Accelerated Oral Healing by Angelica gigas Nakai from Hot Melt Extrusion Technology: An In Vitro Study
Abstract
:1. Introduction
2. Materials and Methods
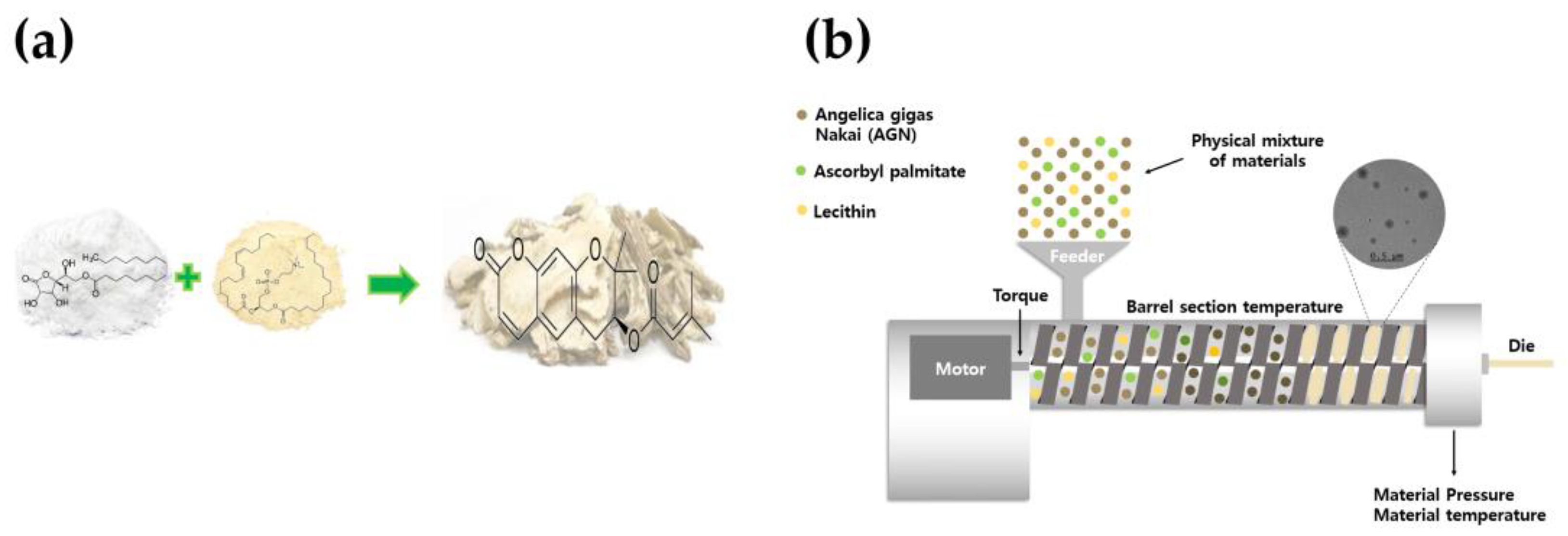
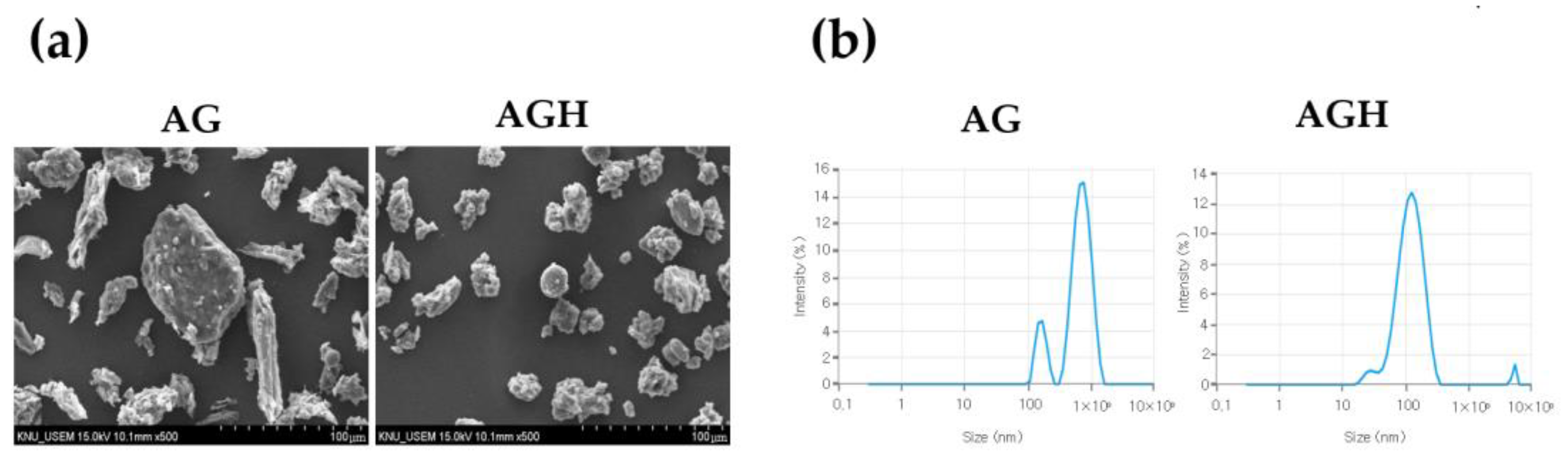
2.1. Preparation of AG by HME Technology
2.2. Culture of Human Gingival Fibroblasts
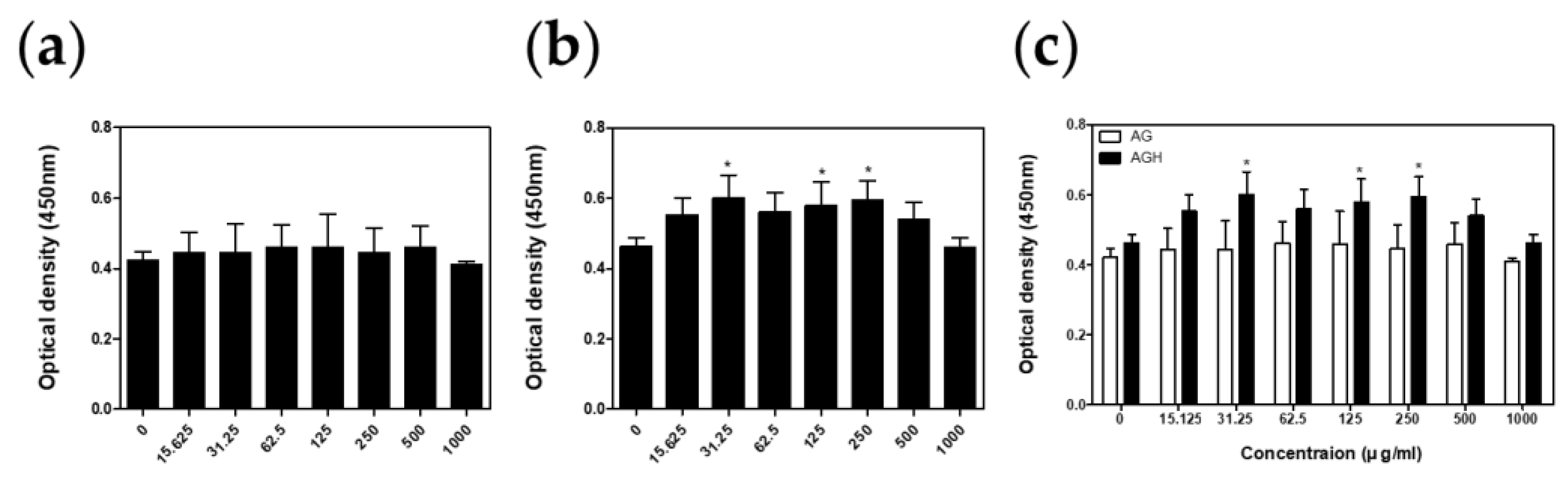
2.3. Cell Viability Test
2.4. Nitric Oxide (NO) Assay
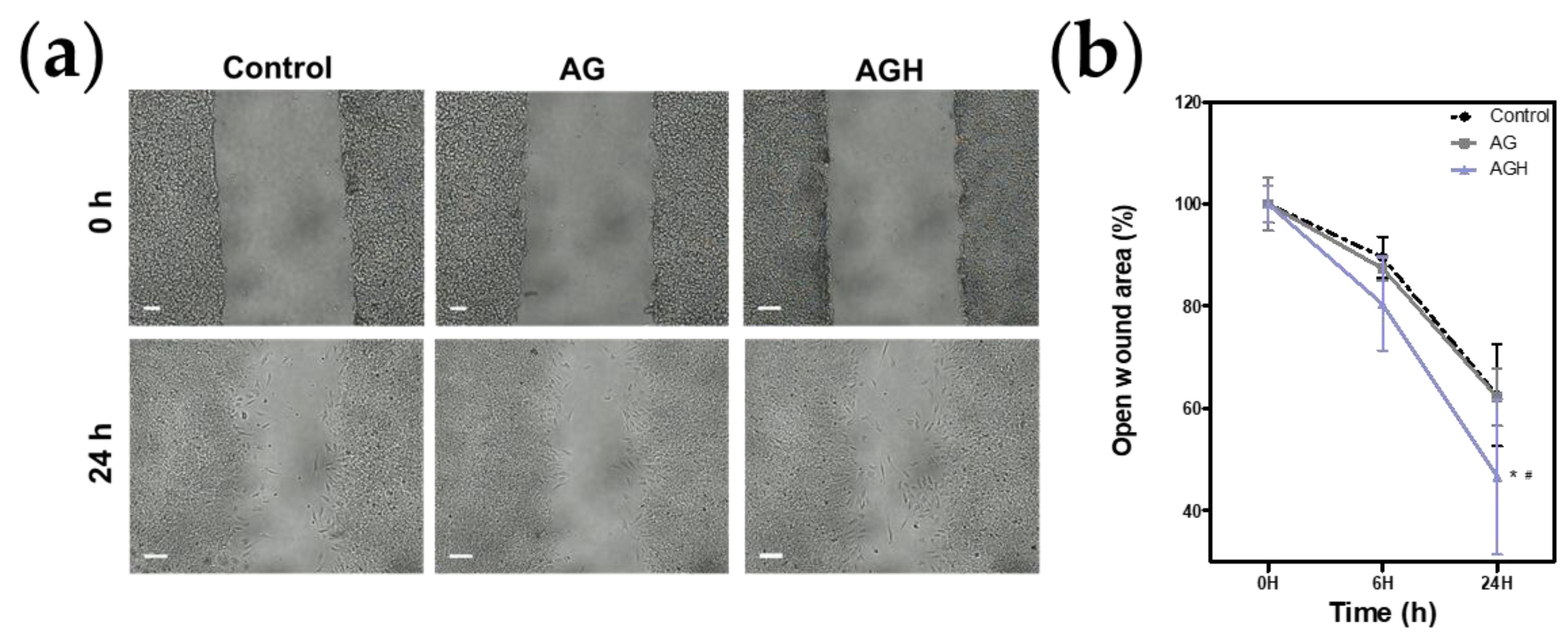
2.5. Cell Proliferation/Cell Migration Assay
2.6. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.7. Statistical Analysis
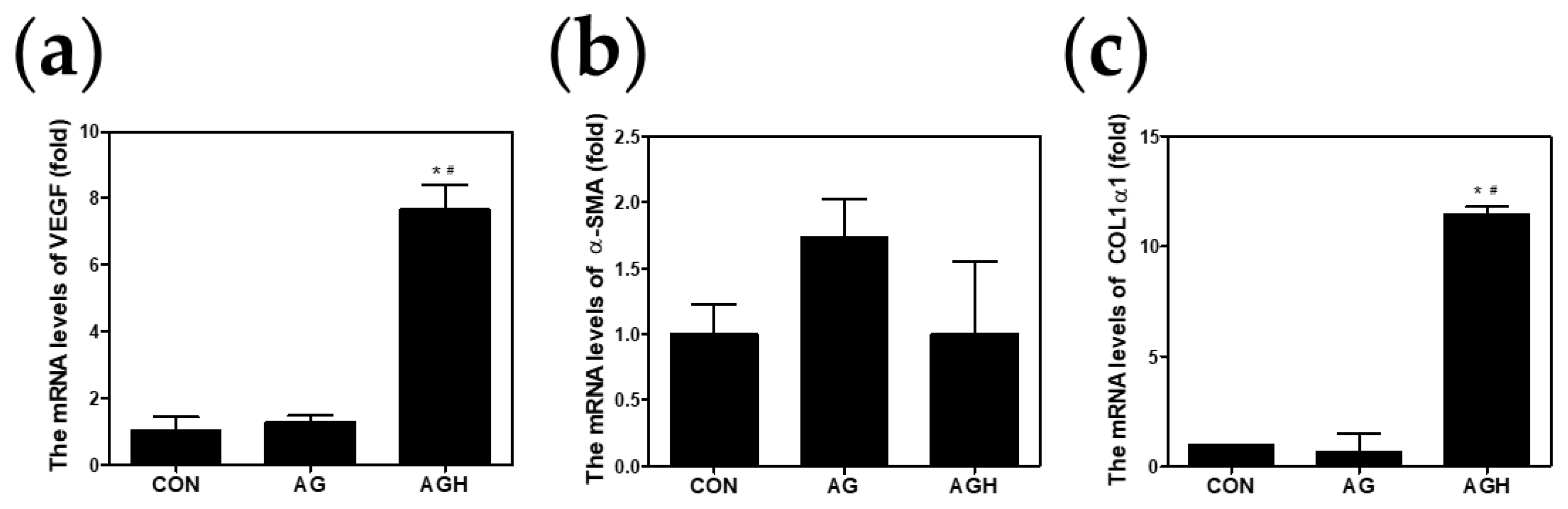
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Waasdorp, M.; Krom, B.P.; Bikker, F.J.; van Zuijlen, P.P.M.; Niessen, F.B.; Gibbs, S. The Bigger Picture: Why Oral Mucosa Heals Better Than Skin. Biomolecules 2021, 11, 1165. [Google Scholar] [CrossRef] [PubMed]
- Sedghi, L.; DiMassa, V.; Harrington, A.; Lynch, S.V.; Kapila, Y.L. The oral microbiome: Role of key organisms and complex networks in oral health and disease. Periodontol. 2000 2021, 87, 107–131. [Google Scholar] [CrossRef] [PubMed]
- Graça, M.F.P.; Miguel, S.P.; Cabral, C.S.D.; Correia, I.J. Hyaluronic acid-Based wound dressings: A review. Carbohydr. Polym. 2020, 241, 116364. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, K.E.; Nam, O.H.; Chae, Y.K.; Lee, M.H.; Kweon, D.K.; Kim, M.S.; Lee, H.S.; Choi, S.C. Orodispersible hyaluronic acid film delivery for oral wound healing in rats. J. Dent. Sci. 2022, 17, 1595–1603. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, K.E.; Kang, S.W.; Park, S.H.; Chae, Y.K.; Lee, M.H.; Kweon, D.K.; Choi, S.C.; Nam, O.H. Effect of orodispersible hyaluronic acid film on palatal mucosa wound healing. Oral Dis. 2023, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, C.; Tarun Kumar, A.B.; Mehta, D.S. Comparative evaluation of free gingival graft and AlloDerm(®) in enhancing the width of attached gingival: A clinical study. Contemp. Clin. Dent. 2015, 6, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Bosco, A.F.; Bosco, J.M. An alternative technique to the harvesting of a connective tissue graft from a thin palate: Enhanced wound healing. Int. J. Periodontics Restor. Dent. 2007, 27, 133–139. [Google Scholar]
- Hämmerle, C.H.; Giannobile, W.V. Biology of soft tissue wound healing and regeneration--consensus report of Group 1 of the 10th European Workshop on Periodontology. J. Clin. Periodontol. 2014, 41 (Suppl. S15), S1–S5. [Google Scholar] [CrossRef]
- Del Pizzo, M.; Modica, F.; Bethaz, N.; Priotto, P.; Romagnoli, R. The connective tissue graft: A comparative clinical evaluation of wound healing at the palatal donor site. A preliminary study. J. Clin. Periodontol. 2002, 29, 848–854. [Google Scholar] [CrossRef]
- He, Z.; Wang, Y.; Chen, Y.; Geng, F.; Jiang, Z.; Li, X. Angelica gigas Nakai: An overview on its chemical composition and pharmacological activity. Biochem. Syst. Ecol. 2023, 111, 104717. [Google Scholar] [CrossRef]
