real-time three-dimensional echocardiography. Eur. J. Echocardiogr. 2009, 10, 738–744. [Google Scholar] [CrossRef]
- Nemes, A.; Kormányos, Á.; Domsik, P.; Kalapos, A.; Gyenes, N.; Lengyel, C. Correlations between left atrial volumes and strains in healthy adults: Detailed analysis from the three-dimensional speckle tracking echocardiographic MAGYAR-Healthy Study. J. Clin. Ultrasound 2021, 49, 650–658. [Google Scholar] [CrossRef]
- Nemes, A.; Kormányos, Á. Right atrial volumes and strains in healthy adults: Is the Frank-Starling mechanism working?—Detailed analysis from a three-dimensional speckle-tracking echocardiographic MAGYAR-Healthy Study. Quant. Imaging Med. Surg. 2023, 13, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Nemes, A.; Domsik, P.; Kalapos, A.; Forster, T. Is three-dimensional speckle-tracking echocardiography able to identify different patterns of left atrial dysfunction in selected disorders?: Short summary of the MAGYAR-Path Study. Int. J. Cardiol. 2016, 220, 535–537. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart. J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef]
- Galderisi, M.; Cosyns, B.; Edvardsen, T.; Cardim, N.; Delgado, V.; Di Salvo, G.; Donal, E.; Sade, L.E.; Ernande, L.; Garbi, M.; et al. Standardization of adult transthoracic echocardiography reporting in agreement with recent chamber quantification, diastolic function, and heart valve disease recommendations: An expert consensus document of the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 1301–1310. [Google Scholar] [PubMed]
- Kleijn, S.A.; Aly, M.F.A.; Terwee, C.B.; van Rossum, A.C.; Kamp, O. Comparison between direct volumetric and speckle tracking methodologies for left ventricular and left atrial chamber quantification by three-dimensional echocardiography. Am. J. Cardiol. 2011, 108, 1038–1044. [Google Scholar] [CrossRef]
- Nagaya, M.; Kawasaki, M.; Tanaka, R.; Onishi, N.; Sato, N.; Ono, K.; Watanabe, T.; Minatoguchi, S.; Miwa, H.; Goto, Y.; et al. Quantitative validation of left atrial structure and function by two-dimensional and three-dimensional speckle tracking echocardiography: A comparative study with three-dimensional computed tomography. J. Cardiol. 2013, 62, 188–194. [Google Scholar] [CrossRef]
- Jacob, R.; Dierberger, B.; Kissling, G. Functional significance of the Frank-Starling mechanism under physiological and pathophysiological conditions. Eur. Heart J. 1992, 13 (Suppl. E), 7–14. [Google Scholar] [CrossRef]
