Three-Dimensional Imaging-Guided Lung Anatomic Segmentectomy: A Single-Center Preliminary Experiment
Abstract
:1. Introduction
2. Materials and Methods
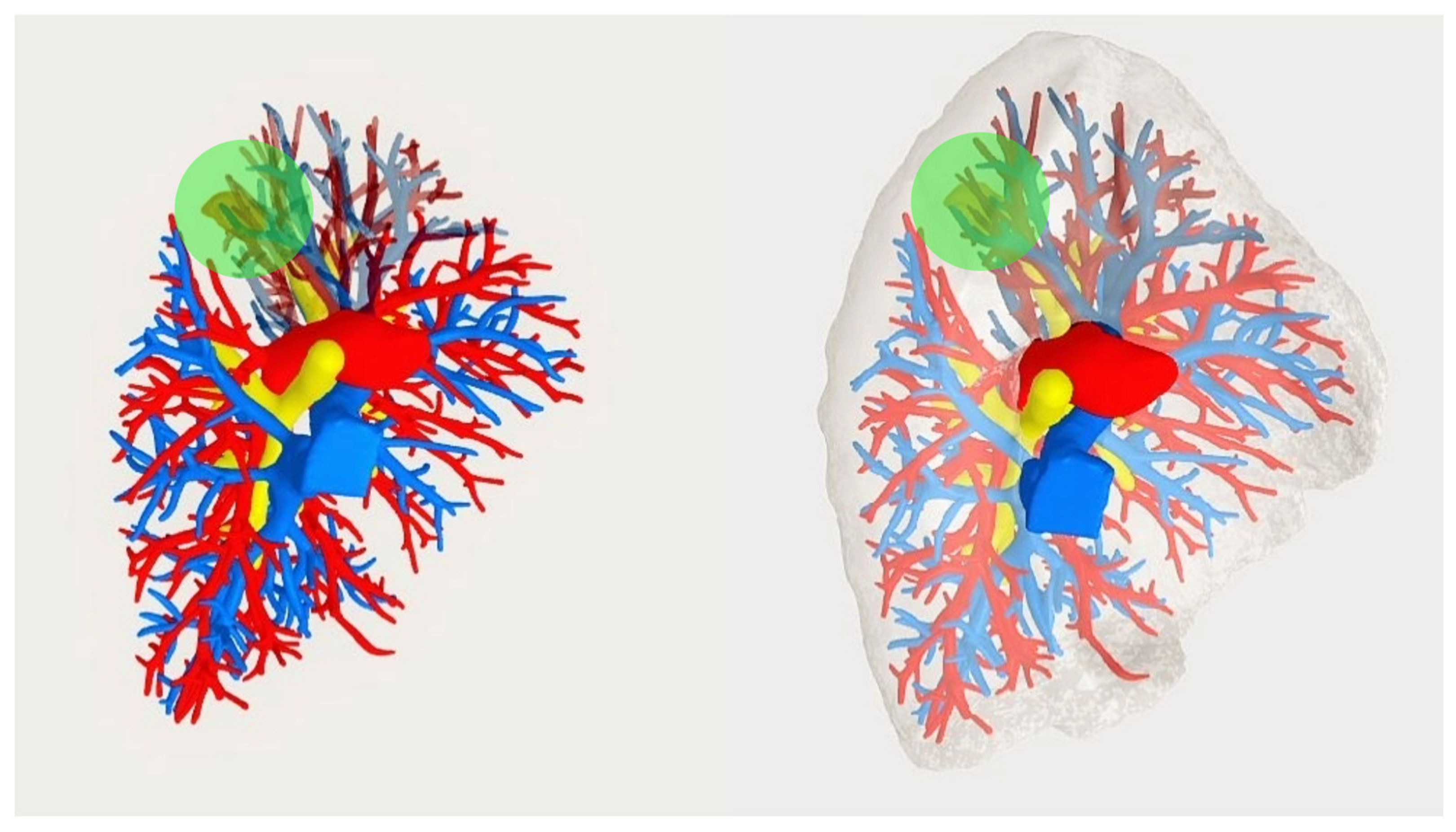
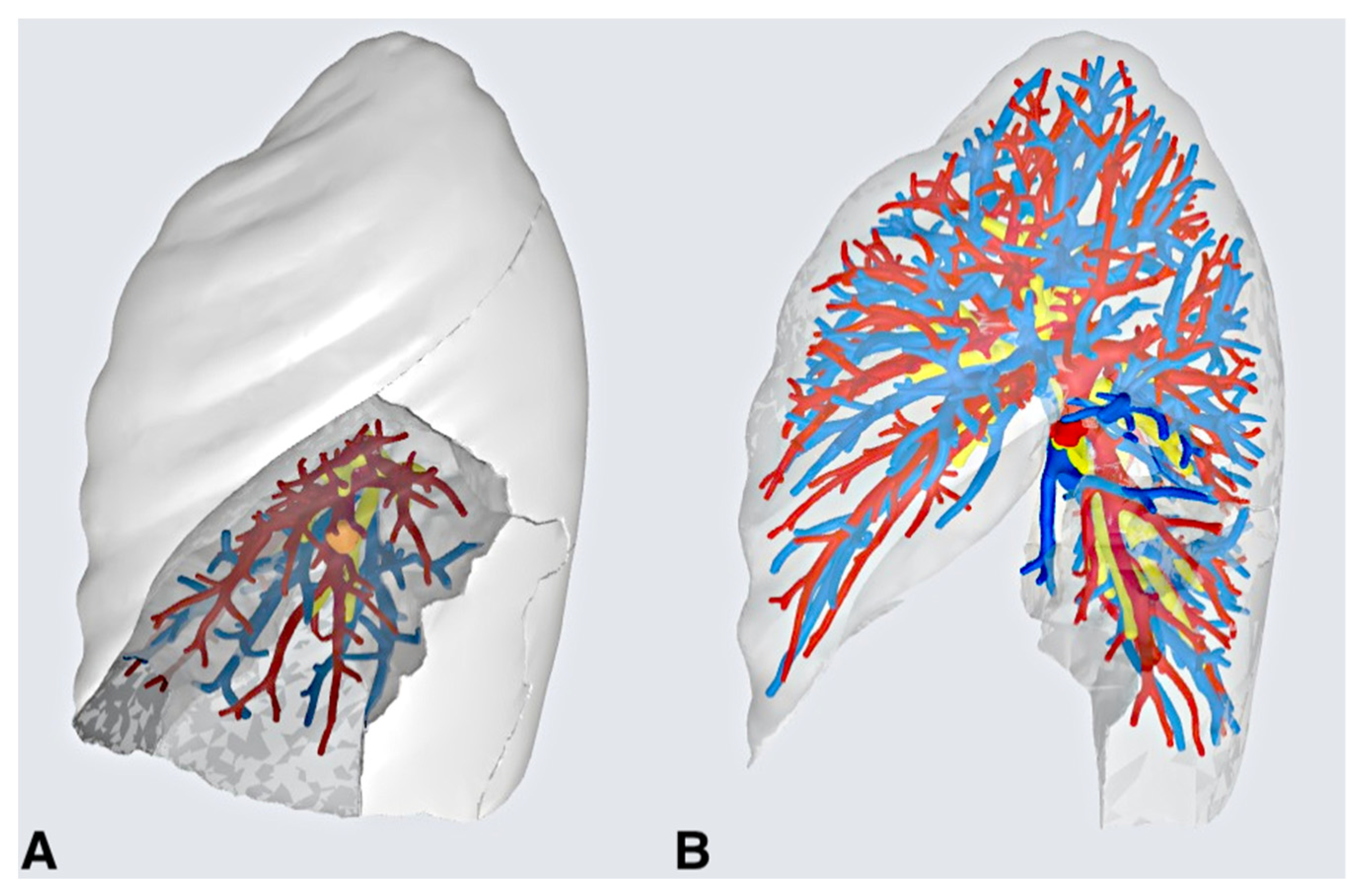
2.1. Creating the 3D Model and Pre-operative Evaluations
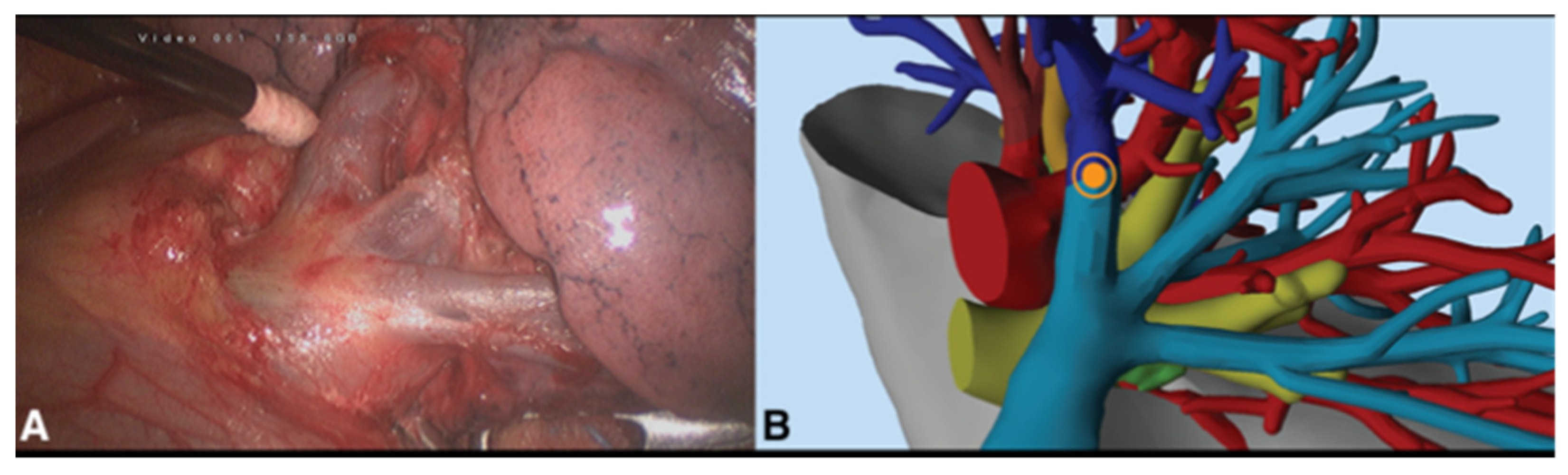
2.2. Surgical Procedure
2.3. Variables and Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Donington, J.; Schumacher, L.; Yanagawa, J. Surgical Issues for Operable Early-Stage Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2022, 40, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Bassiri, A.; Badrinathan, A.; Alvarado, C.E.; Kwak, M.; Sinopoli, J.; Vargas, L.T.; Linden, P.A.; Towe, C.W. Evaluating the Optimal Time between Diagnosis and Surgical Intervention for Early-Stage Lung Cancer. J. Surg. Res. 2023, 292, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, S.; Shimizu, K.; Mogi, A.; Kuwano, H. VATS segmentectomy: Past, present, and future. Gen. Thorac. Cardiovasc. Surg. 2018, 66, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Altorki, N.K.; Wang, X.; Wigle, D.; Gu, L.; Darling, G.; Ashrafi, A.S.; Landrenau, R.; Miller, D.; Liberman, M.; Jones, D.R.; et al. Perioperative mortality and morbidity after sublobar versus lobar resection for early-stage non-small-cell lung cancer: Post-hoc analysis of an international, randomised, phase 3 trial (CALGB/Alliance 140503). Lancet Respir. Med 2018, 6, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Saji, H.; Okada, M.; Tsuboi, M.; Nakajima, R.; Suzuki, K.; Aokage, K.; Aoki, T.; Okami, J.; Yoshino, I.; Ito, H.; et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): A multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022, 399, 1607–1617. [Google Scholar] [CrossRef] [PubMed]