- Sarker, S.D.; Nahar, L. Natural medicine: The genus Angelica. Curr Med Chem 2004, 11, 1479–1500. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, L.; Jiang, C.; Xing, C.; Kim, S.H.; Lü, J. Anti-cancer and other bioactivities of Korean Angelica gigas Nakai (AGN) and its major pyranocoumarin compounds. Anticancer Agents Med. Chem. 2012, 12, 1239–1254. [Google Scholar] [CrossRef] [PubMed]
- Her, Y.; Lee, T.K.; Ahn, J.H.; Lim, S.S.; Kang, B.G.; Park, J.S.; Kim, B.; Sim, H.; Lee, J.C.; Kim, H.S.; et al. Chemical Composition of a Novel Distillate from Fermented Mixture of Nine Anti-Inflammatory Herbs and Its UVB-Protective Efficacy in Mouse Dorsal Skin via Attenuating Collagen Disruption and Inflammation. Molecules 2020, 26, 124. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.Y.; Kang, E.S.; Park, J.H.; Cho, B.O.; Jang, S.I. Anti-inflammatory effect of red ginseng marc, Artemisia scoparia, Paeonia japonica and Angelica gigas extract mixture in LPS-stimulated RAW 264.7 cells. Biomed. Rep. 2022, 17, 63. [Google Scholar] [CrossRef]
- Han, J.; Jin, W.; Ho, N.A.; Hong, J.; Kim, Y.J.; Shin, Y.; Lee, H.; Suh, J.W. Decursin and decursinol angelate improve wound healing by upregulating transcription of genes encoding extracellular matrix remodeling proteins, inflammatory cytokines, and growth factors in human keratinocytes. Biochem. Biophys. Res. Commun. 2018, 499, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, A.R.; Haque, M. Preparation of Medicinal Plants: Basic Extraction and Fractionation Procedures for Experimental Purposes. J. Pharm. Bioallied Sci. 2020, 12, 1–10. [Google Scholar] [CrossRef]