- Anwar, A.M.; Gelejinse, M.L.; Soliman, O.I.I.; Nemes, A.; ten Cate, F.J. Left atrial Frank-Starling law assessed by real-time three-dimensional echocardiographic left atrial volume changes. Heart 2007, 93, 1393–1397. [Google Scholar] [CrossRef]
- Moustafa, S.; Zuhairy, H.; Youssef, M.A.; Alvarez, N.; Connelly, M.S.; Prieur, T.; Mookadam, F. Right and Left Atrial Dissimilarities in Normal Subjects Explored by Speckle Tracking Echocardiography. Echocardiography 2015, 32, 1392–1399. [Google Scholar] [CrossRef]
- Müller, H.; Reverdin, S.; Burri, H.; Shah, D.; Lerch, R. Measurement of left and right atrial volume in patients undergoing ablation for atrial arrhythmias: Comparison of a manual versus semiautomatic algorithm of real time 3D echocardiography. Echocardiography 2014, 31, 499–507. [Google Scholar] [CrossRef]
- Butova, X.; Myachina, T.; Simonova, R.; Kochurova, A.; Mukhlynina, E.; Kopylova, G.; Shchepkin, D.; Khokhlova, A. The inter-chamber differences in the contractile function between left and right atrial cardiomyocytes in atrial fibrillation in rats. Front. Cardiovasc. Med. 2023, 10, 1203093. [Google Scholar] [CrossRef] [PubMed]
- Kaimori, R.; Iwakawa, H.; Suzuki, N.; Aokawa, M.; Tashiro, H.; Terata, K.; Watanabe, H. Asymmetric remodeling between the left and right atria in patients with advanced interatrial block and atrial fibrillation. J. Electrocardiol. 2023, 80, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Mallavarapu, A.; Taksande, A. Dilated Cardiomyopathy in Children: Early Detection and Treatment. Cureus 2022, 14, e31111. [Google Scholar] [CrossRef] [PubMed]
- Palyam, V.; Azam, A.T.; Odeyinka, O.; Alhashimi, R.; Thoota, S.; Ashok, T.; Sange, I. Hypertrophic Cardiomyopathy and Atrial Fibrillation: A Review. Cureus 2022, 14, e21101. [Google Scholar] [CrossRef] [PubMed]
- Dini, F.L.; Pugliese, N.R.; Ameri, P.; Attanasio, U.; Badagliacca, R.; Correale, M.; Mercurio, V.; Tocchetti, C.G.; Agostoni, P.; Palazzuoli, A.; et al. Right ventricular failure in left heart disease: From pathophysiology to clinical manifestations and prognosis. Heart Fail. Rev. 2023, 28, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Atas, H.; Kepez, A.; Bozbay, M.; Gencosmanoglu, D.S.; Cincin, A.; Sunbul, M.; Bozbay, A.Y.; Darvishova, R.; Ergun, T. Assessment of left atrial volume and function in patients with psoriasis by using real time three-dimensional echocardiography. Wien Klin. Wochenschr. 2015, 127, 858–863. [Google Scholar] [CrossRef] [PubMed]
