The Efficacy of Immunotherapy and Clinical Utility of Comprehensive Genomic Profiling in Adenoid Cystic Carcinoma of Head and Neck
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Characterization on the C-CAT Database
2.2. Patient Population and Characterization at Tokyo Medical and Dental University (TMDU)
2.3. Comprehensive Genomic Profiling (CGP) Analysis
2.4. Expert Panel Discussion
2.5. Efficacy Evaluation of ICI Therapy
2.6. Biomarker Assessment
3. Results
3.1. Comprehensive Patient Population and Genetic Characterization of CGP in C-CAT Data
3.2. Outcomes of ICI Therapy in C-CAT Data
3.3. Outcomes of ICI Therapy and Sequencing Results in TMDU
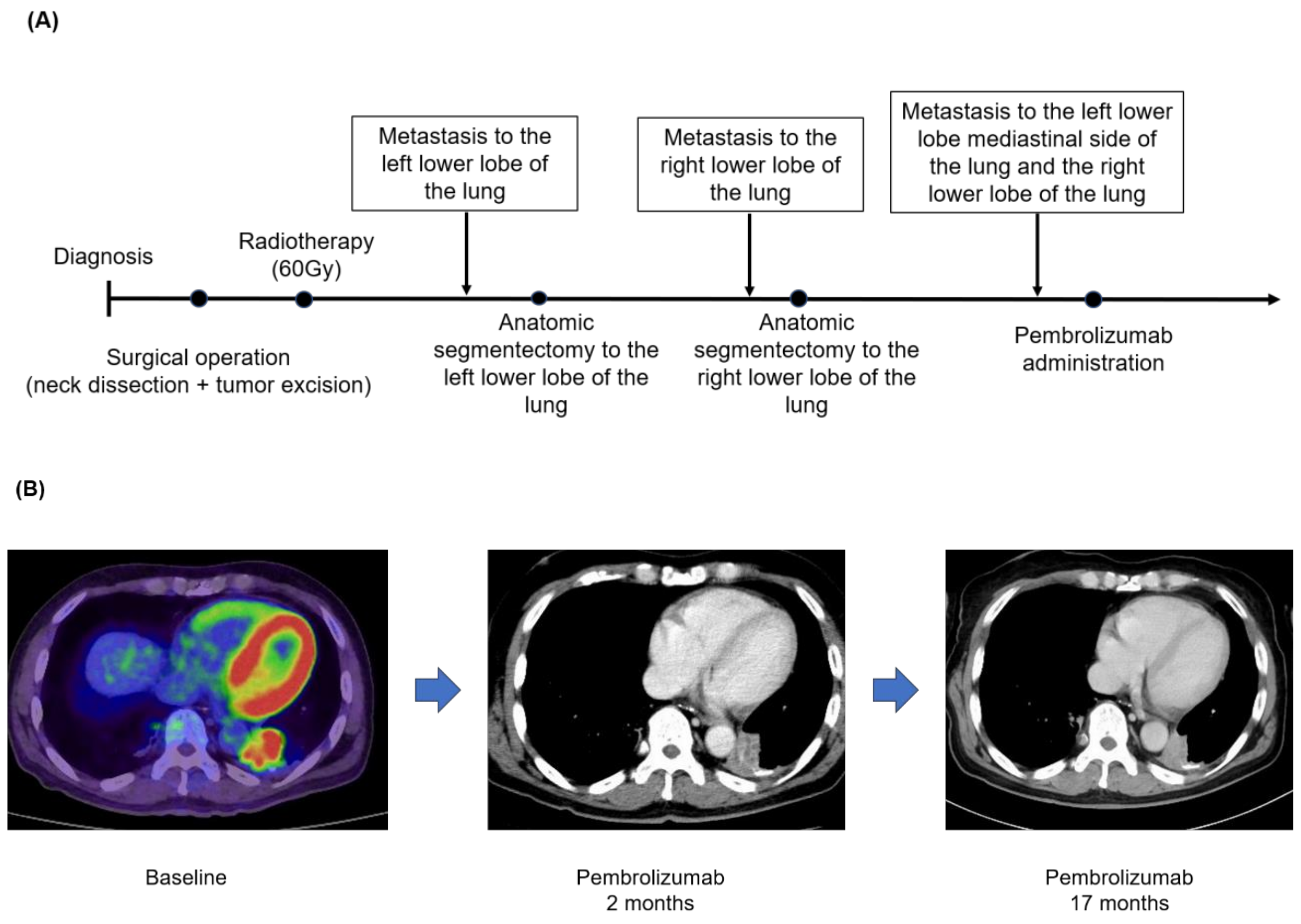
3.4. Case Presentation (Case No.3 in Table 5A,B)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Atallah, S.; Casiraghi, O.; Fakhry, N.; Wassef, M.; Uro-Coste, E.; Espitalier, F.; Sudaka, A.; Kaminsky, M.C.; Dakpe, S.; Digue, L.; et al. A prospective multicentre REFCOR study of 470 cases of head and neck Adenoid cystic carcinoma: Epidemiology and prognostic factors. Eur. J. Cancer 2020, 130, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Jeong, I.S.; Roh, J.L.; Cho, K.J.; Choi, S.H.; Nam, S.Y.; Kim, S.Y. Risk factors for survival and distant metastasis in 125 patients with head and neck adenoid cystic carcinoma undergoing primary surgery. J. Cancer Res. Clin. Oncol. 2020, 146, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Van Weert, S.; Bloemena, E.; van der Waal, I.; de Bree, R.; Rietveld, D.H.; Kuik, J.D.; Leemans, C.R. Adenoid cystic carcinoma of the head and neck: A single-center analysis of 105 consecutive cases over a 30-year period. Oral Oncol. 2013, 49, 824–829. [Google Scholar] [CrossRef] [PubMed]
- Lorini, L.; Ardighieri, L.; Bozzola, A.; Romani, C.; Bignotti, E.; Buglione, M.; Guerini, A.; Lombardi, D.; Deganello, A.; Tomasoni, M.; et al. Prognosis and management of recurrent and/or metastatic head and neck adenoid cystic carcinoma. Oral Oncol. 2021, 115, 105213. [Google Scholar] [CrossRef] [PubMed]
- Adelstein, D.; Gillison, M.L.; Pfister, D.G.; Spencer, S.; Adkins, D.; Brizel, D.M.; Burtness, B.; Busse, P.M.; Caudell, J.J.; Cmelak, A.J.; et al. NCCN Guidelines Insights: Head and Neck Cancers, Version 2.2017. J. Natl. Compr. Cancer Netw. 2017, 15, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Ferris, R.L. Immunology and Immunotherapy of Head and Neck Cancer. J. Clin. Oncol. 2015, 33, 3293–3304. [Google Scholar] [CrossRef]
- Cohen, E.E.W.; Bell, R.B.; Bifulco, C.B.; Burtness, B.; Gillison, M.L.; Harrington, K.J.; Le, Q.T.; Lee, N.Y.; Leidner, R.; Lewis, R.L.; et al. The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of squamous cell carcinoma of the head and neck (HNSCC). J. Immunother. Cancer 2019, 7, 184. [Google Scholar] [CrossRef]
- Burtness, B.; Harrington, K.J.; Greil, R.; Soulieres, D.; Tahara, M.; de Castro, G., Jr.; Psyrri, A.; Baste, N.; Neupane, P.; Bratland, A.; et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet 2019, 394, 1915–1928. [Google Scholar] [CrossRef]
- Ferris, R.L.; Blumenschein, G., Jr.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef]
- Haendel, M.A.; Chute, C.G.; Robinson, P.N. Classification, Ontology, and Precision Medicine. N. Engl. J. Med. 2018, 379, 1452–1462. [Google Scholar] [CrossRef]
- Schlauch, D.; Fu, X.; Jones, S.F.; Burris, H.A., 3rd; Spigel, D.R.; Reeves, J.; McKenzie, A.J. Tumor-Specific and Tumor-Agnostic Molecular Signatures Associated With Response to Immune Checkpoint Inhibitors. JCO Precis. Oncol. 2021, 5, 1625–1638. [Google Scholar] [CrossRef] [PubMed]
- Blons, H.; Cabelguenne, A.; Carnot, F.; Laccourreye, O.; de Waziers, I.; Hamelin, R.; Brasnu, D.; Beaune, P.; Laurent-Puig, P. Microsatellite analysis and response to chemotherapy in head-and-neck squamous-cell carcinoma. Int. J. Cancer 1999, 84, 410–415. [Google Scholar] [CrossRef]
- Noji, R.; Tohyama, K.; Kugimoto, T.; Kuroshima, T.; Hirai, H.; Tomioka, H.; Michi, Y.; Tasaki, A.; Ohno, K.; Ariizumi, Y.; et al. Comprehensive Genomic Profiling Reveals Clinical Associations in Response to Immune Therapy in Head and Neck Cancer. Cancers 2022, 14, 3476. [Google Scholar] [CrossRef] [PubMed]
- Tchekmedyian, V.; Sherman, E.J.; Dunn, L.; Fetten, J.V.; Michel, L.S.; Kriplani, A.; Morris, L.; Ostrovnaya, I.; Katabi, N.; Haque, S.; et al. A phase II trial cohort of nivolumab plus ipilimumab in patients (Pts) with recurrent/metastatic adenoid cystic carcinoma (R/M ACC). J. Clin. Oncol. 2019, 37, 6084. [Google Scholar] [CrossRef]
- Mosconi, C.; de Arruda, J.A.A.; de Farias, A.C.R.; Oliveira, G.A.Q.; de Paula, H.M.; Fonseca, F.P.; Mesquita, R.A.; Silva, T.A.; Mendonça, E.F.; Batista, A.C. Immune microenvironment and evasion mechanisms in adenoid cystic carcinomas of salivary glands. Oral Oncol. 2019, 88, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Guazzo, E.; Cooper, C.; Wilkinson, L.; Feng, S.; King, B.; Simpson, F.; Porceddu, S.; Panizza, B.; Coward, J.I.G. Therapeutic implications of immune-profiling and EGFR expression in salivary gland carcinoma. Head Neck 2021, 43, 768–777. [Google Scholar] [CrossRef]
- Frampton, G.M.; Fichtenholtz, A.; Otto, G.A.; Wang, K.; Downing, S.R.; He, J.; Schnall-Levin, M.; White, J.; Sanford, E.M.; An, P.; et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat. Biotechnol. 2013, 31, 1023–1031. [Google Scholar] [CrossRef]
- Sunami, K.; Ichikawa, H.; Kubo, T.; Kato, M.; Fujiwara, Y.; Shimomura, A.; Koyama, T.; Kakishima, H.; Kitami, M.; Matsushita, H.; et al. Feasibility and utility of a panel testing for 114 cancer-associated genes in a clinical setting: A hospital-based study. Cancer Sci. 2019, 110, 1480–1490. [Google Scholar] [CrossRef]
- Matsudera, S.; Kano, Y.; Aoyagi, Y.; Tohyama, K.; Takahashi, K.; Kumaki, Y.; Mitsumura, T.; Kimura, K.; Onishi, I.; Takemoto, A.; et al. A Pilot Study Analyzing the Clinical Utility of Comprehensive Genomic Profiling Using Plasma Cell-Free DNA for Solid Tumor Patients in Japan (PROFILE Study). Ann. Surg. Oncol. 2021, 28, 8497–8505. [Google Scholar] [CrossRef]
- Naito, Y.; Aburatani, H.; Amano, T.; Baba, E.; Furukawa, T.; Hayashida, T.; Hiyama, E.; Ikeda, S.; Kanai, M.; Kato, M.; et al. Clinical practice guidance for next-generation sequencing in cancer diagnosis and treatment (edition 2.1). Int. J. Clin. Oncol. 2021, 26, 233–283. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Büttner, R.; Longshore, J.W.; López-Ríos, F.; Merkelbach-Bruse, S.; Normanno, N.; Rouleau, E.; Penault-Llorca, F. Implementing TMB measurement in clinical practice: Considerations on assay requirements. ESMO Open 2019, 4, e000442. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.S.; Ochoa, A.; Jayakumaran, G.; Zehir, A.; Valero Mayor, C.; Tepe, J.; Makarov, V.; Dalin, M.G.; He, J.; Bailey, M.; et al. Genetic hallmarks of recurrent/metastatic adenoid cystic carcinoma. J. Clin. Investig. 2019, 129, 4276–4289. [Google Scholar] [CrossRef] [PubMed]
- Ferrarotto, R.; Mitani, Y.; Diao, L.; Guijarro, I.; Wang, J.; Zweidler-McKay, P.; Bell, D.; William, W.N., Jr.; Glisson, B.S.; Wick, M.J.; et al. Activating NOTCH1 Mutations Define a Distinct Subgroup of Patients With Adenoid Cystic Carcinoma Who Have Poor Prognosis, Propensity to Bone and Liver Metastasis, and Potential Responsiveness to Notch1 Inhibitors. J. Clin. Oncol. 2017, 35, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.; Broun, A.; Ge, K. Lysine Demethylase KDM6A in Differentiation, Development, and Cancer. Mol. Cell Biol. 2020, 40, e00341-20. [Google Scholar] [CrossRef]
- Brill, L.B., 2nd; Kanner, W.A.; Fehr, A.; Andrén, Y.; Moskaluk, C.A.; Löning, T.; Stenman, G.; Frierson, H.F., Jr. Analysis of MYB expression and MYB-NFIB gene fusions in adenoid cystic carcinoma and other salivary neoplasms. Mod. Pathol. 2011, 24, 1169–1176. [Google Scholar] [CrossRef]
- Rettig, E.M.; Tan, M.; Ling, S.; Yonescu, R.; Bishop, J.A.; Fakhry, C.; Ha, P.K. MYB rearrangement and clinicopathologic characteristics in head and neck adenoid cystic carcinoma. Laryngoscope 2015, 125, E292–E299. [Google Scholar] [CrossRef]
- Humtsoe, J.O.; Kim, H.S.; Jones, L.; Cevallos, J.; Boileau, P.; Kuo, F.; Morris, L.G.T.; Ha, P. Development and Characterization of MYB-NFIB Fusion Expression in Adenoid Cystic Carcinoma. Cancers 2022, 14, 2263. [Google Scholar] [CrossRef]
- Xu, L.H.; Zhao, F.; Yang, W.W.; Chen, C.W.; Du, Z.H.; Fu, M.; Ge, X.Y.; Li, S.L. MYB promotes the growth and metastasis of salivary adenoid cystic carcinoma. Int. J. Oncol. 2019, 54, 1579–1590. [Google Scholar] [CrossRef]
- Liu, X.; Chen, D.; Lao, X.; Liang, Y. The value of MYB as a prognostic marker for adenoid cystic carcinoma: Meta-analysis. Head Neck 2019, 41, 1517–1524. [Google Scholar] [CrossRef]
- Ho, A.L.; Dunn, L.; Sherman, E.J.; Fury, M.G.; Baxi, S.S.; Chandramohan, R.; Dogan, S.; Morris, L.G.; Cullen, G.D.; Haque, S.; et al. A phase II study of axitinib (AG-013736) in patients with incurable adenoid cystic carcinoma. Ann. Oncol. 2016, 27, 1902–1908. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.S.; Gay, L.M.; Wang, K.; Vergilio, J.A.; Suh, J.; Ramkissoon, S.; Somerset, H.; Johnson, J.M.; Russell, J.; Ali, S.; et al. Comprehensive genomic profiles of metastatic and relapsed salivary gland carcinomas are associated with tumor type and reveal new routes to targeted therapies. Ann. Oncol. 2017, 28, 2539–2546. [Google Scholar] [CrossRef] [PubMed]
- Witte, H.M.; Gebauer, N.; Lappöhn, D.; Umathum, V.G.; Riecke, A.; Arndt, A.; Steinestel, K. Prognostic Impact of PD-L1 Expression in Malignant Salivary Gland Tumors as Assessed by Established Scoring Criteria: Tumor Proportion Score (TPS), Combined Positivity Score (CPS), and Immune Cell (IC) Infiltrate. Cancers 2020, 12, 873. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.B.; Delord, J.P.; Doi, T.; Piha-Paul, S.A.; Liu, S.V.; Gilbert, J.; Algazi, A.P.; Damian, S.; Hong, R.L.; Le Tourneau, C.; et al. Pembrolizumab for the Treatment of Advanced Salivary Gland Carcinoma: Findings of the Phase 1b KEYNOTE-028 Study. Am. J. Clin. Oncol. 2018, 41, 1083–1088. [Google Scholar] [CrossRef]
- Tsui, C.; Kretschmer, L.; Rapelius, S.; Gabriel, S.S.; Chisanga, D.; Knöpper, K.; Utzschneider, D.T.; Nüssing, S.; Liao, Y.; Mason, T.; et al. MYB orchestrates T cell exhaustion and response to checkpoint inhibition. Nature 2022, 609, 354–360. [Google Scholar] [CrossRef]
- Ferrarotto, R.; Sousa, L.G.; Feng, L.; Mott, F.; Blumenschein, G.; Altan, M.; Bell, D.; Bonini, F.; Li, K.; Marques-Piubelli, M.L.; et al. Phase II Clinical Trial of Axitinib and Avelumab in Patients With Recurrent/Metastatic Adenoid Cystic Carcinoma. J. Clin. Oncol. 2023, 41, 2843–2851. [Google Scholar] [CrossRef]

| (A) | ||||||||||
| n (%) | Best response | ORR | DCR | |||||||
| CR | PR | SD | PD | NE | ||||||
| All patients | 59 (100) | 2 (3) | 3 (5) | 26 (44) | 11 (19) | 17 (29) | 5 (8) | 31 (53) | ||
| TMB | 59 (100) | |||||||||
| Low (≤5) | 54 (92) | 2 (4) | 3 (6) | 23 (43) | 10 (19) | 16 (31) | 5/54 (9) | 28/54 (52) | ||
| Intermediate (5–10) | 4 (7) | 0 (0) | 0 (0) | 2 (50) | 1 (25) | 1 (25) | 0/4 (0) | 2/4 (50) | ||
| High (>10) | 1 (2) | 0 (0) | 0 (0) | 1 (100) | 0 (0) | 0 (0) | 0/1 (0) | 1/1 (100) | ||
| (B) | ||||||||||
| n (%) | Age (y) | Outcome (%) | ||||||||
| Median | Range | ORR | DCR | |||||||
| All patients | 59 (100) | 61 | 34–77 | 5/59 (8) | 31/59 (53) | |||||
| Male | 27 (46) | 60 | 36–77 | 2/27 (7) | 15/27 (56) | |||||
| Female | 32 (54) | 62 | 34–77 | 3/32 (9) | 16/32 (50) | |||||
| (A) | ||||||||
| n (%) | Best response | ORR | DCR | |||||
| CR | PR | SD | PD | NE | ||||
| All patients | 28 (100) | 1 (4) | 1 (4) | 8 (29) | 7 (25) | 11 (39) | 2 (7) | 10 (36) |
| TMB | 28 (100) | |||||||
| Low (≤5) | 25 (89) | 1 (4) | 1 (4) | 6 (24) | 7 (28) | 10 (40) | 2/25 (8) | 8/25 (32) |
| Intermediate (5–10) | 3 (11) | 0 (0) | 0 (0) | 2 (67) | 0 (0) | 1 (33) | 0/0 (0) | 2/3 (67) |
| High (>10) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0/0 (0) | 0/0 (0) |
| (B) | ||||||||
| n (%) | Best response | ORR | DCR | |||||
| CR | PR | SD | PD | NE | ||||
| All patients | 31 (100) | 1 (3) | 2 (6) | 18 (58) | 4 (13) | 6 (19) | 3 (10) | 21 (68) |
| TMB | 31 (100) | |||||||
| Low (≤5) | 29 (90) | 1 (3) | 2 (7) | 17 (59) | 3 (10) | 6 (21) | 3/29 (11) | 20/29 (69) |
| Intermediate (5–10) | 1 (7) | 0 (0) | 0 (0) | 0 (0) | 1 (100) | 0 (0) | 0/1 (0) | 0/1 (0) |
| High (>10) | 1 (4) | 0 (0) | 0 (0) | 1 (100) | 0 (0) | 0 (0) | 0/1 (0) | 1/1 (100) |
| n | Best Response (%) | ORR | DCR | |||||
|---|---|---|---|---|---|---|---|---|
| CR | PR | SD | PD | NE | ||||
| NOTCH1 | 13 | 0 (0) | 0 (0) | 6 (46) | 3 (23) | 4 (30) | 0/13 (0) | 6/13 (46) |
| BRAF | 4 | 0 (0) | 0 (0) | 2 (50) | 0 (0) | 2 (50) | 0/4 (0) | 2/4 (50) |
| KDM6A | 5 | 0 (0) | 0 (0) | 1 (20) | 1 (20) | 3 (60) | 0/5 (0) | 1/5 (20) |
| MYB * | 5 | 1 (20) | 1 (20) | 0 (0) | 2 (40) | 1 (20) | 2/5 (40) | 2/5 (40) |
| Case No. | Age(yr)/Gender | Tumor Mutational Burden | Immune Checkpoint Inhibitor | Treatment Line | Best Response |
|---|---|---|---|---|---|
| 1 | 53/F | 5.04 mut/Mb | Pembrolizumab | 1st line | CR |
| 2 | 55/M | 0 mut/Mb | Nivolumab | 2nd line | CR |
| 3 | 63/M | 2 mut/Mb | Pembrolizumab | 1st line | PR |
| 4 | 62/F | 1.26 mut/Mb | Pembrolizumab | 2nd line | PR |
| 5 | 34/F | 1 mut/Mb | Nivolumab | 3rd line | PR |
| (A) | ||||||
| Case No. | Age(yr)/Gender | Primary site | Tumor Mutational Burden | Combined positive score | Immune checkpoint inhibitor | Best Response |
| 1 | 57/M | Mandibular gingival | 1.26 mut/Mb | 2–3% | Pembrolizumab | SD |
| 2 | 61/M | Palate | 1.26 mut/Mb | 1–3% | Pembrolizumab | PD |
| 3 | 70/M | Submandibular gland | 1.26 mut/Mb | 1–3% | Pembrolizumab | SD |
| 4 | 49/F | Submandibular gland | 2.52 mut/Mb | 1–5% | Pembrolizumab | SD |
| (B) | ||||||
| Case No. | Genomic Findings | |||||
| 1 | NOTCH1 S990fs*34,H1544fs*66 U2AF1 S34F | |||||
| 2 | TP53 R273C | |||||
| 3 | BRAF G469A EP300 R202* MLL2 G61fs*69 MYB-NFIB fusion MYB rearrangement intron 14 NOTCH1 H2428fs*6 TERT promoter -124C>T TP53 C176W-subclonal | |||||
| 4 | RPTOR D1101Y TERT promoter -124C>T CEBPA P222T CARD11 L601P,T670M SPEN R3403H | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naito, T.; Noji, R.; Kugimoto, T.; Kuroshima, T.; Tomioka, H.; Fujiwara, S.; Suenaga, M.; Harada, H.; Kano, Y. The Efficacy of Immunotherapy and Clinical Utility of Comprehensive Genomic Profiling in Adenoid Cystic Carcinoma of Head and Neck. Medicina 2023, 59, 2111. https://doi.org/10.3390/medicina59122111
Naito T, Noji R, Kugimoto T, Kuroshima T, Tomioka H, Fujiwara S, Suenaga M, Harada H, Kano Y. The Efficacy of Immunotherapy and Clinical Utility of Comprehensive Genomic Profiling in Adenoid Cystic Carcinoma of Head and Neck. Medicina. 2023; 59(12):2111. https://doi.org/10.3390/medicina59122111
Chicago/Turabian StyleNaito, Takahiro, Rika Noji, Takuma Kugimoto, Takeshi Kuroshima, Hirofumi Tomioka, Shun Fujiwara, Mitsukuni Suenaga, Hiroyuki Harada, and Yoshihito Kano. 2023. "The Efficacy of Immunotherapy and Clinical Utility of Comprehensive Genomic Profiling in Adenoid Cystic Carcinoma of Head and Neck" Medicina 59, no. 12: 2111. https://doi.org/10.3390/medicina59122111