- Ashour, E.A.; Majumdar, S.; Alsheteli, A.; Alshehri, S.; Alsulays, B.; Feng, X.; Gryczke, A.; Kolter, K.; Langley, N.; Repka, M.A. Hot melt extrusion as an approach to improve solubility, permeability and oral absorption of a psychoactive natural product, piperine. J. Pharm. Pharmacol. 2016, 68, 989–998. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Smith, M.K.; French, J.L.; Kowalski, K.G.; Hutmacher, M.M.; Ewy, W. Decision-Making in Drug development: Application of a model based framework for assessing trial performance. In Clinical Trial Simulations; Springer: Cham, Switzerland, 2011; pp. 61–83. [Google Scholar]
- Pimparade, M.B.; Vo, A.; Maurya, A.S.; Bae, J.; Morott, J.T.; Feng, X.; Kim, D.W.; Kulkarni, V.I.; Tiwari, R.; Vanaja, K.; et al. Development and evaluation of an oral fast disintegrating anti-allergic film using hot-melt extrusion technology. Eur. J. Pharm. Biopharm. 2017, 119, 81–90. [Google Scholar] [CrossRef]
- Ryu, S.; Lee, H.Y.; Nam, S.-H.; Baek, J.-S. Antifungal Activity of Angelica gigas with Enhanced Water Solubility of Decursin and Decursinol Angelate by Hot-Melt Extrusion Technology against Candida albicans. Int. J. Transl. Med. 2022, 2, 515–521. [Google Scholar] [CrossRef]
- Jiang, Y.; Piao, J.; Cho, H.J.; Kang, W.S.; Kim, H.Y. Improvement in antiproliferative activity of Angelica gigas Nakai by solid dispersion formation via hot-melt extrusion and induction of cell cycle arrest and apoptosis in HeLa cells. Biosci. Biotechnol. Biochem. 2015, 79, 1635–1643. [Google Scholar] [CrossRef]
- Nam, O.H.; Ro, S.T.; Lee, H.W.; Jeong, J.; Chae, Y.K.; Lee, K.E.; Choi, S.C.; Kang, S.W. Evaluation of delphinidin as a storage medium for avulsed teeth. BMC Oral Health 2023, 23, 21. [Google Scholar] [CrossRef]