- D’Ascenzi, F.; Pelliccia, A.; Natali, B.M.; Zacà, V.; Cameli, M.; Alvino, F.; Malandrino, A.; Palmitesta, P.; Zorzi, A.; Corrado, D.; et al. Morphological and functional adaptation of left and right atria induced by training in highly trained female athletes. Circ. Cardiovasc. Imaging 2014, 7, 222–229. [Google Scholar] [CrossRef]

| Data | Measures |
|---|---|
| Right atrium | |
| End-systolic maximum RA volume (Vmax, mL) | 47.0 ± 14.3 * |
| Early diastolic RA volume (VpreA, mL) | 33.7 ± 11.2 * |
| Late diastolic RA volume (Vmin, mL) | 26.2 ± 9.8 * |
| Total RA stroke volume (TASV, mL) | 20.9 ± 9.3 |
| Total RA emptying fraction (TAEF, %) | 44.1 ± 13.2 * |
| Passive RA stroke volume (PASV, mL) | 13.3 ± 7.8 |
| Passive RA emptying fraction (PAEF, %) | 27.9 ± 12.5 * |
| Active RA stroke volume (AASV, mL) | 7.5 ± 4.6 |
| Active RA emptying fraction (AAEF, %) | 22.6 ± 11.6 * |
| Left atrium | |
| End-systolic maximum LA volume (Vmax, mL) | 40.7 ± 13.1 |
| Early diastolic LA volume (VpreA, mL) | 27.6 ± 11.9 |
| Late diastolic LA volume (Vmin, mL) | 19.6 ± 8.3 |
| Total LA stroke volume (TASV, mL) | 21.2 ± 8.0 |
| Total LA emptying fraction (TAEF, %) | 52.2 ± 12.0 |
| Passive LA stroke volume (PASV, mL) | 13.1 ± 5.6 |
| Passive LA emptying fraction (PAEF, %) | 33.4 ± 12.9 |
| Active LA stroke volume (AASV, mL) | 8.1 ± 5.5 |
| Active LA emptying fraction (AAEF, %) | 28.1 ± 11.7 |
| LA-Vmax < 27.6 mL (n = 26) | 27.6 mL ≤ LA-Vmax ≤ 53.8 mL (n = 124) | 53.8 mL < LA-Vmax (n = 29) | LA-VpreA < 15.7 mL (n = 25) | 15.7 mL ≤ LA-VpreA ≤ 39.5 mL (n = 125) | 39.5 mL < LA-VpreA (n = 29) | LA-Vmin < 11.3 mL (n = 25) | 11.3 mL ≤ LA-Vmin ≤ 27.9 mL (n = 125) | 27.9 mL < LA-Vmin (n = 29) | |
|---|---|---|---|---|---|---|---|---|---|
| Vmax (mL) | 35.7 ± 11.3 * | 47.6 ± 13.5 *† | 54.6 ± 13.9 *†‡ | 36.5 ± 11.0 * | 47.1 ± 12.7 *† | 55.0 ± 17.6 *†‡ | 36.6 ± 11.7 * | 46.9 ± 12.8 *† | 56.8 ± 15.7 †‡ |
| VpreA (mL) | 23.3 ± 6.8 * | 34.1 ± 10.3 *† | 41.4 ± 10.8 *†‡ | 22.2 ± 6.3 * | 33.6 ± 9.1 *† | 43.2 ± 13.6 *†‡ | 23.3 ± 7.8 * | 33.2 ± 9.0 *† | 44.8 ± 12.3 †‡ |
| Vmin (mL) | 18.0 ± 5.4 * | 26.3 ± 9.0 *† | 32.8 ± 10.5 †‡ | 17.2 ± 5.8 * | 25.9 ± 8.4 *† | 34.5 ± 11.3 †‡ | 17.3 ± 6.3 * | 25.8 ± 8.3 *† | 35.7 ± 10.0 †‡ |
| TASV (mL) | 17.7 ± 8.5 * | 21.3 ± 9.2 † | 21.7 ± 10.1 * | 19.3 ± 7.8 | 21.2 ± 9.2 | 20.6 ± 10.6 * | 19.3 ± 8.3 | 21.1 ± 9.3 | 21.1 ± 10.3 * |
| TAEF (%) | 48.2 ± 11.9 | 44.4 ± 12.7 * | 39.2 ± 15.1 *†‡ | 52.2 ± 11.7 * | 44.4 ± 12.6 *† | 36.3 ± 13.0 *†‡ | 52.0 ± 12.6 * | 44.4 ± 12.5 *† | 36.1 ± 12.7 *†‡ |
| PASV (mL) | 12.4 ± 7.5 * | 13.6 ± 7.4 | 13.1 ± 9.1 | 14.3 ± 6.8 | 13.5 ± 7.5 | 11.8 ± 9.4 | 13.3 ± 6.7 | 13.7 ± 7.6 | 12.0 ± 9.2 |
| PAEF (%) | 33.2 ± 13.6 | 28.0 ± 11.5 *† | 22.7 ± 13.6 †‡ | 38.1 ± 11.9 * | 27.9 ± 11.3 *† | 20.0 ± 12.6 †‡ | 35.6 ± 13.7 * | 28.3 ± 11.3 *† | 19.9 ± 12.3 †‡ |
| AASV (mL) | 5.3 ± 2.8 * | 7.8 ± 4.5 † | 8.6 ± 5.6 *† | 5.0 ± 2.4 * | 7.7 ± 4.3 † | 8.8 ± 6.2 *† | 6.0 ± 4.0 | 7.5 ± 4.4 | 9.1 ± 5.7 *† |
| AAEF (%) | 22.4 ± 9.5 | 23.0 ± 11.5 * | 21.3 ± 13.4 * | 23.1 ± 10.3 | 23.1 ± 11.6 * | 20.1 ± 12.2 * | 25.5 ± 12.1 * | 22.5 ± 11.5 * | 20.0 ± 10.6 * |
| LA-Vmax < 27.6 mL (n = 26) | 27.6 mL ≤ LA-Vmax ≤ 53.8 mL (n = 124) | 53.8 mL < LA-Vmax (n = 29) | LA-VpreA < 15.7 mL (n = 25) | 15.7 mL ≤ LA-VpreA ≤ 39.5 mL (n = 125) | 39.5 mL < LA-VpreA (n = 29) | LA-Vmin < 11.3 mL (n = 25) | 11.3 mL ≤ LA-Vmin ≤ 27.9 mL (n = 125) | 27.9 mL < LA-Vmin (n = 29) | |
|---|---|---|---|---|---|---|---|---|---|
| Vmax (mL) | 24.0 ± 3.5 | 38.7 ± 6.4 † | 64.3 ± 6.8 †‡ | 26.2 ± 5.4 | 38.5 ± 8.5 † | 62.4 ± 9.2 †‡ | 27.0 ± 5.3 | 38.9 ± 9.1 † | 60.4 ± 10.5 †‡ |
| VpreA (mL) | 15.3 ± 4.0 | 25.6 ± 7.0 † | 47.4 ± 10.4 †‡ | 12.9 ± 2.3 | 25.3 ± 5.9 † | 50.0 ± 7.8 †‡ | 14.0 ± 3.3 | 25.7 ± 7.1 † | 47.7 ± 8.9 †‡ |
| Vmin (mL) | 11.7 ± 3.5 | 18.3 ± 5.7 † | 31.9 ± 8.0 †‡ | 9.6 ± 2.2 | 18.3 ± 5.0 † | 33.3 ± 6.9 †‡ | 9.0 ± 1.6 | 18.3 ± 4.1 † | 34.3 ± 5.4 †‡ |
| TASV (mL) | 12.2 ± 3.6 | 20.4 ± 5.4 † | 32.4 ± 7.3 †‡ | 16.6 ± 5.2 | 20.2 ± 6.9 † | 29.1 ± 9.0 †‡ | 18.0 ± 4.6 | 20.6 ± 7.8 | 26.1 ± 8.8 †‡ |
| TAEF (%) | 50.9 ± 13.1 | 52.8 ± 12.0 | 50.6 ± 10.9 | 62.0 ± 10.0 | 51.8 ± 11.6 † | 46.1 ± 10.7 †‡ | 65.6 ± 7.2 | 51.8 ± 10.9 † | 42.3 ± 9.1 †‡ |
| PASV (mL) | 8.7 ± 3.4 | 13.1 ± 4.8 † | 16.9 ± 7.0 †‡ | 13.3 ± 4.1 | 13.2 ± 5.7 | 12.6 ± 5.9 | 12.9 ± 4.3 | 13.2 ± 5.6 | 12.7 ± 6.3 |
| PAEF (%) | 36.3 ± 13.5 | 34.3 ± 12.4 | 26.7 ± 12.0 †‡ | 50.0 ± 8.0 | 33.5 ± 11.0 † | 19.8 ± 7.9 †‡ | 47.2 ± 10.9 | 33.7 ± 11.1 † | 20.6 ± 8.7 †‡ |
| AASV (mL) | 3.6 ± 2.8 | 7.3 ± 3.5 † | 15.6 ± 7.2 †‡ | 3.3 ± 1.9 | 7.1 ± 3.4 † | 16.5 ± 6.9 †‡ | 5.0 ± 3.1 | 7.4 ± 4.9 † | 13.4 ± 6.3 †‡ |
| AAEF (%) | 22.5 ± 14.3 | 28.3 ± 10.7 † | 32.3 ± 11.5 † | 25.0 ± 12.9 | 27.6 ± 11.3 | 32.7 ± 11.7 †‡ | 33.3 14.0 | 28.2 ± 11.5 † | 27.2 ± 9.2 † |
| RA-Vmax < 32.7 mL (n = 31) | 32.7 mL ≤ RA-Vmax ≤ 61.3 mL (n = 116) | 61.3 mL < RA-Vmax (n = 32) | RA-VpreA < 22.5 mL (n = 27) | 22.5 mL ≤ RA-VpreA ≤ 44.9 mL (n = 130) | 44.9 mL < RA-VpreA (n = 22) | RA-Vmin < 16.4 mL (n = 27) | 16.4 mL ≤ RA-Vmin ≤ 36.0 mL (n = 122) | 36.0 mL < RA-Vmin (n = 30) | |
|---|---|---|---|---|---|---|---|---|---|
| Vmax (mL) | 28.8 ± 3.0 | 45.5 ± 7.6 *† | 71.0 ± 7.6 *†‡ | 30.4 ± 4.8 | 46.9 ± 11.0 *† | 68.1 ± 11.3 *†‡ | 30.7 ± 6.3 | 46.6 ± 11.1 *† | 63.4 ± 13.1 *†‡ |
| VpreA (mL) | 20.8 ± 4.8 | 33.1 ± 7.5 *† | 47.9 ± 10.6 *†‡ | 18.3 ± 3.1 | 33.5 ± 6.2 *† | 53.9 ± 8.6 *†‡ | 19.1 ± 4.3 | 32.8 ± 6.3 *† | 50.5 ± 9.4 *†‡ |
| Vmin (mL) | 16.2 ± 4.2 | 25.6 ± 7.6 *† | 37.6 ± 8.6 *†‡ | 13.9 ± 3.0 | 25.7 ± 6.3 *† | 43.9 ± 5.3 *†‡ | 13.2 ± 2.5 | 24.9 ± 5.0 *† | 43.0 ± 4.5 *†‡ |
| TASV (mL) | 12.7 ± 3.5 * | 19.9 ± 7.4 † | 33.4 ± 7.5 *†‡ | 16.6 ± 5.3 | 21.2 ± 9.5 † | 24.2 ± 10.1 † | 17.5 ± 6.7 | 21.7 ± 9.3 † | 20.4 ± 10.6 |
| TAEF (%) | 44.2 ± 12.1 * | 43.6 ± 13.9 * | 47.2 ± 10.3 | 53.5 ± 11.6 | 43.8 ± 12.7 *† | 34.3 ± 10.1 *†‡ | 55.4 ± 11.6 | 45.0 ± 11.0 *† | 30.2 ± 10.9 *†‡ |
| PASV (mL) | 8.0 ± 4.0 * | 12.3 ± 6.0 † | 23.1 ± 8.4 *†‡ | 12.1 ± 4.7 | 13.4 ± 8.0 | 14.2 ± 9.0 | 11.5 ± 5.2 | 13.8 ± 7.9 | 12.9 ± 8.88 |
| PAEF (%) | 28.0 ± 14.1 * | 27.0 ± 11.8 * | 32.6 ± 11.8 ‡ | 39.0 ± 11.4 | 27.0 ± 11.6 *† | 20.0 ± 10.6 *†‡ | 36.7 ± 13.4 | 28.1 ± 11.3 *† | 19.2 ± 10.6 *†‡ |
| AASV (mL) | 4.7 ± 2.1 * | 7.5 ± 4.5 † | 10.3 ± 5.3 †‡ | 4.4 ± 2.0 | 7.8 ± 4.5 † | 9.9 ± 5.8 †‡ | 5.9 ± 3.4 | 7.9 ± 4.3 † | 7.5 ± 6.2 * |
| AAEF (%) | 22.5 ± 8.5 * | 23.1 ± 12.7 * | 21.4 ± 9.2 * | 24.4 ± 10.0 | 23.1 ± 12.1 * | 17.7 ± 8.4 *†‡ | 29.6 ± 11.0 | 23.4 ± 10.6 *† | 13.3 ± 10.0 *†‡ |
| RA-Vmax < 32.7 mL (n = 31) | 32.7 mL ≤ RA-Vmax ≤ 61.3 mL (n = 116) | 61.3 mL < RA-Vmax (n = 32) | RA-VpreA < 22.5 mL (n = 27) | 22.5 mL ≤ RA-VpreA ≤ 44.9 mL (n = 130) | 44.9 mL < RA-VpreA (n = 22) | RA-Vmin < 16.4 mL (n = 27) | 16.4 mL ≤ RA-Vmin ≤ 36.0 mL (n = 122) | 36.0 mL < RA-Vmin (n = 30) | |
|---|---|---|---|---|---|---|---|---|---|
| Vmax (mL) | 32.6 ± 11.9 | 40.0 ± 10.6 † | 50.5 ± 15.0 †‡ | 29.9 ± 8.6 | 41.0 ± 11.7 † | 52.2 ± 15.0 †‡ | 31.7 ± 9.8 | 40.2 ± 11.5 † | 50.8 ± 14.8 †‡ |
| VpreA (mL) | 21.5 ± 11.1 | 27.0 ± 9.9 † | 34.7 ± 14.5 †‡ | 17.2 ± 6.5 | 27.9 ± 10.4 † | 39.0 ± 14.5 †‡ | 18.5 ± 7.0 | 27.0 ± 10.0 † | 38.6 ± 14.3 †‡ |
| Vmin (mL) | 15.0 ± 6.8 | 19.3 ± 7.4 † | 24.5 ± 9.5 †‡ | 12.2 ± 4.0 | 19.7 ± 7.3 † | 27.8 ± 9.5 †‡ | 12.5 ± 4.7 | 19.2 ± 7.1 † | 27.2 ± 9.0 †‡ |
| TASV (mL) | 17.6 ± 6.9 | 20.7 ± 7.3 † | 26.1 ± 8.5 †‡ | 17.7 ± 7.4 | 21.3 ± 7.8 † | 24.4 ± 8.3 † | 19.1 ± 7.5 | 21.0 ± 7.7 | 23.6 ± 9.0 † |
| TAEF (%) | 54.1 ± 11.2 | 51.8 ± 12.7 | 52.1 ± 10.3 | 57.8 ± 12.1 | 51.9 ± 11.9 † | 46.9 ± 9.3 †‡ | 59.5 ± 11.3 | 52.0 ± 11.6 † | 46.1 ± 10.6 †‡ |
| PASV (mL) | 11.1 ± 4.2 | 12.9 ± 5.3 | 15.9 ± 6.6 †‡ | 12.7 ± 5.5 | 13.1 ± 5.5 | 13.3 ± 5.8 | 13.2 ± 5.7 | 13.3 ± 5.4 | 12.2 ± 5.9 |
| PAEF (%) | 36.0 ± 12.5 | 33.0 ± 12.9 | 33.0 ± 12.8 | 42.2 ± 12.4 | 32.7 ± 12.3 † | 26.5 ± 11.0 †‡ | 41.5 ± 11.2 | 33.6 ± 12.3 † | 25.0 ± 11.5 †‡ |
| AASV (mL) | 6.5 ± 5.0 | 7.7 ± 4.9 | 10.2 ± 6.6 †‡ | 5.0 ± 3.6 | 8.2 ± 5.4 † | 11.2 ± 6.2 †‡ | 5.9 ± 3.6 | 7.7 ± 5.1 | 11.4 ± 7.2 †‡ |
| AAEF (%) | 28.2 ± 11.3 | 28.0 ± 12.2 | 28.1 ± 10.1 | 27.3 ± 12.0 | 28.4 ± 12.1 | 27.5 ± 8.9 | 31.1 ± 11.0 | 27.5 ± 12.1 | 27.8 ± 10.2 |
| Intraobserver Agreement | Interobserver Agreement | |||
|---|---|---|---|---|
| Mean ± 2 SD Difference in Values Obtained by two Measurements of the Same Observer | ICC between Measurements of the Same Observer | Mean ± 2 SD Difference in Values Obtained by two Observers | ICC between Independent Measurements of two Observers | |
| LA-Vmax | 0.4 ± 3.4 mL | 0.96 (p < 0.001) | 0.4 ± 4.9 mL | 0.96 (p < 0.001) |
| LA-VpreA | 0.4 ± 3.4 mL | 0.97 (p < 0.001) | 0.4 ± 4.0 mL | 0.97 (p < 0.001) |
| LA-Vmin | −1.1 ± 5.1 mL | 0.87 (p < 0.001) | −1.0 ± 4.6 mL | 0.88 (p < 0.001) |
| RA-Vmax | 1.1 ± 5.9 mL | 0.95 (p < 0.001) | 1.1 ± 5.4 mL | 0.97 (p < 0.001) |
| RA-VpreA | −1.6 ± 7.8 mL | 0.88 (p < 0.001) | −1.5 ± 7.9 mL | 0.91 (p < 0.001) |
| RA-Vmin | 0.9 ± 4.8 mL | 0.93 (p < 0.001) | 0.9 ± 4.2 mL | 0.95 (p < 0.001) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nemes, A.; Kormányos, Á.; Ambrus, N.; Lengyel, C. Similarities and Differences between Three-Dimensional Speckle-Tracking Echocardiography-Derived Left and Right Atrial Volumes and Volume-Based Functional Properties in the Same Healthy Adults—A Detailed Analysis from the MAGYAR-Healthy Study. Medicina 2023, 59, 2051. https://doi.org/10.3390/medicina59122051
Nemes A, Kormányos Á, Ambrus N, Lengyel C. Similarities and Differences between Three-Dimensional Speckle-Tracking Echocardiography-Derived Left and Right Atrial Volumes and Volume-Based Functional Properties in the Same Healthy Adults—A Detailed Analysis from the MAGYAR-Healthy Study. Medicina. 2023; 59(12):2051. https://doi.org/10.3390/medicina59122051
Chicago/Turabian StyleNemes, Attila, Árpád Kormányos, Nóra Ambrus, and Csaba Lengyel. 2023. "Similarities and Differences between Three-Dimensional Speckle-Tracking Echocardiography-Derived Left and Right Atrial Volumes and Volume-Based Functional Properties in the Same Healthy Adults—A Detailed Analysis from the MAGYAR-Healthy Study" Medicina 59, no. 12: 2051. https://doi.org/10.3390/medicina59122051






