1. Introduction
Chemoradiotherapy (CRT) is currently the standard treatment for locally advanced head and neck cancer [
1,
2]. However, CRT causes mucositis, dermatitis, muscle and nerve dysfunction, and tissue fibrosis, resulting in post-treatment dysphagia [
3]. Among the associated adverse events associated with CRT, dysphagia is a major detriment to quality of life. Several recent studies have reported the relationship between nutritional status and swallowing function; however, patients with head and neck cancer are prone to undernutrition because of the impaired food passage associated with the primary tumor, swallowing pain associated with radiation-induced mucositis, anorexia, and nausea associated with chemotherapy during treatment. Therefore, many studies have reported the importance of nutritional management and early intervention of swallowing rehabilitation in preventing dysphagia [
4,
5,
6].
Swallowing exercises are often used in rehabilitation during CRT to increase muscle strength and prevent muscle atrophy. In general, muscle training is a well-established intervention for muscle strengthening, as it is believed to produce muscle hypertrophy effects and changes in the firing threshold and discharge rate of motor units (MUs), in addition to increasing the muscle output power [
7,
8]. Muscle strength evaluation using high-density surface electromyography (HD-sEMG) can provide detailed information about MUs. Changes in MU recruitment patterns as a result of muscle-strengthening exercises can be detected via analysis of the sEMG spatial distribution [
9,
10,
11,
12].
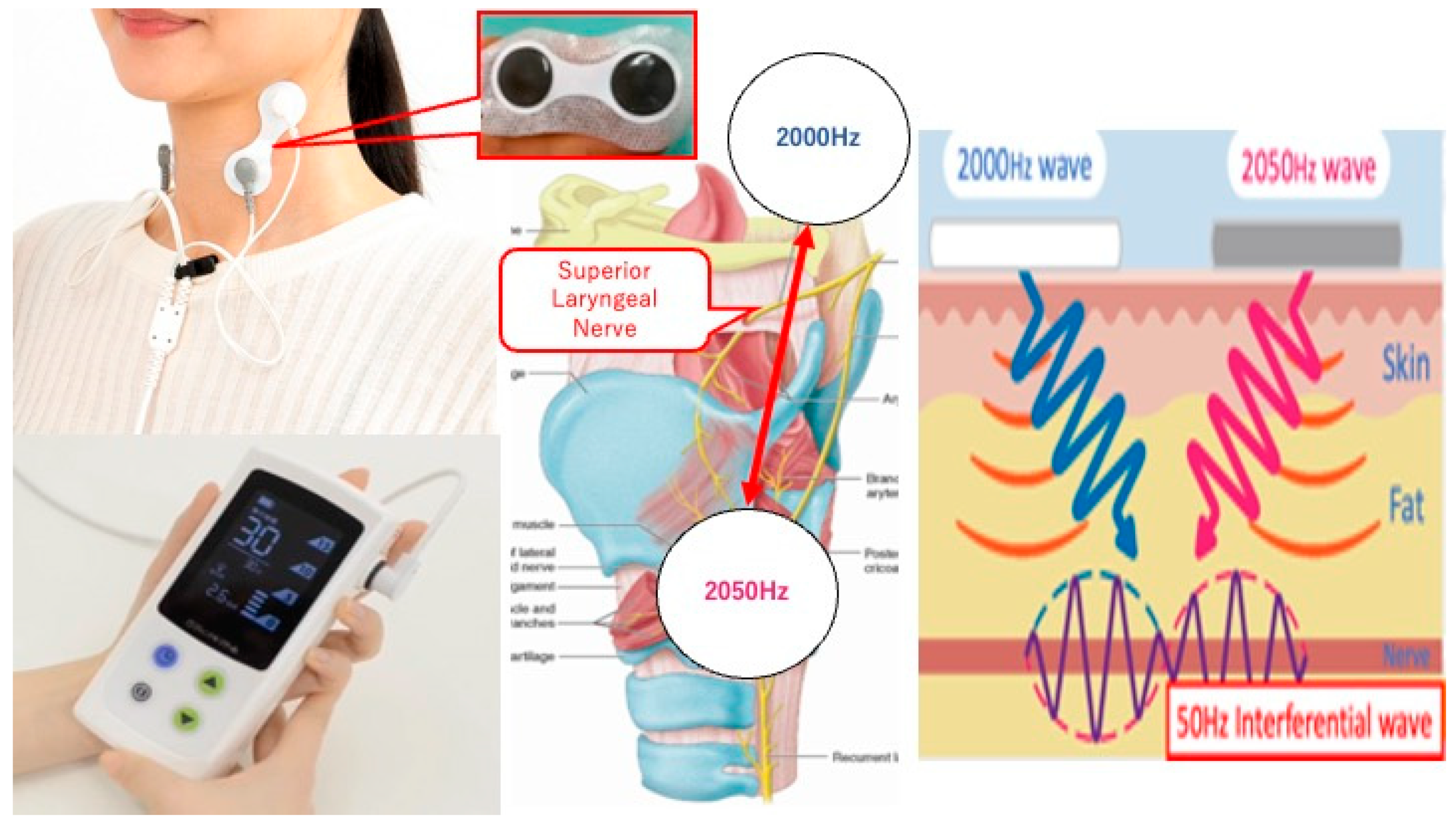
In 2022, we conducted a prospective study to evaluate the safety and feasibility of transcutaneous electrical sensory stimulation (TESS) therapy using an interferential current device (IFCD) named “Gentle-Stim” (Food Care Co., Ltd. Kanagawa, Japan, medical device certification number: 227AHBZX00026000) in patients with head and neck squamous cell carcinoma (HNSCC) undergoing CRT (
Figure 1) [
13]. The primary endpoint was the feasibility of TESS for such patients; it was concluded that TESS was feasible until the end of treatment. To establish further studies examining the efficacy of TESS in patients with head and neck cancer receiving CRT, we included physical assessment, tongue pressure, and HD-sEMG of the suprahyoid muscle group (SHMG) as secondary endpoints because in order to study the effectiveness of TEES, swallowing function must be measured in some way, and these measurement values must be specifically quantified.
Few studies have investigated changes in the recruitment patterns of swallowing MUs in the SHMG during CRT. Therefore, the present study evaluated the effects of swallowing exercises in patients with HNSCC undergoing CRT. We also aimed to determine the significance of sEMG in evaluating swallowing function. Here, we present our results and discuss the existing literature.
Gentle-Stim is an inferential current device manufactured by Food Care Co., Ltd. (Kanagawa, Japan). Electrodes placed across the hyoid bone and thyroid cartilage create interference waves, stimulate the superior laryngeal nerve, and improve laryngeal sensation.
2. Materials and Methods
2.1. Ethics
The study protocol was approved on 17 March 2022 by the Certified Clinical Research Committee of Hiroshima University (certification number: CRB210005), registered with the Japan Registry of Clinical Trials (jRCTs062220008), and submitted to the Ministry of Health, Labour and Welfare. Written informed consent was obtained from each participant, and the study was conducted in accordance with the tenets of the Declaration of Helsinki.
2.2. Study Objectives and Eligibility Criteria
This single-center, exploratory, single-arm prospective study was conducted to evaluate the safety of TESS in patients enrolled and treated between 13 April 2022 and 30 March 2023. Ten patients with locally advanced head and neck cancer who underwent CRT were selected from Hiroshima University Hospital. The eligibility criteria were (1) patients who underwent CRT for head and neck cancer at Hiroshima University Hospital; (2) patients who received 70 Gy of radiation to the laryngopharyngeal area, including the nasopharynx, oropharynx, hypopharynx, or larynx; (3) patients >20 years old at the time of consent; and (4) patients who provided written consent for participation in this study. The exclusion criteria were (1) patients with a history of radiation therapy administration in the head and neck region; (2) patients with a history of tracheostomy; (3) patients with a history of radiation therapy mainly in an area other than the laryngopharyngeal area; (4) patients with pacemakers and implantable cardioverter-defibrillators; (5) patients with difficulty wearing an IFCD on the neck; (6) patients with many inconveniences in daily life (performance status 2 or higher); (7) pregnant and breastfeeding patients and those of reproductive age; and (8) patients who were judged to be inappropriate by the principal investigator or the research coordinator. This study was designed to evaluate the safety and feasibility of TESS using IFCD in patients with HNSCC undergoing CRT. The details of the 10 eligible cases were previously reported [
13]. A supplementary objective was to assess the efficacy of HD-sEMG in swallowing exercises.
2.3. Measurement Items
2.3.1. Physical Status
Patients’ height and weight were measured, and their body mass index (BMI) was calculated. Their skeletal muscle mass was calculated using bioelectrical impedance analysis with the InBody S10 (InBody Japan Co., Ltd., Tokyo, Japan), and their skeletal muscle mass index (SMI) was calculated [
14].
2.3.2. HD-sEMG
The muscle activity of the SHMG was measured using HD-sEMG. The electrodes were 64 channels in 13 rows and 5 columns of a 1 mm diameter sheet (GR04MM1305, OT Bioelettronica Co. Ltd., Torino, Italy). The electrode sheet was affixed to the anterior cervical midline between the mandible and hyoid bone (
Figure 2). To detect stable muscle activity, 50 bipolar surface electromyographic (sEMG) signals were derived from 55 electrodes, with the exclusion of the electrodes at both ends. Signals from each electrode were captured using an 18-bit A/D converter and a bandpass filter of 10–500 Hz (EMG-USB2+, OT Bioelettronica). The EMG signals were analyzed using analysis software (MATLAB 2019a, MathWorks, Inc., Natick, MA, USA), and the root mean square (RMS) of the amplitude was calculated as the index of muscle activity. The RMS value was calculated as the average of values derived from the 50 bipolar sEMG signals. Furthermore, the RMS values were normalized with respect to the values obtained at 0% of MVC. Also, the changes in the spatial distribution pattern of muscle activity within the electrode were determined using the coefficient of variation (CoV), (SD/Ave × 100, CoV force) relative to the RMS value.
The electrode sheet was affixed to the anterior cervical midline between the mandible and hyoid bone. Signals from each electrode were captured and the EMG signals were analyzed using analysis software (MATLAB 2019a, MathWorks, Inc., MA, USA).
2.3.3. Measurements of Tongue Muscle Strength
Tongue muscle strength was measured using a tongue strength meter (Takei Scientific Instruments Co. Ltd., Niigata, Japan). The maximum tongue muscle strength produced by pushing the tongue depressor was measured using isometric contraction for 3 s. The highest value was considered the maximum tongue strength. A ramp-up task for progressive muscle power exertion was performed against the maximum voluntary contraction (MVC) obtained during tongue raising. The examinee progressively increased the muscle strength at 10% MVC/s from 0% to 80% MVC while receiving visual feedback of the tongue muscle strength data displayed on the monitor. The HD-sEMG was recorded during this task in accordance with the ramp-up task [
15]. Among the EMG signals from 0% to 80% MVC, the EMG data at three points (50%, 60%, and 65% MVC) were analyzed.
2.3.4. Tongue Pressure
Tongue pressure was measured using the JMS tongue pressure monitor TPM-01 (JMS Co., Ltd., Hiroshima, Japan). A tongue pressure probe was inserted into the oral cavity, and the base of the probe was lightly cupped by the upper and lower incisors to fix the mandible. The probe was fixed between the anterior part of the tongue and hard palate, and the patient’s tongue was pressed against the probe as hard as possible (maximum tongue pressure value). The maximum tongue pressure (MTP) was measured twice, and the larger value was used as the maximum value.
2.4. Swallowing Muscle Exercise
Swallowing exercises were performed five days a week for eight weeks, with mouth-opening training, cervical isometric contraction exercises, and Shaker exercises. For the mouth-opening training, three sets of 20 opening movements were performed at the maximum opening position. For the cervical isometric contraction exercise comprised 15 sets of 5 s contractions were performed. In the Shaker exercise, one set of 60 s of continuous head raising and 30 repetitions of head raising were performed.
2.5. Statistical Analysis
Patients’ height, weight, BMI, SMI, and tongue pressure were measured before and after treatment. Two parameters, the CoV and RMS, were calculated using the HD-sEMG. The Wilcoxon signed-rank test was used to compare the results before and after each treatment. The rate of change (measured value before start–measured value after treat-ment/measured value before start × 100) was calculated. The CoV and RMS were measured before and after treatment, and the rate of change before and after treatment was calculated. Correlation coefficients were calculated for each of the three physical assessment items and the two parameters of the HD-sEMG. Statistical analyses were performed using JMP Pro ver.16.2.0 (SAS Institute Inc., Cary, NC, USA). The threshold for statistical significance was set at p < 0.05 for each parameter.
3. Results
The 10 enrolled patients were all males. The clinical data of the 10 patients are presented in
Table 1. The median patient age was 67 (45–76) years, and the ECOG PS performance status score was 0. The primary sites were the nasopharynx (one patient), hypopharynx (seven patients), and larynx (one patient), with one remaining unknown (one patient). Two, four, and four patients had clinical stages II, IVa, and IVb, respectively. For the treatment details shown in
Table 2, six cases received induction chemotherapy (docetaxel, cisplatin, 5-FU) before CRT and four cases received two cycles of tri-weekly cisplatin, five cases received three cycles of tri-weekly cisplatin, and one case received seven cycles of weekly cetuximab during irradiation. As for the irradiation dose of radiation, nine patients received a prescribed dose of 70 Gy/35 fractions and one patient received 66 Gy/33 fractions; the patient was irradiated with a large field for an unknown primary cancer, resulting in severe mucositis, and RT was terminated based on clinical judgment. All patients were treated using intensity-modulated radiation therapy (IMRT) as the radiation technique. Combination chemotherapy consisting of cisplatin was administered to all patients, except one who received cetuximab owing to decreased renal function.
The values of the CoV and RMS correlation coefficients between the rate of change in body weight and HD-sEMG parameters were, respectively, CoV p = 0.28, r = 0.37 and RMS p = 0.009, r = 0.77 at 50% MVC, CoV p = 0.05, r = 0.63 and RMS p = 0.005, r = 0.80 at 60% MVC, and CoV p = 0.05, r = 0.63 and RMS p = 0.005, r = 0.80 at 65% MVC. The values of the CoV and RMS correlation coefficients between the rate of change in SMI and HD-sEMG parameters were, respectively, CoV p = 0.21, r = 0.43 and RMS p = 0.04, r = 0.65 at 50% MVC, CoV p = 0.08, r = 0.57 and RMS p = 0.01, r = 0.76 at 60% MVC, CoV p = 0.012, r = 0.74 and RMS p = 0.023, r = 0.70 at 65% MVC. The values of the CoV and RMS correlation coefficients between the rate of change in tongue pressure and HD-sEMG parameters were, respectively, CoV p = 0.97, r = −0.013 and RMS p = 0.97, r = −0.01 at 50% MVC, CoV p = 0.73, r = 0.12 and RMS p = 0.73, r = 0.12 at 60% MVC, and CoV p = 0.93, r = 0.03 and RMS p = 0.93, r = 0.03 at 65% MVC.
In HD-sEMG, a significant correlation was observed between the rate of change in body weight and the rate of change in the CoV and RMS. Additionally, a significant correlation was also found between the rate of change in SMI and the rate of change in the CoV and RMS. However, no significant correlation was observed between the rate of change in tongue pressure and the rate of change in the CoV and RMS. On the other hand, there were no significant differences in BMI, SMI, tongue pressure, the CoV, or the RMS before and after treatment (
Table 3).
4. Discussion
Although CRT for head and neck cancer has been reported to cause tissue scarring, muscle atrophy, and muscle weakness [
3,
16], there are no detailed reports on the effects of CRT on the MU of swallowing muscles. In the present study, the SHMG continuously performed swallowing exercises using TESS during CRT; we evaluated the changes in the MU of the swallowing muscle using HD-sEMG and obtained several findings.
Muscle strength is regulated by the muscle fibers and the nerve factors that activate them. Nerve factors control the recruitment and discharge rate of the MU during muscle contractions; the higher the number of MUs and discharge rate, the higher the muscle strength [
9,
10,
11,
12]. HD-sEMG is a method to spatially evaluate the MU firing behavior and can detect the MU recruitment pattern distribution and changes. The RMS reflects the muscle activation quantification of the MU, whereas the CoV indicates the spatial distribution of the muscle activity [
10,
17,
18]. Muscle-strengthening exercises are thought to increase muscle strength by stimulating hypertrophy of the muscle fibers, lowering the recruitment threshold, and increasing the discharge rate [
7,
8], which has been proven in actual clinical practice [
18].
In the present study, patients underwent eight weeks of muscle-strengthening exercises for the SHMG with TESS. Therefore, it was assumed that muscle exercises would increase the RMS and CoV before and after treatment; however, no such increase was observed. This may be because patients could not perform sufficient strength training due to the pain, fatigue, and nausea caused by mucositis, dermatitis, and stomatitis during CRT. Most of the training performed in this study involved weight-bearing and required physical exertion. Therefore, it seems likely that the patients undergoing CRT could not perform aggressive high-intensity exercises. The second factor is a decline in nutritional status. A high correlation was found between body weight or SMI change rate, an indicator of nutritional status, and RMS or CoV change rate. Patients with decreased body weight and SMI had a decreased discharge rate and distribution of MUs, that is, decreased swallowing muscle strength. Recently, the concept of sarcopenia has been proposed, with many reports that low nutrition causes dysphagia [
19,
20,
21]. Regarding the causal relationship between undernutrition and dysphagia, a vicious cycle has been postulated in which undernutrition causes a decrease in the mass of swallowing-related muscles, muscle at-rophy, and contractility of muscle fibers, resulting in dysphagia [
22]. Therefore, the present study’s results support the finding that the number of MUs decreases in an undernourished state [
23]. As patients with head and neck cancer are at high risk of secondary sarcopenia due to the malnutrition and hypercatabolism associated with dysphagia during CRT, exercise and nutritional interventions are needed during treatment [
24,
25].
Although it has been hypothesized that tongue-raising movements correlate with electromyograms of the SHMG [
26], no correlation was found between the rate of change in tongue pressure and the rate of change in the MU of the SHMG in the present study. Because the irradiation sites of the patients were mainly located around the larynx, the irradiation dose to the oral cavity was limited. Tongue pressure is thought to be generated by the internal and external lingual muscles and the SHMG, and the limited effect of radiation on the lingual muscles suggests that tongue pressure was not involved in the changes in the MU of the SHMG. Additionally, we believe that TESS did not affect the strength of the SHMG, as the TESS targeted the sensory nerve. Although the safety of TESS in CRT for HNSCC has been demonstrated in our previous study, no significant changes were observed before and after treatment. However, significant correlations were observed between the rate of weight loss and SMI reduction and the rate of change in the recruitment of MU of the SHMG. The results suggest that it would be needed to observe changes over time in HD-sEMG in future studies to demonstrate its efficacy in dysphagia.
This study had some limitations. First, the sample size was not enough to reach a definitive conclusion and this study was only a pre- and post-treatment comparison, and it is not clear at what stage during treatment each of the parameters changed. Therefore, whether the effects of muscle-strengthening exercises were limited to the first few weeks of treatment was not adequately measured. We realize the need to investigate the changes over time from a prophylactic perspective to reduce adverse events. As most head and neck cancer patients are male in general, all patients were male in this study. It should be mentioned that the gender difference in muscle mass could be relevant to HD-sEMG, muscle strength, and tongue pressure. Although we focused on the body weight, SMI, and MU of the SHMG, there were many acute adverse events during CRT, including mucositis, dermatitis, and vomiting. It was not clear which of these adverse events strongly influenced the treatment completion rate, oral intake maintenance rate, or length of hospital stay; therefore, continued data collection is needed.
5. Conclusions
Muscle-strengthening exercises were performed in patients undergoing CRT for head and neck cancer, and their effects before and after treatment were investigated using HD-sEMG. Although no significant changes were observed before and after treatment, significant correlations were observed between the rate of weight loss and SMI reduction and the rate of change in the recruitment of MU of the SHMG. Therefore, we believe that nutritional supplementation is necessary in addition to muscle strengthening during CRT. A detailed investigation of the changes over time may help us understand the effects of muscle strengthening and adverse events in detail.
Author Contributions
Investigation, data curation, formal analysis, visualization, writing—original draft, K.Y. (Kohei Yoshikawa); conceptualization, methodology, project administration, visualization, writing—review and editing, T.H.; investigation, data curation, Y.S., K.Y. (Kohei Yumii), N.C., T.T., K.T., M.N. and T.K.; data curation and validation, T.I. and Y.H.; investigation, data curation, formal analysis, visualization, Y.N.; supervision, writing—review and editing, T.U., Y.M. and S.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and the study protocol was approved 17 March 2022 by the Certified Clinical Research Committee of Hiroshima University (certification number: CRB210005), registered with the Japan Registry of Clinical Trials (jRCTs062220008), and submitted by the Ministry of Health, Labour and Welfare.
Informed Consent Statement
Written informed consent was obtained from all patients involved in the study.
Data Availability Statement
The data presented in this study are available upon reasonable request from the corresponding authors.
Acknowledgments
We thank Taizo Hirata for their support in conceptualizing this study. We also thank Yuka Nagano and Mikako Kato for performing the TESS rehabilitation.
Conflicts of Interest
Food Care Corporation provided the consumables used to attach the IFCDs. Conflicts related to this study were appropriately managed using the standards for man-aging conflicts of interest in clinical research under the Clinical Research Act. The funders had no role in the study design; collection, analyses, or interpretation of data; writing of the manuscript; or decision to publish the results.
References
- NCCN Clinical Practice Guidelines in Oncology, Head and Neck Cancers. Available online: https://www.nccn.org/guidelines/guidelines-process/transparency-process-and-recommendations/GetFileFromFileManagerGuid?FileManagerGuidId=c0d39f8d-46a2-4662-b373-467ca4cacd96 (accessed on 18 May 2023).
- Pignon, J.; Le Maître, A.; Maillard, E.; Maillard, E.; Bourhis, J.; MACH-NC Collaborative Group. Meta-analysis of chemotherapy in head and neck cancer: An update on 93 randomised trials and 17346 patients. Radiother. Oncol. 2009, 92, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Nie, W.; Zhou, X.; Guo, W.; Mou, J.; Yong, J.; Wu, T.; Liu, X. Review of prophylactic swallowing interventions for head and neck cancer. Int. J. Nurs. Stud. 2021, 123, 104074. [Google Scholar] [CrossRef] [PubMed]
- Kotz, T.; Federman, A.D.; Kao, J.; Milman, L.; Packer, S.; Lopez-Prieto, C.; Forsythe, K.; Genden, E.M. Prophylactic swallowing exercises in patients with head and neck cancer undergoing chemoradiation: A randomized trial. Arch. Otolaryngol. Head Neck Surg. 2012, 138, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Carnaby-Mann, G.; Crary, M.A.; Schmalfuss, I.; Amdur, R. ‘Pharyngocise’: Randomized controlled trial of preventative exercises to maintain muscle structure and swallowing function during head-and-neck chemoradiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 210–219. [Google Scholar] [CrossRef]
- Tang, Y.; Shen, Q.; Wang, Y.; Lu, K.; Wang, Y.; Peng, Y. A randomized prospective study of rehabilitation therapy in the treatment of radiation-induced dysphagia and trismus. Strahlenther. Onkol. 2011, 187, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, R.; Loenneke, J.P.; Thiebaud, R.S.; Abe, T. Low-load bench press training to fatigue results in muscle hypertrophy similar to high-load bench press training. Int. J. Clin. Med. 2013, 4, 114–121. [Google Scholar] [CrossRef]
- Mitchell, C.J.; Churchward-Venne, T.A.; West, D.W.; Burd, N.A.; Breen, L.; Baker, S.K.; Phillips, S.M. Resistance exercise load does not determine training-mediated hypertrophic gains in young men. J. Appl. Physiol. 2012, 113, 71–77. [Google Scholar] [CrossRef]
- Holtermann, A.; Grönlund, C.; Stefan Karlsson, J.S.; Roeleveld, K. Spatial distribution of active muscle fibre characteristics in the upper trapezius muscle and its dependency on contraction level and duration. J. Electromyogr. Kinesiol. 2008, 18, 372–381. [Google Scholar] [CrossRef]
- Holtermann, A.; Roeleveld, K. EMG amplitude distribution changes over the upper trapezius muscle are similar in sustained and ramp contractions. Acta Physiol. 2006, 186, 159–168. [Google Scholar] [CrossRef]
- Merletti, R.; Holobar, A.; Farina, D. Analysis of motor units with high-density surface electromyography. J. Electromyogr. Kinesiol. 2008, 18, 879–890. [Google Scholar] [CrossRef]
- Koyama, Y.; Sugimoto, A.; Hamano, T.; Kasahara, T.; Toyokura, M.; Masakado, Y. Proposal for a modified jaw opening exercise for dysphagia: A randomized, controlled trial. Tokai J. Exp. Clin. Med. 2017, 42, 71–78. [Google Scholar]
- Hamamoto, T.; Sato, Y.; Yumii, K.; Chikuie, N.; Taruya, T.; Horibe, Y.; Ishino, T.; Ueda, T.; Takeno, S.; Yoshimura, K. Evaluation of the safety of percutaneous sensory nerve stimulation in patients with head and neck cancer receiving chemoradiotherapy. J. Pers. Med. 2023, 13, 1129. [Google Scholar] [CrossRef]
- Baumgartner, R.N.; Koehler, K.M.; Gallagher, D.; Romero, L.; Heymsfield, S.B.; Ross, R.R.; Garry, P.J.; Lindeman, R.D. Epidemiology of sarcopenia among the elderly in New Mexico. Am. J. Epidemiol. 1998, 147, 755–763. [Google Scholar] [CrossRef]
- Maeda, N.; Komiya, M.; Nishikawa, Y.; Morikawa, M.; Tsutsumi, S.; Tashiro, T.; Fukui, K.; Kimura, H.; Urabe, Y. Effect of acute static stretching on the activation patterns using high-density surface electromyography of the gastrocnemius muscle during ramp-up task. Sensors 2021, 21, 4841. [Google Scholar] [CrossRef]
- Wall, L.R.; Ward, E.C.; Cartmill, B.; Hill, A.J. Physiological changes to the swallowing mechanism following (chemo)radiotherapy for head and neck cancer: A systematic review. Dysphagia 2013, 28, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, I.H. Summary comments. Am. J. Clin. Nutr. 1989, 50, 1231–1233. [Google Scholar] [CrossRef]
- Hudson, H.M.; Daubert, C.R.; Mills, R.H. The interdependency of protein-energy malnutrition, aging, and dysphagia. Dysphagia 2000, 15, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Robbins, J.; Gangnon, R.E.; Theis, S.M.; Kays, S.A.; Hewitt, A.L.; Hind, J.A. The effects of lingual exercise on swallowing in older adults. J. Am. Geriatr. Soc. 2005, 53, 1483–1489. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, Y.; Kuroda, R. Relationship between thinness and swallowing function in Japanese older adults: Implications for sarcopenic dysphagia. J. Am. Geriatr. Soc. 2012, 60, 1785–1786. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, I.; Fujiu-Kurachi, M.; Arai, H.; Hyodo, M.; Kagaya, H.; Maeda, K.; Mori, T.; Nishioka, S.; Oshima, F.; Ogawa, S.; et al. Sarcopenia and dysphagia: Position paper by four professional organizations. Geriatr. Gerontol. Int. 2019, 19, 91–97. [Google Scholar] [CrossRef]
- Saito, T.; Hayashi, K.; Nakazawa, H.; Yagihashi, F.; Oikawa, L.O.; Ota, T. A significant association of malnutrition with dysphagia in acute patients. Dysphagia 2018, 33, 258–265. [Google Scholar] [CrossRef]
- Piasecki, M.; Ireland, A.; Piasecki, J.; Stashuk, D.W.; Swiecicka, A.; Rutter, M.K.; Jones, D.A.; McPhee, J.S. Failure to expand the motor unit size to compensate for declining motor unit numbers distinguishes sarcopenic from non-sarcopenic older men. J. Physiol. 2018, 596, 1627–1637. [Google Scholar] [CrossRef] [PubMed]
- Momosaki, R.; Yasunaga, H.; Matsui, H.; Horiguchi, H.; Fushimi, K.; Abo, M. Effect of dysphagia rehabilitation on oral intake in elderly patients with aspiration pneumonia. Geriatr. Gerontol. Int. 2015, 15, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, Y.; Uchida, K.; Jeong, S.; Yamaga, M. Effects of nutritional supplements on muscle mass and activities of daily living in elderly rehabilitation patients with decreased muscle mass: A randomized controlled trial. J. Nutr. Health Aging 2016, 20, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Palmer, P.M.; Luschei, E.S.; Jaffe, D.; McCulloch, T.M. Contributions of individual muscles to the submental surface electromyogram during swallowing. J. Speech Lang. Hear. Res. 1999, 42, 1378–1391. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).