- Kim, H.B.; Ryu, S.; Baek, J.S. The Effect of Hot-Melt Extrusion of Mulberry Leaf on the Number of Active Compounds and Antioxidant Activity. Plants 2022, 11, 3019. [Google Scholar] [CrossRef]
- Wang, W.; Kang, Q.; Liu, N.; Zhang, Q.; Zhang, Y.; Li, H.; Zhao, B.; Chen, Y.; Lan, Y.; Ma, Q.; et al. Enhanced dissolution rate and oral bioavailability of Ginkgo biloba extract by preparing solid dispersion via hot-melt extrusion. Fitoterapia 2015, 102, 189–197. [Google Scholar] [CrossRef]
- Tambe, S.M.; Jain, D.D.; Mehta, C.H.; Ashwini, T.; Yogendra Nayak, U.; Amin, P.D. Hot-melt extruded in situ gelling systems (MeltDrops Technology): Formulation development, in silico modelling and in vivo studies. Eur. J. Pharm. Biopharm. 2023, 188, 108–124. [Google Scholar] [CrossRef]
- Hwang, I.; Kang, C.-Y.; Park, J.-B. Advances in hot-melt extrusion technology toward pharmaceutical objectives. J. Pharm. Investig. 2017, 47, 123–132. [Google Scholar] [CrossRef]
- Jadhav, P.; Gokarna, V.; Deshpande, V.; Vavia, P. Bioavailability Enhancement of Olmesartan Medoxomil Using Hot-Melt Extrusion: In-Silico, In-Vitro, and In-Vivo Evaluation. AAPS PharmSciTech 2020, 21, 254. [Google Scholar] [CrossRef]
- Fan, W.; Wu, J.; Gao, M.; Zhang, X.; Zhu, W. Preparation of Solid Dispersion of Polygonum Cuspidatum Extract by Hot Melt Extrusion to Enhance Oral Bioavailability of Resveratrol. Molecules 2023, 28, 737. [Google Scholar] [CrossRef]
- Jiang, X.; Zhao, Y.; Guan, Q.; Xiao, S.; Dong, W.; Lian, S.; Zhang, H.; Liu, M.; Wang, Z.; Han, J. Amorphous solid dispersions of cyclosporine A with improved bioavailability prepared via hot melt extrusion: Formulation, physicochemical characterization, and in vivo evaluation. Eur. J. Pharm. Sci. 2022, 168, 106036. [Google Scholar] [CrossRef]
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef]
- Rafa, H.; Saoula, H.; Belkhelfa, M.; Medjeber, O.; Soufli, I.; Toumi, R.; de Launoit, Y.; Moralès, O.; Nakmouche, M.; Delhem, N.; et al. IL-23/IL-17A axis correlates with the nitric oxide pathway in inflammatory bowel disease: Immunomodulatory effect of retinoic acid. J. Interferon Cytokine Res. 2013, 33, 355–368. [Google Scholar] [CrossRef]
- Rafa, H.; Amri, M.; Saoula, H.; Belkhelfa, M.; Medjeber, O.; Boutaleb, A.; Aftis, S.; Nakmouche, M.; Touil-Boukoffa, C. Involvement of interferon-γ in bowel disease pathogenesis by nitric oxide pathway: A study in Algerian patients. J. Interferon Cytokine Res. 2010, 30, 691–697. [Google Scholar] [CrossRef]
- Pereira, L.M.; Hatanaka, E.; Martins, E.F.; Oliveira, F.; Liberti, E.A.; Farsky, S.H.; Curi, R.; Pithon-Curi, T.C. Effect of oleic and linoleic acids on the inflammatory phase of wound healing in rats. Cell Biochem. Funct. 2008, 26, 197–204. [Google Scholar] [CrossRef]
- Pastar, I.; Stojadinovic, O.; Yin, N.C.; Ramirez, H.; Nusbaum, A.G.; Sawaya, A.; Patel, S.B.; Khalid, L.; Isseroff, R.R.; Tomic-Canic, M. Epithelialization in Wound Healing: A Comprehensive Review. Adv. Wound Care 2014, 3, 445–464. [Google Scholar] [CrossRef]
- Cassidy, C.M.; Tunney, M.M.; Caldwell, D.L.; Andrews, G.P.; Donnelly, R.F. Development of novel oral formulations prepared via hot melt extrusion for targeted delivery of photosensitizer to the colon. Photochem. Photobiol. 2011, 87, 867–876. [Google Scholar] [CrossRef]
- Bao, P.; Kodra, A.; Tomic-Canic, M.; Golinko, M.S.; Ehrlich, H.P.; Brem, H. The role of vascular endothelial growth factor in wound healing. J. Surg. Res. 2009, 153, 347–358. [Google Scholar] [CrossRef]
- Lee, J.-H.; Lee, S.-N.; Kim, M.-G.; Kim, M.-H.; Kim, H.-J.; Jo, H.-J.; Leem, K.-H. Effects of Angelica gigantis Radix extracts on the collagenase activity and procollagen synthesis in HS68 human fibroblasts and tyrosinase activity. Korea J. Herbol. 2011, 26, 29–33. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, J.R.; Lee, H.Y.; Park, Y.-J.; Chae, Y.K.; An, H.-J.; Baek, J.-S.; Nam, O.H. Accelerated Oral Healing by Angelica gigas Nakai from Hot Melt Extrusion Technology: An In Vitro Study. Medicina 2023, 59, 2066. https://doi.org/10.3390/medicina59122066
Ye JR, Lee HY, Park Y-J, Chae YK, An H-J, Baek J-S, Nam OH. Accelerated Oral Healing by Angelica gigas Nakai from Hot Melt Extrusion Technology: An In Vitro Study. Medicina. 2023; 59(12):2066. https://doi.org/10.3390/medicina59122066
Chicago/Turabian StyleYe, Ju Ri, Ha Yeon Lee, Yea-Jin Park, Yong Kwon Chae, Hyo-Jin An, Jong-Suep Baek, and Ok Hyung Nam. 2023. "Accelerated Oral Healing by Angelica gigas Nakai from Hot Melt Extrusion Technology: An In Vitro Study" Medicina 59, no. 12: 2066. https://doi.org/10.3390/medicina59122066