- Aokage, K.; Suzuki, K.; Saji, H.; Wakabayashi, M.; Kataoka, T.; Sekino, Y.; Fukuda, H.; Endo, M.; Hattori, A.; Mimae, T.; et al. Segmentectomy for ground-glass-dominant lung cancer with a tumour diameter of 3 cm or less including ground-glass opacity (JCOG1211): A multicentre, single-arm, confirmatory, phase 3 trial. Lancet Respir. Med. 2023, 11, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Huang, Z.; Qin, Y.; Jiao, W. Progress in three-dimensional computed tomography reconstruction in anatomic pulmonary segmentectomy. Thorac. Cancer 2022, 13, 1881–1887. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Q.; Zhang, X.; Yin, H.; Fu, Y.; Cao, M.; Zhao, X. Application of three-dimensional (3D) reconstruction in the treatment of video-assisted thoracoscopic complex segmentectomy of the lower lung lobe: A retrospective study. Front. Surg. 2022, 9, 968199. [Google Scholar] [CrossRef] [PubMed]
- Sardari Nia, P.; Olsthoorn, J.R.; Heuts, S.; Maessen, J.G. Interactive 3D Reconstruction of Pulmonary Anatomy for Preoperative Planning, Virtual Simulation, and Intraoperative Guiding in Video-Assisted Thoracoscopic Lung Surgery. Innovations 2019, 14, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.; Chen, K.; Jin, R.; Zheng, B.; Gong, X.; Nie, Q.; Jiang, B.; Zhong, W.; Chen, C.; Li, H. Three-dimensional computed tomography reconstruction in video-assisted thoracoscopic segmentectomy (DRIVATS): A prospective, multicenter randomized controlled trial. Front. Surg. 2022, 9, 941582. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Feng, J.; Li, Y.; Liu, B.; Tao, R.; Wang, Z.; Zhao, H.; Zhang, Y.; Wang, J.; Zhang, G. Application of three-dimensional reconstruction of left upper lung lobes in anatomical segmental resection. Thorac. Cancer 2022, 13, 1176–1183. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, Z.; Qi, Q.; Zhang, K.; Sui, X.; Wang, X.; Weng, W.; Wang, S.; Zhao, H.; Sun, C.; et al. A fully automated noncontrast CT 3-D reconstruction algorithm enabled accurate anatomical demonstration for lung segmentectomy. Thorac. Cancer 2022, 13, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Frangi, A.F.; Niessen, W.J.; Vincken, K.L.; Viergever, M.A. Multiscale vessel enhancement filtering. In Medical Image Computing and Computer-AssistedIntervention—Miccai’98; Wells, W.M., Colchester, A., Delp, S., Eds.; Springer: Berlin/Heidelberg, Germany, 1998; Volume 1496, pp. 130–137. [Google Scholar]
