Coronary Microvascular Dysfunction and Hypertension: A Bond More Important than We Think
Abstract
:1. Introduction
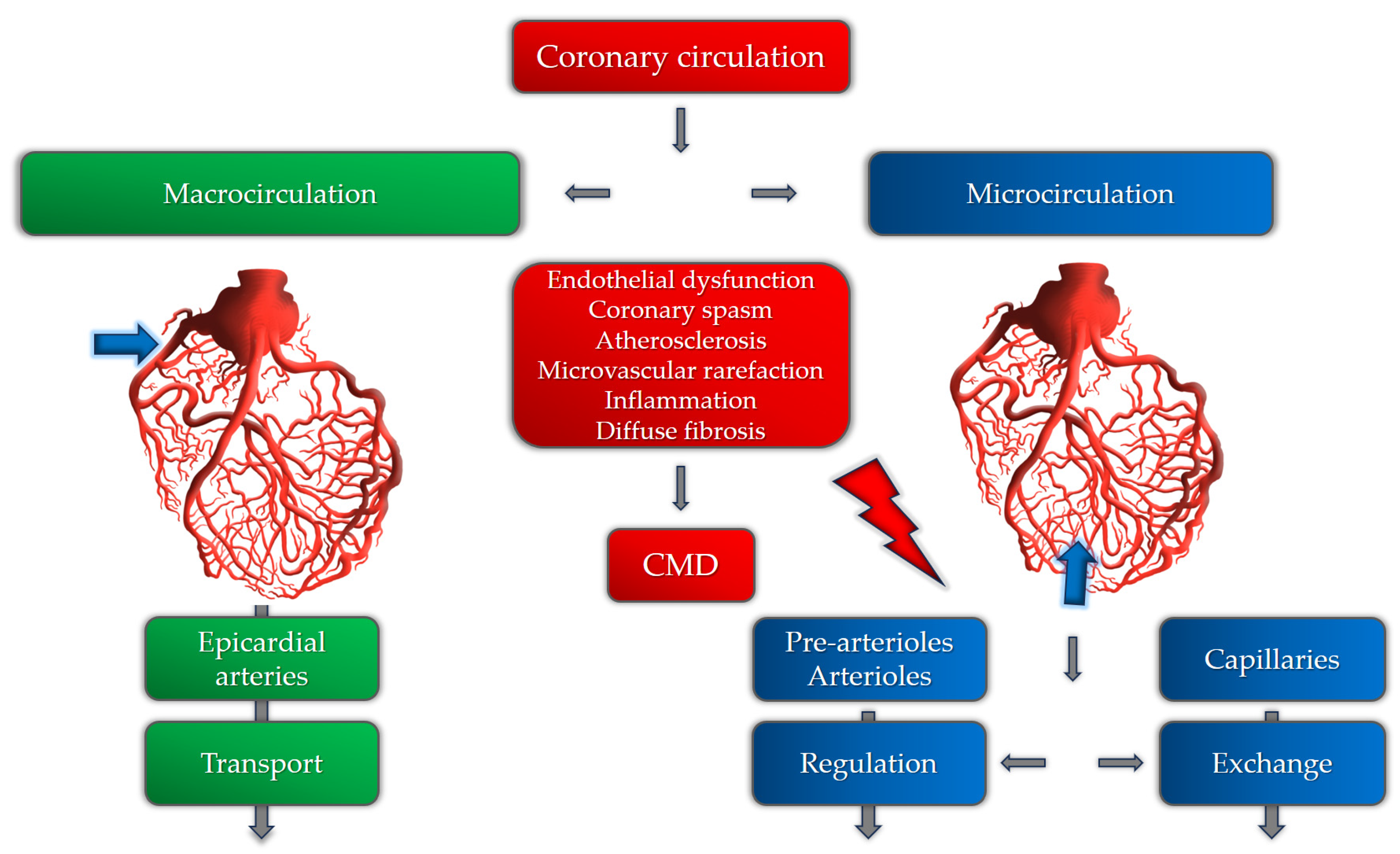
2. Pathogenetic Mechanisms of Coronary Microvascular Dysfunction
Microvascular Angina and Endothelial Dysfunction
3. Additional Risk Factors
3.1. Sex-Related Differences in Patients with Coronary Microvascular Dysfunction and Hypertension
3.2. Metabolic Syndrome
3.3. Obesity
3.4. Diabetes Mellitus
3.5. Hypercholesterolemia
3.6. Obstructive Sleep Apnea
3.7. Smoking
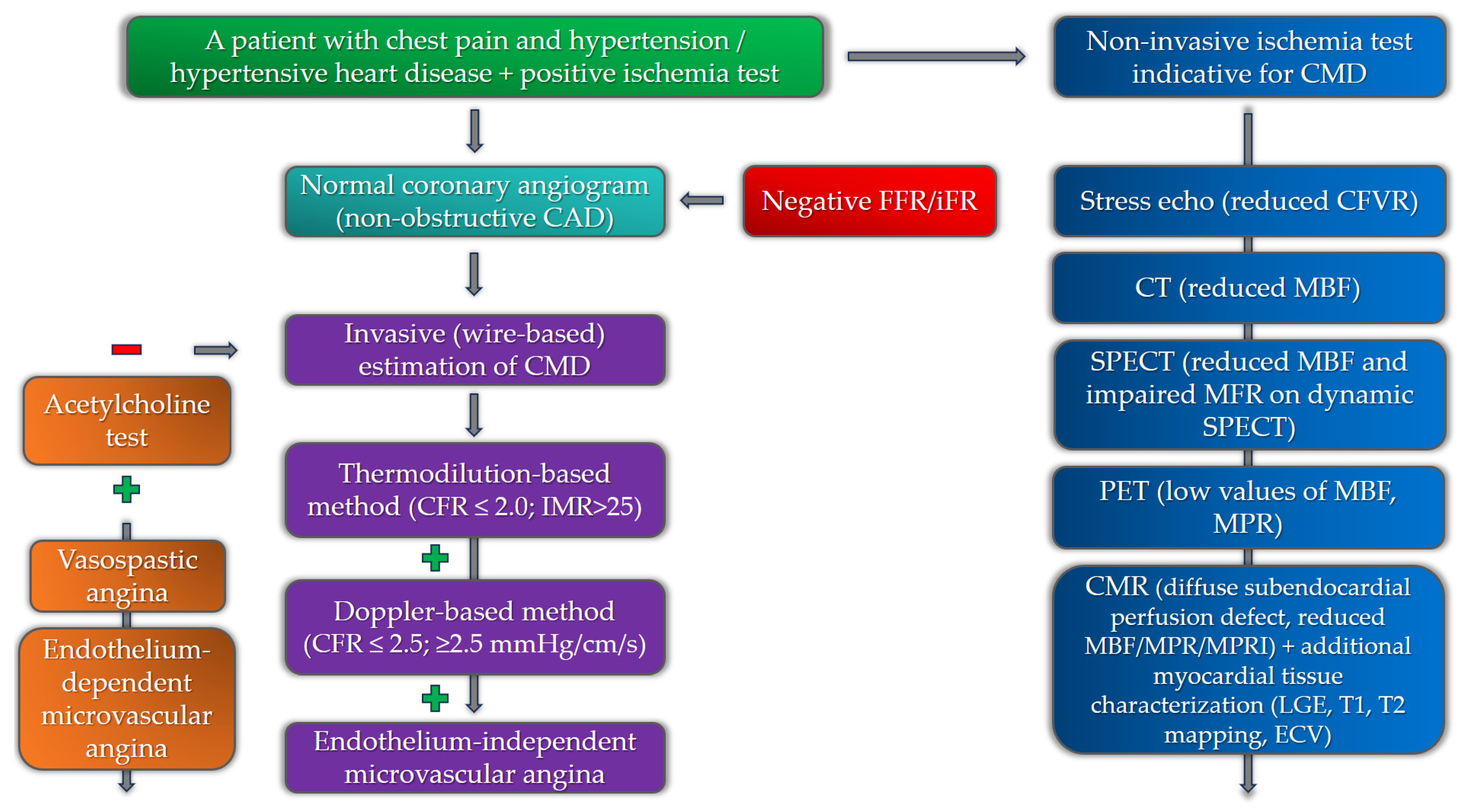
4. Diagnostics for Coronary Microvascular Dysfunction in Patients with Hypertension
4.1. Non-Invasive Diagnostics
4.1.1. Echocardiography
4.1.2. Computerized Tomography (CT)
4.1.3. Single-Photon Emission Computed Tomography (SPECT)
4.1.4. Positron Emission Tomography (PET)
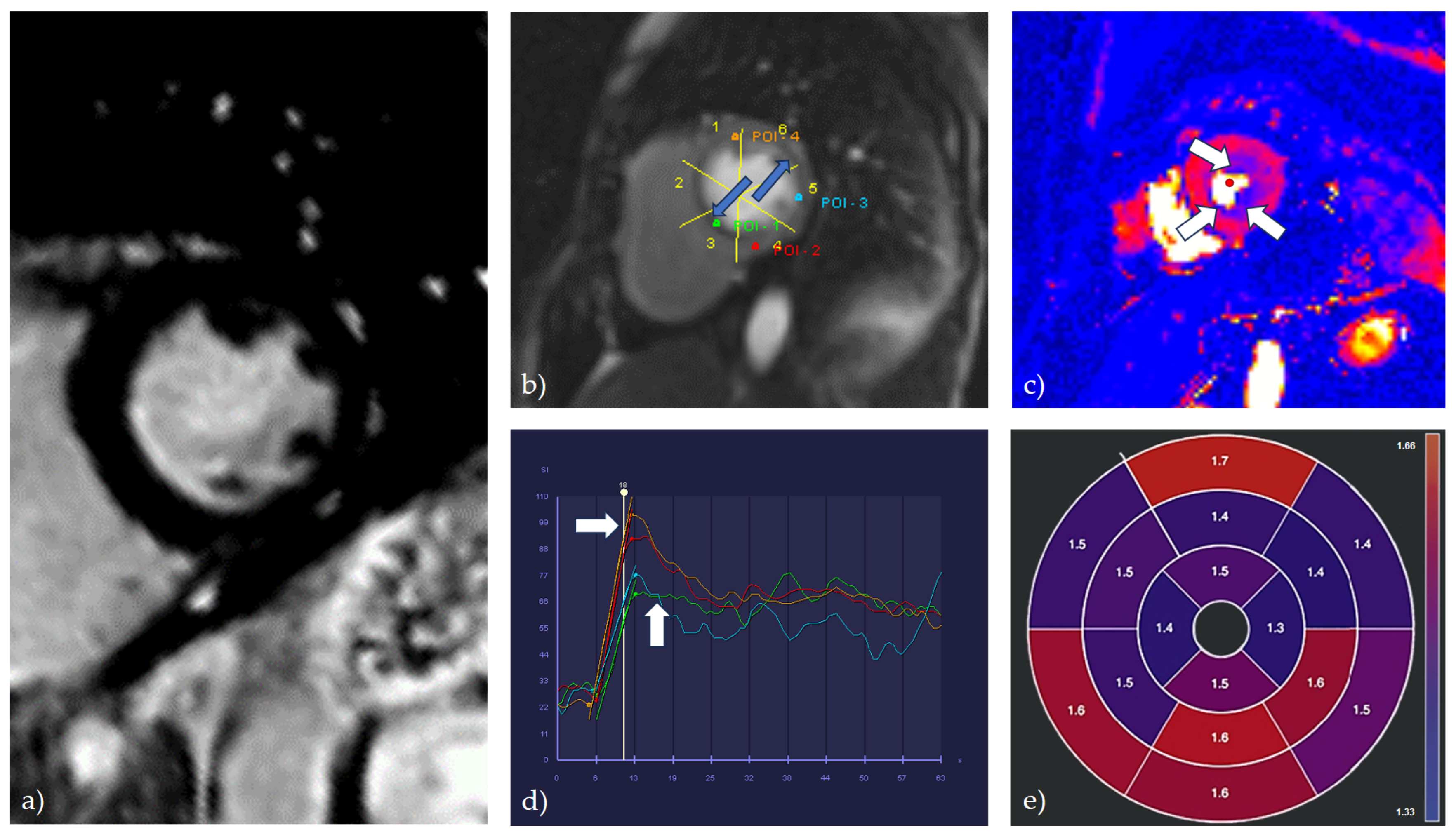
4.1.5. Cardiac Magnetic Resonance (CMR)
4.2. Invasive Diagnostics
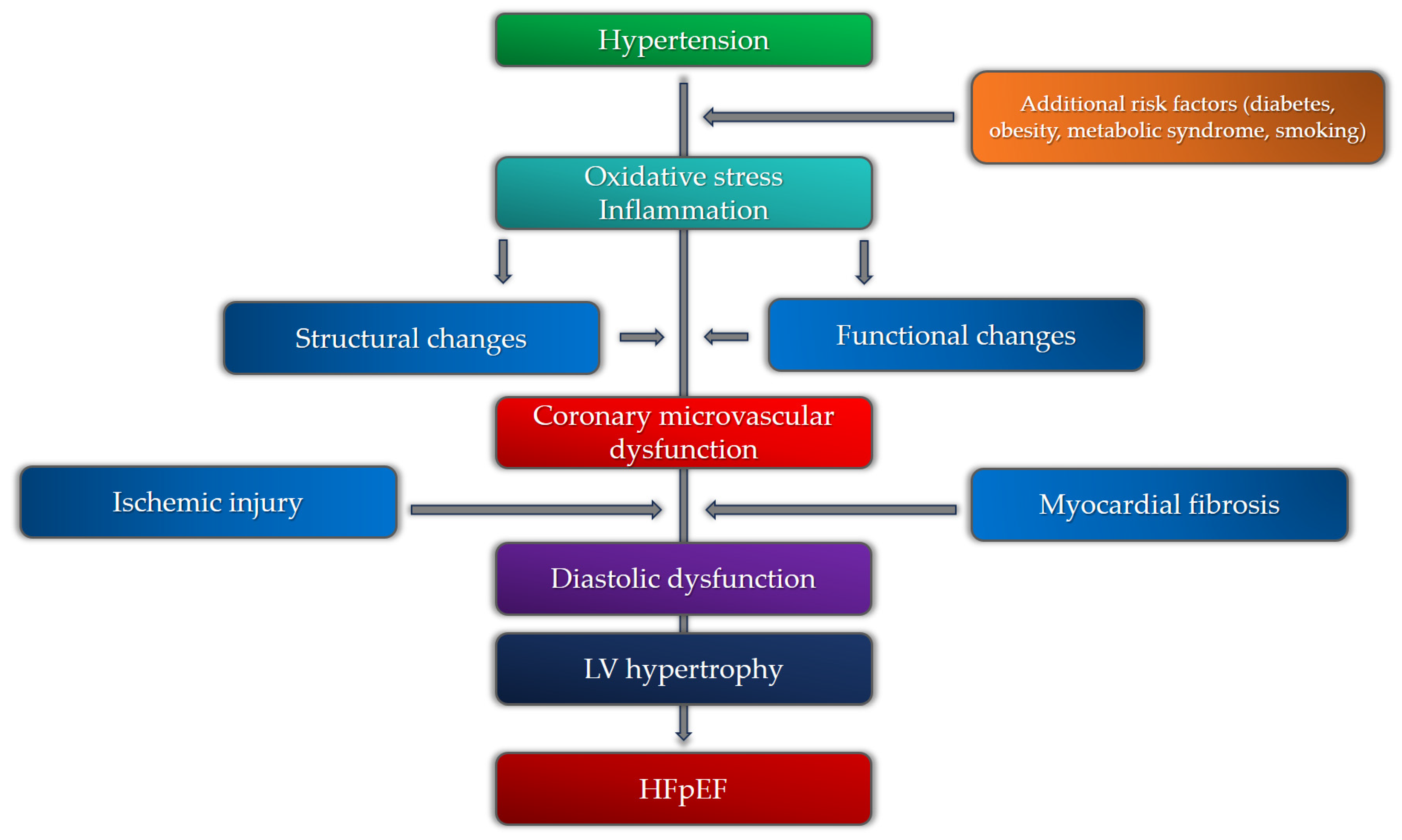
5. Coronary Microvascular Dysfunction, Hypertension, and HFpEF
6. Coronary Microvascular Dysfunction, Hypertension, and Atrial Fibrillation
7. Management of Coronary Microvascular Dysfunction in Patients with Hypertension
8. Prognosis
9. Future Perspectives
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, B.; Perel, P.; Mensah, G.A.; Ezzati, M. Global epidemiology, health burden and effective interventions for elevated blood pressure and hypertension. Nat. Rev. Cardiol. 2021, 18, 785–802. [Google Scholar] [CrossRef]
- Vasan, R.S.; Song, R.J.; Xanthakis, V.; Beiser, A.; DeCarli, C.; Mitchell, G.F.; Seshadri, S. Hypertension-Mediated Organ Damage: Prevalence, Correlates, and Prognosis in the Community. Hypertension 2022, 79, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Benas, D.; Triantafyllidi, H.; Birmpa, D.; Fambri, A.; Schoinas, A.; Thymis, I.; Kostelli, G.; Ikonomidis, I. Hypertension-Mediated Organ Damage in Young Patients with First-Diagnosed And Never Treated Systolic Hypertension. Curr. Vasc. Pharmacol. 2023, 21, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wei, J.; AlBadri, A.; Zarrini, P.; Merz, C.N.B. Coronary Microvascular Dysfunction—Epidemiology, Pathogenesis, Prognosis, Diagnosis, Risk Factors and Therapy. Circ. J. 2016, 81, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Bradley, C.; Berry, C. Definition and epidemiology of coronary microvascular disease. J. Nucl. Cardiol. 2022, 29, 1763–1775. [Google Scholar] [CrossRef]
- Vancheri, F.; Longo, G.; Vancheri, S.; Henein, M. Coronary Microvascular Dysfunction. J. Clin. Med. 2020, 9, 2880. [Google Scholar] [CrossRef]
- Godo, S.; Takahashi, J.; Yasuda, S.; Shimokawa, H. Endothelium in Coronary Macrovascular and Microvascular Diseases. J. Cardiovasc. Pharmacol. 2021, 78 (Suppl. S6), S19–S29. [Google Scholar] [CrossRef]
- Labazi, H.; Trask, A.J. Coronary microvascular disease as an early culprit in the pathophysiology of diabetes and metabolic syndrome. Pharmacol. Res. 2017, 123, 114–121. [Google Scholar] [CrossRef]
- Horton, W.B.; Barrett, E.J. Microvascular Dysfunction in Diabetes Mellitus and Cardiometabolic Disease. Endocr. Rev. 2021, 42, 29–55. [Google Scholar] [CrossRef]
- Kibel, A.; Selthofer-Relatic, K.; Drenjancevic, I.; Bacun, T.; Bosnjak, I.; Kibel, D.; Gros, M. Coronary microvascular dysfunction in diabetes mellitus. J. Int. Med. Res. 2017, 45, 1901–1929. [Google Scholar] [CrossRef]
- Lee, M.P.; Glynn, R.J.; Schneeweiss, S.; Lin, K.J.; Patorno, E.; Barberio, J.; Levin, R.; Evers, T.; Wang, S.V.; Desai, R.J. Risk Factors for Heart Failure with Preserved or Reduced Ejection Fraction Among Medicare Beneficiaries: Application of Competing Risks Analysis and Gradient Boosted Model. Clin. Epidemiol. 2020, 12, 607–616. [Google Scholar] [CrossRef]
- Paulus, W.J.; Tschöpe, C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J. Am. Coll. Cardiol. 2013, 62, 263–271. [Google Scholar] [CrossRef]
- Crea, F.; Montone, R.A.; Rinaldi, R. Pathophysiology of Coronary Microvascular Dysfunction. Circ. J. 2022, 86, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- Masi, S.; Rizzoni, D.; Taddei, S.; Widmer, R.J.; Montezano, A.C.; Lüscher, T.F.; Schiffrin, E.L.; Touyz, R.M.; Paneni, F.; Lerman, A.; et al. Assessment and pathophysiology of microvascular disease: Recent progress and clinical implications. Eur. Heart J. 2021, 42, 2590–2604. [Google Scholar] [CrossRef] [PubMed]
- Suda, A.; Takahashi, J.; Hao, K.; Kikuchi, Y.; Shindo, T.; Ikeda, S.; Sato, K.; Sugisawa, J.; Matsumoto, Y.; Miyata, S.; et al. Coronary Functional Abnormalities in Patients with Angina and Nonobstructive Coronary Artery Disease. J. Am. Coll. Cardiol. 2019, 74, 2350–2360. [Google Scholar] [CrossRef] [PubMed]
- Sorop, O.; Heinonen, I.; van Kranenburg, M.; van de Wouw, J.; de Beer, V.J.; Nguyen, I.T.N.; Octavia, Y.; van Duin, R.W.B.; Stam, K.; van Geuns, R.-J.; et al. Multiple common comorbidities produce left ventricular diastolic dysfunction associated with coronary microvascular dysfunction, oxidative stress, and myocardial stiffening. Cardiovasc. Res. 2018, 114, 954–964. [Google Scholar] [CrossRef]
- Kwiecinski, J.; Lennen, R.J.; Gray, G.A.; Borthwick, G.; Boswell, L.; Baker, A.H.; Newby, D.E.; Dweck, M.R.; Jansen, M.A. Progression and regression of left ventricular hypertrophy and myocardial fibrosis in a mouse model of hypertension and concomitant cardiomyopathy. J. Cardiovasc. Magn. Reson. 2020, 22, 57. [Google Scholar] [CrossRef] [PubMed]
- De Moraes, R.; Tibirica, E. Early Functional and Structural Microvascular Changes in Hypertension Related to Aging. Curr. Hypertens. Rev. 2017, 13, 24–32. [Google Scholar] [CrossRef]
- Hermann, M.; Flammer, A.; Lüscher, T.F. Nitric oxide in hypertension. J. Clin. Hypertens. 2006, 8, 17–29. [Google Scholar] [CrossRef]
- Da Silva, G.M.; da Silva, M.C.; Nascimento, D.V.G.; Lima Silva, E.M.; Gouvêa, F.F.F.; de França Lopes, L.G.; Araújo, A.V.; Ferraz Pereira, K.N.; de Queiroz, T.M. Nitric Oxide as a Central Molecule in Hypertension: Focus on the Vasorelaxant Activity of New Nitric Oxide Donors. Biology 2021, 10, 1041. [Google Scholar] [CrossRef]
- Montezano, A.C.; Touyz, R.M. Reactive oxygen species, vascular Noxs, and hypertension: Focus on translational and clinical research. Antioxid. Redox Signal. 2014, 20, 164–182. [Google Scholar] [CrossRef] [PubMed]
- Brandt, M.M.; Cheng, C.; Merkus, D.; Duncker, D.J.; Sorop, O. Mechanobiology of Microvascular Function and Structure in Health and Disease: Focus on the Coronary Circulation. Front. Physiol. 2021, 12, 771960. [Google Scholar] [CrossRef] [PubMed]
- Pries, A.R.; Badimon, L.; Bugiardini, R.; Camici, P.G.; Dorobantu, M.; Duncker, D.J.; Escaned, J.; Koller, A.; Piek, J.J.; de Wit, C. Coronary vascular regulation, remodelling, and collateralization: Mechanisms and clinical implications on behalf of the working group on coronary pathophysiology and microcirculation. Eur. Heart J. 2015, 36, 3134–3146. [Google Scholar] [CrossRef] [PubMed]
- E Konst, R.; Guzik, T.J.; Kaski, J.-C.; Maas, A.H.E.M.; E Elias-Smale, S. The pathogenic role of coronary microvascular dysfunction in the setting of other cardiac or systemic conditions. Cardiovasc. Res. 2020, 116, 817–828. [Google Scholar] [CrossRef]
- Ding, J.; Wai, K.L.; McGeechan, K.; Ikram, M.K.; Kawasaki, R.; Xie, J.; Klein, R.; Klein, B.B.; Cotch, M.F.; Wang, J.J.; et al. Retinal vascular caliber and the development of hypertension: A meta-analysis of individual participant data. J. Hypertens. 2014, 32, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Dharmashankar, K.; Widlansky, M.E. Vascular endothelial function and hypertension: Insights and directions. Curr. Hypertens. Rep. 2010, 12, 448–455. [Google Scholar] [CrossRef]
- Goto, K.; Ohtsubo, T.; Kitazono, T. Endothelium-Dependent Hyperpolarization (EDH) in Hypertension: The Role of Endothelial Ion Channels. Int. J. Mol. Sci. 2018, 19, 315. [Google Scholar] [CrossRef]
- Kostov, K. The Causal Relationship between Endothelin-1 and Hypertension: Focusing on Endothelial Dysfunction, Arterial Stiffness, Vascular Remodeling, and Blood Pressure Regulation. Life 2021, 11, 986. [Google Scholar] [CrossRef]
- Hamilos, M.; Petousis, S.; Parthenakis, F. Interaction between platelets and endothelium: From pathophysiology to new therapeutic options. Cardiovasc. Diagn. Ther. 2018, 8, 568–580. [Google Scholar] [CrossRef] [PubMed]
- Aribas, E.; Roeters van Lennep, J.E.; E Elias-Smale, S.; Piek, J.J.; Roos, M.; Ahmadizar, F.; Arshi, B.; Duncker, D.J.; Appelman, Y.; Kavousi, M. Prevalence of microvascular angina among patients with stable symptoms in the absence of obstructive coronary artery disease: A systematic review. Cardiovasc. Res. 2022, 118, 763–771. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Fearon, W.F.; Honda, Y.; Tanaka, S.; Pargaonkar, V.; Fitzgerald, P.J.; Lee, D.P.; Stefanick, M.; Yeung, A.C.; Tremmel, J.A. Effect of Sex Differences on Invasive Measures of Coronary Microvascular Dysfunction in Patients with Angina in the Absence of Obstructive Coronary Artery Disease. JACC Cardiovasc. Interv. 2015, 8, 1433–1441. [Google Scholar] [CrossRef]
- Loperena, R.; Harrison, D.G. Oxidative Stress and Hypertensive Diseases. Med. Clin. N. Am. 2017, 101, 169–193. [Google Scholar] [CrossRef]
- Reynolds, H.R.; Merz, C.N.B.; Berry, C.; Samuel, R.; Saw, J.; Smilowitz, N.R.; de Souza, A.C.D.A.; Sykes, R.; Taqueti, V.R.; Wei, J. Coronary Arterial Function and Disease in Women with No Obstructive Coronary Arteries. Circ. Res. 2022, 130, 529–551. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, M.; Shufelt, C.; Mehta, P.K.; Gill, E.; Berman, D.S.; Li, D.; Sharif, B.; Li, N.; Merz, C.N.B.; Thomson, L.E. Cardiac risk factors and myocardial perfusion reserve in women with microvascular coronary dysfunction. Cardiovasc. Diagn. Ther. 2013, 3, 146–152. [Google Scholar] [CrossRef]
- Barnabas, O.; Wang, H.; Gao, X.-M. Role of estrogen in angiogenesis in cardiovascular diseases. J. Geriatr. Cardiol. 2013, 10, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Tunc, E.; Eve, A.A.; Madak-Erdogan, Z. Coronary Microvascular Dysfunction and Estrogen Receptor Signaling. Trends Endocrinol. Metab. 2020, 31, 228–238. [Google Scholar] [CrossRef]
- Pizzi, C.; Santarella, L.; Costa, M.G.; Manfrini, O.; Flacco, M.E.; Capasso, L.; Chiarini, S.; Di Baldassarre, A.; Manzoli, L. Pathophysiological mechanisms linking de-pression and atherosclerosis: An overview. J. Biol. Regul. Homeost. Agents 2012, 26, 775–782. [Google Scholar]
- Van der Meer, R.E.; Maas, A.H. The Role of Mental Stress in Ischaemia with No Obstructive Coronary Artery Disease and Coronary Vasomotor Disorders. Eur. Cardiol. Rev. 2021, 1, e37. [Google Scholar] [CrossRef] [PubMed]
- Mottillo, S.; Filion, K.B.; Genest, J.; Joseph, L.; Pilote, L.; Poirier, P.; Rinfret, S.; Schiffrin, E.L.; Eisenberg, M.J. The metabolic syndrome and cardiovascular risk: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2010, 56, 1113–1132. [Google Scholar] [CrossRef] [PubMed]
- Chilian, W.; Nystoriak, M.A.; Sisakian, H.; Ohanyan, V. Coronary microvascular disease during metabolic syndrome: What is known and unknown: Pathological consequences of redox imbalance for endothelial K+ channels. Int. J. Cardiol. 2020, 321, 18–19. [Google Scholar] [CrossRef]
- Sucato, V.; Madaudo, C.; Di Fazio, L.; Manno, G.; Vadalà, G.; Novo, S.; Evola, S.; Novo, G.; Galassi, A.R. Impact of Metabolic Syndrome on Coronary Microvascular Dysfunction: A Single Center Experience. Cardiol. Cardiovasc. Med. 2023, 07, 145–150. [Google Scholar] [CrossRef]
- Feng, C.; Abdu, F.A.; Mohammed, A.-Q.; Zhang, W.; Liu, L.; Yin, G.; Feng, Y.; Mohammed, A.A.; Mareai, R.M.; Lv, X.; et al. Prognostic impact of coronary microvascular dysfunction assessed by caIMR in overweight with chronic coronary syndrome patients. Front. Endocrinol. 2022, 13, 922264. [Google Scholar] [CrossRef]
- Mahmoud, I.; Dykun, I.; Kärner, L.; Hendricks, S.; Totzeck, M.; Al-Rashid, F.; Rassaf, T.; Mahabadi, A.A. Epicardial adipose tissue differentiates in patients with and without coronary microvascular dysfunction. Int. J. Obes. 2021, 45, 2058–2063. [Google Scholar] [CrossRef]
- Bajaj, N.S.; Osborne, M.T.; Gupta, A.; Tavakkoli, A.; Bravo, P.E.; Vita, T.; Bibbo, C.F.; Hainer, J.; Dorbala, S.; Blankstein, R.; et al. Coronary Microvascular Dysfunction and Cardiovascular Risk in Obese Patients. J. Am. Coll. Cardiol. 2018, 72, 707–717. [Google Scholar] [CrossRef]
- Sato, R.; von Haehling, S. Revisiting the obesity paradox in heart failure: What is the best anthropometric index to gauge obesity? Eur. Heart J. 2023, 44, 1154–1156. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, T.; Galiero, R.; Caturano, A.; Vetrano, E.; Loffredo, G.; Rinaldi, L.; Catalini, C.; Gjeloshi, K.; Albanese, G.; Di Martino, A.; et al. Coronary Microvascular Dysfunction in Diabetes Mellitus: Pathogenetic Mechanisms and Potential Therapeutic Options. Biomedicines 2022, 10, 2274. [Google Scholar] [CrossRef]
- Ormazabal, V.; Nair, S.; Elfeky, O.; Aguayo, C.; Salomon, C.; Zuñiga, F.A. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc. Diabetol. 2018, 17, 122. [Google Scholar] [CrossRef]
- Russo, I.; Frangogiannis, N.G. Diabetes-associated cardiac fibrosis: Cellular effectors, molecular mechanisms and therapeutic opportunities. J. Mol. Cell. Cardiol. 2016, 90, 84–93. [Google Scholar] [CrossRef]
- Zhang, W.; Singh, S.; Liu, L.; Mohammed, A.-Q.; Yin, G.; Xu, S.; Lv, X.; Shi, T.; Feng, C.; Jiang, R.; et al. Prognostic value of coronary microvascular dysfunction assessed by coronary angiography-derived index of microcirculatory resistance in diabetic patients with chronic coronary syndrome. Cardiovasc. Diabetol. 2022, 21, 222. [Google Scholar] [CrossRef]
- Gallinoro, E.; Paolisso, P.; Candreva, A.; Bermpeis, K.; Fabbricatore, D.; Esposito, G.; Bertolone, D.; Peregrina, E.F.; Munhoz, D.; Mileva, N.; et al. Microvascular Dysfunction in Patients with Type II Diabetes Mellitus: Invasive Assessment of Absolute Coronary Blood Flow and Microvascular Resistance Reserve. Front. Cardiovasc. Med. 2021, 8, 765071. [Google Scholar] [CrossRef]
- Emanuelsson, F.; Nordestgaard, B.G.; Tybjærg-Hansen, A.; Benn, M. Impact of LDL Cholesterol on Microvascular Versus Macrovascular Disease: A Mendelian Randomization Study. J. Am. Coll. Cardiol. 2019, 74, 1465–1476. [Google Scholar] [CrossRef]
- Padró, T.; Vilahur, G.; Badimon, L. Dyslipidemias and Microcirculation. Curr. Pharm. Des. 2018, 24, 2921–2926. [Google Scholar] [CrossRef]
- Avtaar Singh, S.S.; Nappi, F. Pathophysiology and Outcomes of Endothelium Function in Coronary Microvascular Diseases: A Systematic Review of Randomized Controlled Trials and Multicenter Study. Biomedicines 2022, 10, 3010. [Google Scholar] [CrossRef] [PubMed]
- Chen, H. Role of thromboxane A2 signaling in endothelium-dependent contractions of arteries. Prostaglandins Other Lipid Mediat. 2018, 134, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, P.A.; Goodwill, A.G.; James, M.E.; Brock, R.W.; Frisbee, J.C. Hypercholesterolemia and microvascular dysfunction: Interventional strategies. J. Inflamm. 2010, 7, 54. [Google Scholar] [CrossRef]
- Zdravkovic, M.; Popadic, V.; Klasnja, S.; Milic, N.; Rajovic, N.; Divac, A.; Manojlovic, A.; Nikolic, N.; Lukic, F.; Rasiti, E.; et al. Obstructive Sleep Apnea and Cardiovascular Risk: The Role of Dyslipidemia, Inflammation, and Obesity. Front. Pharmacol. 2022, 13, 898072. [Google Scholar] [CrossRef]
- Bozbas, S.S.; Eroglu, S.; Ozyurek, B.A.; Eyuboglu, F.O. Coronary flow reserve is impaired in patients with obstructive sleep apnea. Ann. Thorac. Med. 2017, 12, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Pittilo, M. Cigarette smoking, endothelial injury and cardiovascular disease. Int. J. Exp. Pathol. 2000, 81, 219–230. [Google Scholar] [CrossRef]
- Gullu, H.; Caliskan, M.; Ciftci, O.; Erdogan, D.; Topcu, S.; Yildirim, E.; Yildirir, A.; Muderrisoglu, H. Light cigarette smoking impairs coronary microvascular functions as severely as smoking regular cigarettes. Heart 2007, 93, 1274–1277. [Google Scholar] [CrossRef]
- Haig, C.; Carrick, D.; Carberry, J.; Mangion, K.; Maznyczka, A.; Wetherall, K.; McEntegart, M.; Petrie, M.C.; Eteiba, H.; Lindsay, M.; et al. Current Smoking and Prognosis After Acute ST-Segment Elevation Myocardial Infarction: New Pathophysiological Insights. JACC Cardiovasc. Imaging 2018, 12, 993–1003. [Google Scholar] [CrossRef]
- Lanza, G.A.; Morrone, D.; Pizzi, C.; Tritto, I.; Bergamaschi, L.; De Vita, A.; Villano, A.; Crea, F. Diagnostic approach for coronary microvascular dysfunction in patients with chest pain and no obstructive coronary artery disease. Trends Cardiovasc. Med. 2022, 32, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Fordyce, C.B.; Newby, D.E.; Douglas, P.S. Diagnostic Strategies for the Evaluation of Chest Pain: Clinical Implications from SCOT-HEART and PROMISE. J. Am. Coll. Cardiol. 2016, 67, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Carbone, A.; D’andrea, A.; Sperlongano, S.; Tagliamonte, E.; Mandoli, G.E.; Santoro, C.; Evola, V.; Bandera, F.; Morrone, D.; Malagoli, A.; et al. Echocardiographic assessment of coronary microvascular dysfunction: Basic concepts, technical aspects, and clinical settings. Echocardiography 2021, 38, 993–1001. [Google Scholar] [CrossRef]
- Schroder, J.; Prescott, E. Doppler Echocardiography Assessment of Coronary Microvascular Function in Patients with Angina and No Obstructive Coronary Artery Disease. Front. Cardiovasc. Med. 2021, 8, 723542. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.J.; Lam, C.S.P.; Svedlund, S.; Saraste, A.; Hage, C.; Tan, R.-S.; Beussink-Nelson, L.; Faxén, U.L.; Fermer, M.L.; Broberg, M.A.; et al. Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS-HFpEF. Eur. Heart J. 2018, 39, 3439–3450. [Google Scholar] [CrossRef] [PubMed]
- Völz, S.; Svedlund, S.; Andersson, B.; Li-Ming, G.; Rundqvist, B. Coronary flow reserve in patients with resistant hypertension. Clin. Res. Cardiol. 2016, 106, 151–157. [Google Scholar] [CrossRef]
- Clemmensen, T.S.; Christensen, M.; Løgstrup, B.B.; Kronborg, C.J.S.; Knudsen, U.B. Reduced coronary flow velocity reserve in women with previous pre-eclampsia: Link to increased cardiovascular disease risk. Ultrasound Obstet. Gynecol. 2020, 55, 786–792. [Google Scholar] [CrossRef]
- Gaibazzi, N.; Bergamaschi, L.; Pizzi, C.; Tuttolomondo, D. Resting global longitudinal strain and stress echocardiography to detect coronary artery disease burden. Eur. Heart J. Cardiovasc. Imaging 2023, 24, e86–e88. [Google Scholar] [CrossRef]
- Tagliamonte, E.; Sperlongano, S.; Montuori, C.; Riegler, L.; Scarafile, R.; Carbone, A.; Forni, A.; Radmilovic, J.; Di Vilio, A.; Astarita, R.; et al. Coronary microvascular dysfunction affects left ventricular global longitudinal strain response to dipyridamole stress echocardiography: A pilot study. Heart Vessel. 2023, 38, 470–477. [Google Scholar] [CrossRef]
- Jovanovic, I.; Tesic, M.; Giga, V.; Dobric, M.; Boskovic, N.; Vratonjic, J.; Orlic, D.; Gudelj, O.; Tomasevic, M.; Dikic, M.; et al. Impairment of coronary flow velocity reserve and global longitudinal strain in women with cardiac syndrome X and slow coronary flow. J. Cardiol. 2020, 76, 1–8. [Google Scholar] [CrossRef]
- Nieman, K.; Balla, S. Dynamic CT myocardial perfusion imaging. J. Cardiovasc. Comput. Tomogr. 2020, 14, 303–306. [Google Scholar] [CrossRef]
- Seitun, S.; Clemente, A.; De Lorenzi, C.; Benenati, S.; Chiappino, D.; Mantini, C.; Sakellarios, A.I.; Cademartiri, F.; Bezante, G.P.; Porto, I. Cardiac CT perfusion and FFRCTA: Pathophysiological features in ischemic heart disease. Cardiovasc. Diagn. Ther. 2020, 10, 1954–1978. [Google Scholar] [CrossRef] [PubMed]
- Ihdayhid, A.R.; Fairbairn, T.A.; Gulsin, G.S.; Tzimas, G.; Danehy, E.; Updegrove, A.; Jensen, J.M.; Taylor, C.A.; Bax, J.J.; Sellers, S.L.; et al. Cardiac computed tomography-derived coronary artery volume to myocardial mass. J. Cardiovasc. Comput. Tomogr. 2022, 16, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Van Rosendael, S.E.; van Rosendael, A.R.; Kuneman, J.H.; Patel, M.R.; Nørgaard, B.L.; Fairbairn, T.A.; Nieman, K.; Akasaka, T.; Berman, D.S.; Koweek, L.M.H.; et al. Coronary Volume to Left Ventricular Mass Ratio in Patients with Hypertension. Am. J. Cardiol. 2023, 199, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Djaïleb, L.; Riou, L.; Piliero, N.; Carabelli, A.; Vautrin, E.; Broisat, A.; Leenhardt, J.; Machecourt, J.; Fagret, D.; Vanzetto, G.; et al. SPECT myocardial ischemia in the absence of obstructive CAD: Contribution of the invasive assessment of microvascular dysfunction. J. Nucl. Cardiol. 2018, 25, 1017–1022. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Caobelli, F.; Che, W.; Huang, Y.; Zhang, Y.; Fan, X.; Hu, X.; Xu, C.; Fei, M.; Zhang, J.; et al. The prognostic value of CZT SPECT myocardial blood flow (MBF) quantification in patients with ischemia and no obstructive coronary artery disease (INOCA): A pilot study. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 1940–1953. [Google Scholar] [CrossRef]
- Mathew, R.C.; Bourque, J.M.; Salerno, M.; Kramer, C.M. Cardiovascular Imaging Techniques to Assess Microvascular Dysfunction. JACC Cardiovasc. Imaging 2020, 13, 1577–1590. [Google Scholar] [CrossRef]
- Zhou, W.; Brown, J.M.; Bajaj, N.S.; Chandra, A.; Divakaran, S.; Weber, B.; Bibbo, C.F.; Hainer, J.; Taqueti, V.R.; Dorbala, S.; et al. Hypertensive coronary microvascular dysfunction: A subclinical marker of end organ damage and heart failure. Eur. Heart J. 2020, 41, 2366–2375. [Google Scholar] [CrossRef]
- Patel, A.R.; Salerno, M.; Kwong, R.Y.; Singh, A.; Heydari, B.; Kramer, C.M. Stress Cardiac Magnetic Resonance Myocardial Perfusion Imaging: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2021, 78, 1655–1668. [Google Scholar] [CrossRef]
- Zdravkovic, M.; Klasnja, S.; Popovic, M.; Djuran, P.; Mrda, D.; Ivankovic, T.; Manojlovic, A.; Koracevic, G.; Lovic, D.; Popadic, V. Cardiac Magnetic Resonance in Hypertensive Heart Disease: Time for a New Chapter. Diagnostics 2022, 13, 137. [Google Scholar] [CrossRef]
- Liang, L.; Wang, X.; Yu, Y.; Zhang, Y.; Liu, J.; Chen, M.; Zhang, L.; Jiang, T. T1 Mapping and Extracellular Volume in Cardiomyopathy Showing Left Ventricular Hypertrophy: Differentiation between Hypertrophic Cardiomyopathy and Hypertensive Heart Disease. Int. J. Gen. Med. 2022, 15, 4163–4173. [Google Scholar] [CrossRef] [PubMed]
- Engblom, H.; Xue, H.; Akil, S.; Carlsson, M.; Hindorf, C.; Oddstig, J.; Hedeer, F.; Hansen, M.S.; Aletras, A.H.; Kellman, P.; et al. Fully quantitative cardiovascular magnetic resonance myocardial perfusion ready for clinical use: A comparison between cardiovascular magnetic resonance imaging and positron emission tomography. J. Cardiovasc. Magn. Reson. 2017, 19, 78. [Google Scholar] [CrossRef] [PubMed]
- Zorach, B.; Shaw, P.W.; Bourque, J.; Kuruvilla, S.; Balfour, P.C.; Yang, Y.; Mathew, R.; Pan, J.; Gonzalez, J.A.; Taylor, A.M.; et al. Quantitative cardiovascular magnetic resonance perfusion imaging identifies reduced flow reserve in microvascular coronary artery disease. J. Cardiovasc. Magn. Reson. 2018, 20, 14. [Google Scholar] [CrossRef]
- Thomson, L.E.; Wei, J.; Agarwal, M.; Haft-Baradaran, A.; Shufelt, C.L.; Mehta, P.K.; Gill, E.B.; Johnson, B.D.; Kenkre, T.; Handberg, E.M.; et al. Cardiac magnetic resonance myocardial perfusion reserve index is reduced in women with coronary microvascular dysfunction. A National Heart, Lung, and Blood Institute-sponsored study from the Women’s Ischemia Syndrome Evaluation. Circ. Cardiovasc. Imaging 2015, 8, e002481. [Google Scholar] [CrossRef]
- Rahman, H.; