Predictive Factors for Pregnancy-Related Persistent Pelvic Girdle Pain (PPGP): A Systematic Review
Abstract
:1. Introduction
2. Objective
3. Methods
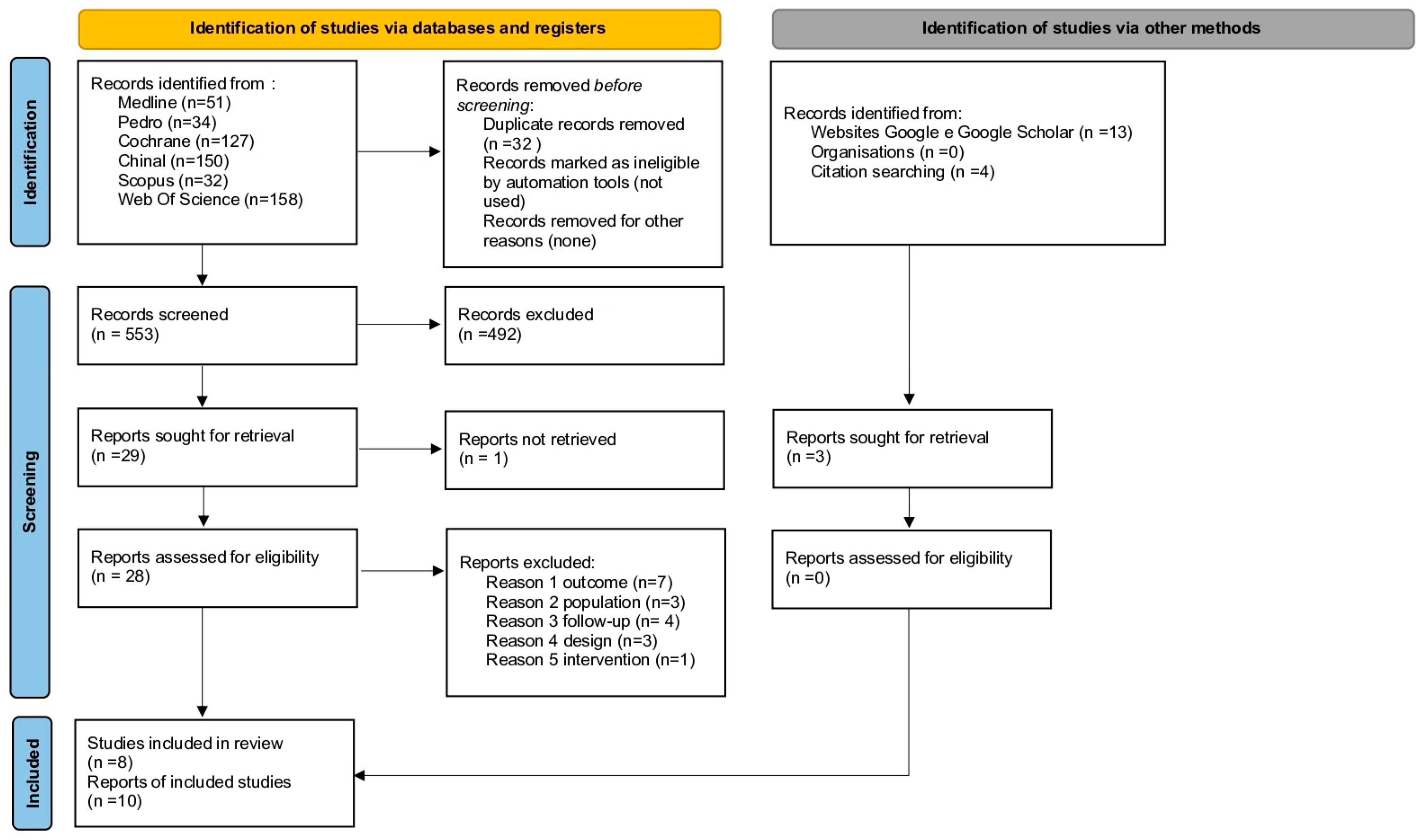
3.1. Study Selection
- PEDRO simple search: Pelvic girdle pain pregnancy: 34 papers (26 RCT, 8 SR).
- COCHRANE: Pelvic girdle pain: 1 Cochrane protocol with 127 trials.
- CINAHL ((pelvic girdle pain OR pelvic girdle pain pregnancy-related OR pelvic girdle pain postpartum)) AND (risk factors or contributing factors or predisposing factors): 150 papers.
- SCOPUS (TITLE-ABS-KEY (pelvic AND girdle AND pain OR pelvic AND girdle AND pain AND pregnancy-related OR pelvic AND girdle AND pain AND postpartum) AND TITLE-ABS-KEY (risk AND factors)): 32 papers.
- WEB OF SCIENCE ((pelvic girdle pain OR pelvic girdle pain pregnancy-related OR pelvic girdle pain postpartum)) AND (risk factors or contributing factors or predisposing factors): 158 papers.
3.2. Assessment of Risk of Bias
4. Data Synthesis
5. Results
Study Selection and Characteristics
6. Risk of Bias of Included Studies
7. Synthesis of Results
8. Principal Findings
9. Comparison with Existing Literature
10. Strengths and Limitations
11. Conclusions and Implication
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Registration
Review Protocol
Abbreviations
| PGP | Pelvic Girdle Pain |
| PPGP | Pregnancy-Related Pelvic Girdle Pain |
| PLB | Pregnancy-Related Low Back Pain |
| LPP | Lumbopelvic Pain |
| PGS | Pelvic Girdle Syndrome |
| SIJ | Sacroiliac Joints |
| LDL | Long Dorsal Sacroiliac Ligament |
| P4 | Posterior Pelvic Pain Provocation Test |
| ASLR | Active Straight Leg Raise |
| BMI | Body Mass Index |
| VAS | Visual Analogic Scale |
| PPT | Pain Pressure Threshold |
| PMI | Pregnancy Mobility Index |
| PSS | Perceived Stress Scale |
| DRI | Disability Rating Index |
| ODI | Oswestry Disability Index |
| PGQ | Pelvic Girdle Questionnaire |
| HR-Qol | Health Related Quality of Life |
| EQ-5D | EuroQol |
| SF-36 | Short Form-36 Health Survey |
| SRH | Self-Rated Health |
| NHP | Nottingham Health Profile |
| PCS | Pain Catastrophizing Scale |
| PSQI | Pittsburgh Sleep Quality Index |
| FABQ | Fear-Avoidance Beliefs Questionnaire |
| NHP | Nottingham Health Profile |
| McGill | McGill Pain Questionnaire |
| NP | Neck Pain |
| TC | Thoracic Pain |
| ROB | Risk of Bias |
| QUIPS | Quality In Prognosis Studies Tool |
| OR | Odd Ratio |
| RR | Relative Risk |
| HR | Hazard Ratio |
| CI | Confidence Interval |
References
- Vleeming, A.; Albert, H.B.; Östgaard, H.C.; Sturesson, B.; Stuge, B. European guidelines for the diagnosis and treatment of pelvic girdle pain. Eur. Spine J. 2008, 17, 794–819. [Google Scholar] [CrossRef] [PubMed]
- Elden, H.; Gutke, A.; Kjellby-Wendt, G.; Fagevik-Olsen, M.; Ostgaard, H.-C. Predictors and consequences of long-term pregnancy-related pelvic girdle pain: A longitudinal follow-up study. BMC Musculoskelet. Disord. 2016, 17, 276. [Google Scholar] [CrossRef] [PubMed]
- Wuytack, F.; Daly, D.; Curtis, E.; Begley, C. Prognostic factors for Pregnancy-related Pelvic Girdle Pain, a Systematic Review. Midwifery 2018, 66, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Kanakaris, N.; Roberts, C.; Giannoudis, P. Pregnancy-related pelvic girdle pain: An update. BMC Med. 2011, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Verstraete, E.H.; Vanderstraeten, G.; Parewijck, W. Pelvic Girdle Pain during or after Pregnancy: A review of recent evidence and a clinical care path proposal. FVV ObGyn. 2013, 5, 33–43. [Google Scholar]
- Aslan, E.; Fynes, M. Symphysial pelvic dysfunction. Curr. Opin. Obstet. Gynecol. 2007, 19, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Depledge, J.; McNair, P.J.; Keal-Smith, C.; Williams, M. Management of symphysis pubis dysfunction during pregnancy using exercise and pelvic support belts. Phys. Ther. 2005, 85, 1290–1300. [Google Scholar] [CrossRef] [PubMed]
- Vermani, E.; Mittal, R.; Weeks, A. Pelvic Girdle Pain and Low Back Pain in Pregnancy: A Review. Pain Pract. 2010, 10, 60–71. [Google Scholar] [CrossRef]
- Mogren, I.M. BMI, pain and hyper-mobility are determinants of long-term outcome for women with low back pain and pelvic pain during pregnancy. Eur. Spine J. 2006, 15, 1093–1102. [Google Scholar] [CrossRef]
- Leadbetter, R.E.; Mawer, D.; Lindow, S.W. The development of a scoring system for symphysis pubis dysfunction. J. Obstet. Gynaecol. 2006, 26, 20–23. [Google Scholar] [CrossRef]
- Gausel, A.; Malmqvist, S.; Andersen, K. Subjective recovery from pregnancy related pelvic girdle pain the first 6 weeks after delivery: A prospective longitudinal cohort study. Eur. Spine J. 2020, 29, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M. The PRISMA 2020 statement: Un update guideline for reporting systematic reviews. BMJ 2021, 29, n71. [Google Scholar] [CrossRef] [PubMed]
- Sjödahl, J.; Gutke, A.; Öberg, B. Predictors for long-term disability in women with persistent postpartum pelvic girdle pain. Eur. Spine J. 2013, 22, 1665–1673. [Google Scholar] [CrossRef] [PubMed]
- Stomp-van den Berg, S.G.; Hendriksen, I.J.; Bruinvels, D.J.; Twisk, J.W.; Van Mechelen, W.; Van Poppel, M.N. Predictors for postpartum pelvic girdle pain in working women: The Mom@Work cohort study. Pain 2012, 153, 2370–2379. [Google Scholar] [CrossRef] [PubMed]
- Albert, H.; Godskesen, M.; Westergaard, J. Prognosis in four syndromes of pregnancyrelated pelvic pain. Acta Obstet. Gynecol. Scand. 2001, 80, 505–510. [Google Scholar] [PubMed]
- Olsson, C.B.; Grooten, W.J.; Nilsson-Wikmar, L.; Harms-Ringdahl, K.; Lundberg, M. Catastrophizing during and after pregnancy: Associations with lumbopelvic pain and postpartum physical ability. Phys. Ther. 2012, 92, 49e57. [Google Scholar] [CrossRef] [PubMed]
- Eisenach, J.C.; Pan, P.H.; Smiley, R.; Lavand’homme, P.; Landau, R.; Houle, T.T. Severity of acute pain after childbirth, but not type of delivery, predicts persistent pain and postpartum depression. Pain 2008, 15, 87–94. [Google Scholar] [CrossRef]
- Vøllestad, N.K.; Stuge, B. Prognostic factors for recovery from postpartum pelvic girdle pain. Eur. Spine J. 2009, 18, 718–726. [Google Scholar] [CrossRef]
- Beales, D.J. Correlations between the active straight leg raise, sleep and somatosensory sensitivity during pregnancy with post-partum lumbopelvic pain: An initial exploration. Scandinavian Association for the Study of Pain. Scand. Assoc. Study Pain 2018, 19, 53–60. [Google Scholar] [CrossRef]
- Bergström, C.; Persson, M.; Mogren, I. Pregnancy-related low back pain and pelvic girdle pain approximately 14 months after pregnancy—Pain status, self-rated health and family situation. BMC Pregnancy Childbirth 2014, 25, 48. [Google Scholar] [CrossRef]
- Bergström, C.; Persson, M.; Mogren, I. Sick leave and healthcare utilisation in women reporting pregnancy related low back pain and/or pelvic girdle pain at 14 months postpartum. Chiropr. Man. Therap. 2016, 15, 7. [Google Scholar] [CrossRef] [PubMed]
- Bergström, C. Prevalence and predictors of persistent pelvic girdle pain 12 years postpartum. BMC Musculoskelet. Disord. 2017, 18, 399. [Google Scholar] [CrossRef] [PubMed]
- Bjelland, E.K.; Stuge, B.; Engdahl, B.; Eberhard-Gran, M. The effect of emotional distress on persistent pelvic girdle pain after delivery: A longitudinal population study. BJOG 2013, 120, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Robinson, H.S.; Mengshoel, A.M.; Veierød, M.B.; Vøllestad, N. Pelvic girdle pain: Potential risk factors in pregnancy in relation to disability and pain intensity three months postpartum. Man. Ther. 2010, 15, 522–528. [Google Scholar] [CrossRef]
- Gausel, A.M.; Kjærmann, I.; Malmqvist, S.; Dalen, I.; Larsen, J.P.; Økland, I. Pelvic girdle pain 3-6 months after delivery in an unselected cohort of Norwegian women. Eur. Spine J. 2016, 25, 1953–1959. [Google Scholar] [CrossRef] [PubMed]
- Robinson, H.S.; Mengshoel, A.M.; Bjelland, E.K.; Vøllestad, N.K. Pelvic girdle pain, clinical tests and disability in late pregnancy. Man. Ther. 2010, 15, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Robinson, H.S.; Vøllestad, N.K.; Veierød, M.B. Clinical course of pelvic girdle pain postpartum—Impact of clinical findings in late pregnancy. Man. Ther. 2014, 19, 190–196. [Google Scholar] [CrossRef]
- Ceprnja, D.; Chipchase, L.; Fahey, P.; Liamputtong, P.; Gupta, A. Prevalence and Factors Associated with Pelvic Girdle Pain During Pregnancy in Australian Women: A Cross-Sectional Study. Spine 2021, 15, 944–949. [Google Scholar] [CrossRef]
- Lindgren, A.; Kristiansson, P. Finger joint laxity, number of previous pregnancies and pregnancy induced back pain in a cohort study. BMC Pregnancy Childbirth 2014, 6, 61. [Google Scholar] [CrossRef]
- Bergström, C.; Persson, M.; Mogren, I. Psychosocial and behavioural characteristics in women with pregnancy-related lumbopelvic pain 12 years postpartum. Chiropr. Man. Therap. 2019, 13, 34. [Google Scholar] [CrossRef]
- Lardon, E.; St-Laurent, A.; Babineau, V.; Descarreaux, M.; Ruchat, S.M. Lumbopelvic pain, anxiety, physical activity and mode of conception: A prospective cohort study of pregnant women. BMJ Open. 2018, 8, e022508. [Google Scholar] [CrossRef] [PubMed]
- Röst, C.C.; Jacqueline, J.; Kaiser, A.; Verhagen, A.P.; Koes, B.W. Prognosis of women with pelvic pain during pregnancy: A long-term follow-up study. Acta Obstet. Gynecol. Scand. 2006, 85, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Fernando, M.; Nilsson-Wikmar, L.; Olsson, C.B. Fear-avoidance beliefs: A predictor for postpartum lumbopelvic pain. Physiother. Res. Int. 2020, 25, e1861. [Google Scholar] [CrossRef] [PubMed]
- Munro, A.; George, R.B.; Chorney, J.; Snelgrove-Clarke, E.; Rosen, N.O. Prevalence and Predictors of Chronic Pain in Pregnancy and Postpartum. J. Obstet. Gynaecol. Can. 2017, 39, 734–741. [Google Scholar] [CrossRef]
- Tang, X.; Gong, L.; Shi, Y.; An, X.; Yi, P.; Tan, M. Personality traits predict regression of pelvic girdle pain after pregnancy: A longitudinal follow-up study. BMC Pregnancy Childbirth 2021, 21, 353. [Google Scholar]
- Kovacs, F.M.; Garcia, E.; Royuela, A.; González, L.; Abraira, V. Spanish Back Pain Research Network. Prevalence and factors associated with low back pain and pelvic girdle pain during pregnancy: A multicenter study conducted in the Spanish National Health Service. Spine 2012, 37, 1516–1533. [Google Scholar] [CrossRef]
- Bakker, E.; van Nimwegen-Matzinger, C.W.; Ekkel-van der Voorden, W.; Nijkamp, M.D.; Völlink, T. Psychological determinants of pregnancy-related lumbopelvic pain: A prospective cohort study. Acta Obstet. Gynecol. Scand. 2013, 92, 797–803. [Google Scholar] [CrossRef]
- Hayden, J.A.; Van Der Windt, D.A.; Cartwright, J.L.; Cote, P.; Bombardier, C. Assessing bias in studies of prognostic factors. Ann. Intern. Med. 2013, 158, 280–286. [Google Scholar] [CrossRef]
- Robinson, H.S.; Veierød, M.B.; Mengshoel, A.M.; Vøllestad, N.K. Pelvic girdle pain—Associations between risk factors in early pregnancy and disability or pain intensity in late pregnancy: A prospective cohort study. BMC Musculoskelet. Disord. 2010, 11, 91. [Google Scholar] [CrossRef]
- Rashidi Fakari, F.; Simbar, M.; Saei Ghare Naz, M. The Relationship between Fear-Avoidance Beliefs and Pain in Pregnant Women with Pelvic Girdle Pain: A Cross-Sectional Study. Int. J. Community Based Nurs. Midwifery 2018, 6, 305–313. [Google Scholar]
- Dørheim, S.K.; Bjorvatn, B.; Eberhard-Gran, M. Insomnia and depressive symptoms in late pregnancy: A population-based study. Behav. Sleep Med. 2012, 10, 152–166. [Google Scholar] [CrossRef] [PubMed]
- Gutke, A.; Josefsson, A.; Oberg, B. Pelvic girdle pain and lumbar pain in relation to postpartum depressive symptoms. Spine 2007, 32, 1430–1436. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, N.; Kitagaki, K.; Perrein, E.; Tsuboi, Y.; Ebina, A.; Kondo, Y.; Murata, S.; Isa, T.; Okumura, M.; Kawaharada, R.; et al. Association between Excessive Weight Gain During Pregnancy and Persistent Low Back and Pelvic Pain After Delivery. Spine 2020, 45, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Gluppe, S.; Ellström Engh, M.; Kari, B. Women with diastasis recti abdominis might have weaker abdominal muscles and more abdominal pain, but no higher prevalence of pelvic floor disorders, low back and pelvic girdle pain than women without diastasis recti abdominis. Physiotherapy 2021, 111, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Clinton, S.C.; Newell, A.; Downey, P.A.; Ferreira, K. Pelvic girdle pain in the antepartum population: Physical therapy clinical practice guidelines linked to the international classification of functioning, disability, and health from the Section on Women’s Health and the Orthopaedic Section of the American Physical Therapy Association. J. Women’s Health Phys. Ther. 2017, 41, 102–125. [Google Scholar]
- Palsson, T.S.; Beales, D.; Slater, H.; O’Sullivan, P.; Graven-Nielsen, T. Pregnancy is characterized by widespread deep-tissue hypersensitivity independent of lumbopelvic pain intensity, a facilitated response to manual orthopedic tests, and poorer self-reported health. J. Pain 2015, 16, 270–282. [Google Scholar] [CrossRef]
- Kamaleri, Y.; Natvig, B.; Ihlebaek, C.M.; Benth, J.S.; Bruusgaard, D. Change in the number of musculoskeletal pain sites: A 14-year prospective study. Pain 2009, 141, 25–30. [Google Scholar] [CrossRef]
- Finan, P.H.; Goodin, B.R.; Smith, M.T. The association of sleep and pain: An update and a path forward. J. Pain 2013, 14, 1539–1552. [Google Scholar] [CrossRef]
- Kjeldgaard, H.K.; Eberhard-Gran, M.; Benth, J.Š.; Vikanes, Å.V. Hyperemesis gravidarum and the risk of emotional distress during and after pregnancy. Arch. Womens Ment. Health 2017, 20, 747–756. [Google Scholar] [CrossRef]
- Albert, H.B.; Godskesen, M.; Korsholm, L.; Westergaard, J.G. Risk factors in developing pregnancy-related pelvic girdle pain. Acta Obstet. Gynecol. Scand. 2006, 85, 539–544. [Google Scholar] [CrossRef]
- Beales, D.; Lutz, A.; Thompson, J.; Wand, B.M.; O’Sullivan, P. Disturbed body perception, reduced sleep, and kinesiophobia in subjects with pregnancy-related persistent lumbopelvic pain and moderate levels of disability: An exploratory study. Man. Ther. 2016, 21, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Sivertsen, B.; Lallukka, T.; Petrie, K.J.; Steingrímsdóttir, Ó.A.; Stubhaug, A.; Nielsen, C.S. Sleep and pain sensitivity in adults. Pain 2015, 156, 1433–1439. [Google Scholar] [CrossRef] [PubMed]
- Wuytack, F.; O’Donovan, M. Outcomes and outcomes measurements used in intervention studies of pelvic girdle pain and lumbopelvic pain: A systematic review. Chiropr. Man. Therap. 2019, 27, 62. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, A.; Gamada, K. Altered musculoskeletal mechanics as risk factors for postpartum pelvic girdle pain: A literature review. J. Phys. Ther. Sci. 2019, 31, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Remus, A.; Smith, V.; Gutke, A.; Mena, J.J.S.; Mørkved, S.; Wikmar, L.N.; Öberg, B.; Olsson, C.; Robinson, H.S.; Stuge, B.; et al. A core outcome set for research and clinical practice in women with pelvic girdle pain: PGP-COS. PLoS ONE 2021, 16, e0247466. [Google Scholar] [CrossRef]
- Fagevik Olsén, M.; Elden, H.; Gutke, A. Evaluation of self-administered tests for pelvic girdle pain in pregnancy. BMC Musculoskelet. Disord. 2014, 15, 138. [Google Scholar] [CrossRef]
- Pennick, V.; Liddle, S.D. Interventions for preventing and treating pelvic and back pain in pregnancy. Cochrane Database Syst. Rev. 2013, 1, CD001139. [Google Scholar]
- Gutke, A.; Sundfeldt, K.; De Baets, L. Lifestyle and Chronic Pain in the Pelvis: State of the Art and Future Directions. J. Clin. Med. 2021, 10, 5397. [Google Scholar] [CrossRef]
| Articles Included or Excluded | Inclusion | Exclusion Reason |
|---|---|---|
| Sjodahl, 2013 [13] | No | Lack of primary outcome (VAS). Baseline time to follow-up does not satisfy our criteria. |
| Van den Berg, 2012 [14] | No | Population from clinical trial. |
| Albert, 2001 [15] | Yes | |
| Olsson, 2012 A [16] | No | Primary outcome: catastrophization. |
| Eisenach, 2008 [17] | No | First follow-up 8 weeks postpartum. |
| Vollestad, 2009 [18] | No | Population from clinical trial. |
| Elden, 2016 [2] | No | Population from clinical trial. Baseline time to follow-up not clear. |
| Beales, 2018 [19] | Yes | |
| Bergström, 2014 [20] | Yes | |
| Bergström, 2016 [21] | No | Primary outcomes do not satisfy our criteria. |
| Bergström, 2017 [22] | Yes | |
| Bjelland, 2012 [23] | No | Primary outcome evaluated like numbers of painful points. |
| Robinson, 2010 B [24] | Yes | |
| Gausel, 2015 [25] | Yes | |
| Olsson, 2012 B [16] | Yes | |
| Robinson, 2010 A [26] | No | Lack of postpartum follow-up. |
| Robinson, 2014 C [27] | Yes | |
| Gausel, 2020 [11] | No | Follow-up does not satisfy our criteria. |
| Ceprnja, 2021 [28] | No | Cross-sectional design. |
| Lindgren, 2014 [29] | No | Unclear terminology (back pain). |
| Bergström, 2019 [30] | No | Cross-sectional design. |
| Lardon, 2018 [31] | No | Primary and secondary outcomes do not satisfy our criteria. |
| Rost, 2006 [32] | No | Therapeutical intervention. |
| Fernando, 2020 [33] | Yes | |
| Munro, 2017 [34] | No | Primary outcome body pain, not specific for PPGP. Full text in French. |
| Xiangsheng, 2021 [35] | Yes | |
| Kovacs, 2012 [36] | No | Cross-sectional design. |
| Bakker, 2013 [37] | No | Lack of postpartum follow-up. |
| Assessment Risk of bias Included Articles through QUIPS Tool | |||||||
|---|---|---|---|---|---|---|---|
| Articles | Study Participation | Study Attrition | Predictive Factor Measurement | Outcome Measurement | Study Confounding | Statistical Analysis and Reporting | Overall |
| Albert, 2001 [15] | − | − | +/− | − | + | +/− | Moderate risk of bias |
| Beales, 2018 [19] | − | − | − | − | + | − | Low risk of bias |
| Bergström, 2014 [20] | − | + | +/− | − | +/− | − | Moderate risk of bias |
| Bergström, 2017 [22] | − | − | − | − | + | − | Low risk of bias |
| Robinson, 2010 [24] | +/− | + | − | − | + | − | Moderate risk of bias |
| Gausel, 2015 [25] | − | + | − | − | + | − | Low risk of bias |
| Olsson, 2012 [16] | − | +/− | − | − | − | − | Low risk of bias |
| Robinson, 2014 [27] | − | − | +/− | − | +/− | − | Low risk of bias |
| Fernando, 2020 [33] | +/− | + | − | +/− | − | − | Moderate risk of bias |
| Xiangsheng, 2021 [35] | +/− | + | − | − | +/− | − | Moderate risk of bias |
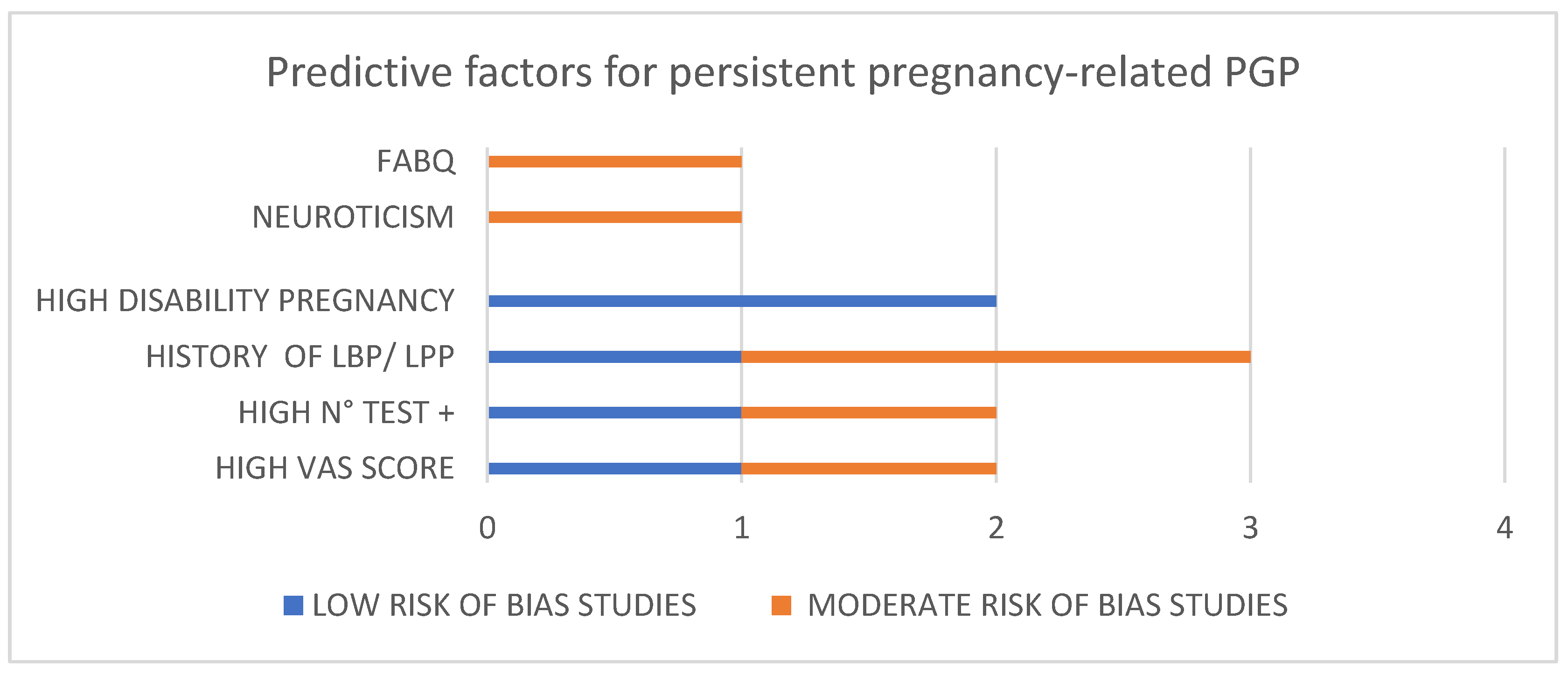
| Prognostic Factor | OR/RR | p Value | References |
|---|---|---|---|
| Pain | |||
| High pain (VAS ≥ 6) | RR = 1.6 | p < 0.05 | Albert, 2001 [15] |
| High sacral PPT | RR = 3.24 | p = 0.008 (personal communication) | Bergström, 2014 [20] |
| Widespread pain | Spearman rho = −0.384 | p = 0.040 | Beales, 2018 [19] |
| OR = 2.03 | p = 0.03 | ||
| Provocation tests | |||
| High number of +test | RR = 10.7 (>16 + response) | p < 0.001 | Albert, 2001 [15] |
| OR = 5.0 (6–8 + test) | p = 0.002 | Robinson, 2010 [24] | |
| Disability | |||
| High pregnancy disability | |||
| OR = 4.03 | p = 0.08 | Bergström, 2017 [22] | |
| OR = 5.2 | p < 0.002 | Gausel, 2015 [25] | |
| HR = 2.14 | p = 0.072 | Olsson, 2012 [16] | |
| LPP/NP/TP | |||
| OR = 2.47 | p < 0.030 | Bergström, 2014 [20] | |
| History LPP | Spearman rho = 0.09 | p < 0.05 | Robinson, 2010 [24] |
| OR = 2.8 | p = 0.017 | Gausel, 2015 [25] | |
| History NP/TP | |||
| OR = 2.50 | p = 0.002 | Bergström, 2017 [22] | |
| Fear | |||
| High fear avoidance | OR = 1.06 | p = 0.03 | Fernando, 2020 [33] |
| Behavior | |||
| Neuroticism | OR = 2.03 | p < 0.001 | Xiangsheng, 2021 [35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burani, E.; Marruganti, S.; Giglioni, G.; Bonetti, F.; Ceron, D.; Cozzi Lepri, A. Predictive Factors for Pregnancy-Related Persistent Pelvic Girdle Pain (PPGP): A Systematic Review. Medicina 2023, 59, 2123. https://doi.org/10.3390/medicina59122123
Burani E, Marruganti S, Giglioni G, Bonetti F, Ceron D, Cozzi Lepri A. Predictive Factors for Pregnancy-Related Persistent Pelvic Girdle Pain (PPGP): A Systematic Review. Medicina. 2023; 59(12):2123. https://doi.org/10.3390/medicina59122123
Chicago/Turabian StyleBurani, Elisa, Sharon Marruganti, Gloria Giglioni, Francesca Bonetti, Daniele Ceron, and Alessandro Cozzi Lepri. 2023. "Predictive Factors for Pregnancy-Related Persistent Pelvic Girdle Pain (PPGP): A Systematic Review" Medicina 59, no. 12: 2123. https://doi.org/10.3390/medicina59122123