- Bédat, B.; Abdelnour-Berchtold, E.; Krueger, T.; Perentes, J.Y.; Zellweger, M.; Triponez, F.; Karenovics, W.; Gonzalez, M. Impact of complex segmentectomies by video-assisted thoracic surgery on peri-operative outcomes. J. Thorac. Dis. 2019, 11, 4109–4118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, M.; Yin, R.; Zhang, Q.; Xu, L. Comparison of the oncologic outcomes of anatomic segmentectomy and lobectomy for early-stage non-small cell lung cancer. Ann. Thorac. Surg. 2015, 99, 728–737. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.G.; Landreneau, J.R.; Schuchert, M.J.; Odell, D.D.; Gu, S.; Pu, J.; Luketich, J.D.; Landreneau, R.J. Preoperative (3-dimensional) computed tomography lung reconstruction before anatomic segmentectomy or lobectomy for stage I non-small cell lung cancer. J. Thorac. Cardiovasc. Surg. 2015, 150, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Iwano, S.; Yokoi, K.; Taniguchi, T.; Kawaguchi, K.; Fukui, T.; Naganawa, S. Planning of segmentectomy using three-dimensional computed tomography angiography with a virtual safety margin: Technique and initial experience. Lung Cancer 2013, 81, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Darras, M.; Ojanguren, A.; Forster, C.; Zellweger, M.; Perentes, J.Y.; Krueger, T.; Gonzalez, M. Short-term local control after VATS segmentectomy and lobectomy for solid NSCLC of less than 2 cm. Thorac. Cancer 2021, 12, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Brunelli, A.; Decaluwe, H.; Gonzalez, M.; Gossot, D.; Petersen, R.H.; Augustin, F.; Assouad, J.; Baste, J.M.; Batirel, H.; Falcoz, P.E.; et al. European Society of Thoracic Surgeons expert consensus recommendations on technical standards of segmentectomy for primary lung cancer. Eur. J. Cardiothorac. Surg. 2023, 63, ezad224. [Google Scholar] [CrossRef] [PubMed]
- Yotsukura, M.; Okubo, Y.; Yoshida, Y.; Nakagawa, K.; Watanabe, S.I. Indocyanine green imaging for pulmonary segmentectomy. JTCVS Tech. 2021, 6, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, S.; Liu, C.; Sun, L.; Yan, M. Three-dimensional reconstruction facilitates thoracoscopic anatomical partial lobectomy by an inexperienced surgeon: A single-institution retrospective review. J. Thorac. Dis. 2021, 13, 5986–5995. [Google Scholar] [CrossRef] [PubMed]
| Patient ID | Sex | Age | CCI | P/y | Tumor Location | Tumor Dimension (CT Scan) [mm] | Pre-Operative Indication | Surgical Intervention | Reason for Changing Indication |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 58 | 7 | - | LUL | 33 | S1 + S2 | S1 + S2 | - |
| 2 | F | 45 | 6 | 4 | RUL | 20 | S2 | S2 | - |
| 3 | F | 63 | 8 | - | LLL | 12 | S7 + S8 | S7 + S8 | - |
| 4 | F | 82 | 7 | - | LUL | 34 | S1 + S2 | LUL * | Ensure negative margins |
| 5 | F | 62 | 5 | - | RLL | 18 | Basal pyramid | Basal pyramid | - |
| 6 | M | 52 | 4 | 3 | LLL | 16 | S9 + S10 | S9 + S10 | - |
| 7 | M | 66 | 5 | - | RUL | 20 | S1 | RUL * | N1+ frozen-examination |
| 8 | M | 61 | 3 | 18 | RUL | 21 | S1 | S1 | - |
| 9 | F | 75 | 5 | - | LLL | 25 | S8 + S9 | LLL * | N2+ frozen-examination |
| 10 | M | 66 | 6 | 50 | LUL | 11 | S1 + S2 | S1 + S2 | - |
| 11 | M | 71 | 5 | 10 | LLL | 21 | S6 | LLL * | N1+ frozen-examination |
| Median (IQR1–3) | 63 (59.5–68.5) | 5.0 (5–6.5) | 0 (0–7) | 20.0 (17–23) |
| Patient ID | Time of Surgery [min] | Number of Parenchymal Charges for the Segmental Plane [n] | Total Liquid Leaks [mL] | Duration of Air Leaks [Days] | Duration of Chest Drainage [Days] | Dismissal [Post-Operative Day] | Pathological Diagnosis | pTNM VIII ed. | StagingVIII ed. | Resectionmargin [mm] | Follow-Up [Months] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 85 | 1 | 760 | 3 | 6 | 7 | Adenocarcinoma | T1cN0 | IA3 | 5 | 9 |
| 2 | 70 | 3 | 950 | 5 | 6 | 7 | Metastasis | - | - | 1 | 9 |
| 3 | 100 | 6 | 100 | 1 | 1 | 3 | Adenocarcinoma | T1aN0 | IA1 | 5 | 12 |
| 4 | 210 | 5 | 1480 | 7 | 9 | 10 | Adenocarcinoma | T1bN0 | IA2 | 2 | 24 |
| 5 | 200 | 4 | 650 | 0 | 3 | 4 | Neuroendocrine tumor | T1bN0 | IA2 | 40 | 24 |
| 6 | 165 | 7 | 500 | 0 | 3 | 5 | Metastasis | - | - | 5 | |
| 7 | 125 | 3 | 310 | 4 | 8 | 9 | Adenocarcinoma | T1bN1a | IIB | 19 | 11 |
| 8 | 110 | 4 | 330 | 0 | 2 | 3 | Adenocarcinoma | T2N0 | IB | 12 | |
| 9 | 205 | 3 | 900 | 1 | 4 | 5 | Adenocarcinoma | T2N2b | IIIA | 12 | 32 |
| 10 | 150 | 4 | 850 | 0 | 3 | 4 | Adenocarcinoma | T1aN0 | IA1 | 35 | 34 |
| 11 | 145 | 4 | 1300 | 0 | 3 | 5 | Adenocarcinoma | T1cN1a | IIB | 4 | 1 |
| Median (IQR1–3) | 115 (105–182.5) | 4 (3–9) | 1030 (415–925) | 1.5 (0–3.5) | 4.5 (3–6) | 6 (3.5–7) | - | - | - | 5 (4–19) | 12 (9–24) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cannone, G.; Verzeletti, V.; Busetto, A.; Lione, L.; Bonis, A.; Nicotra, S.; Rebusso, A.; Mammana, M.; Schiavon, M.; Dell’Amore, A.; et al. Three-Dimensional Imaging-Guided Lung Anatomic Segmentectomy: A Single-Center Preliminary Experiment. Medicina 2023, 59, 2079. https://doi.org/10.3390/medicina59122079
Cannone G, Verzeletti V, Busetto A, Lione L, Bonis A, Nicotra S, Rebusso A, Mammana M, Schiavon M, Dell’Amore A, et al. Three-Dimensional Imaging-Guided Lung Anatomic Segmentectomy: A Single-Center Preliminary Experiment. Medicina. 2023; 59(12):2079. https://doi.org/10.3390/medicina59122079
Chicago/Turabian StyleCannone, Giorgio, Vincenzo Verzeletti, Alberto Busetto, Luigi Lione, Alessandro Bonis, Samuele Nicotra, Alessandro Rebusso, Marco Mammana, Marco Schiavon, Andrea Dell’Amore, and et al. 2023. "Three-Dimensional Imaging-Guided Lung Anatomic Segmentectomy: A Single-Center Preliminary Experiment" Medicina 59, no. 12: 2079. https://doi.org/10.3390/medicina59122079
APA StyleCannone, G., Verzeletti, V., Busetto, A., Lione, L., Bonis, A., Nicotra, S., Rebusso, A., Mammana, M., Schiavon, M., Dell’Amore, A., & Rea, F. (2023). Three-Dimensional Imaging-Guided Lung Anatomic Segmentectomy: A Single-Center Preliminary Experiment. Medicina, 59(12), 2079. https://doi.org/10.3390/medicina59122079






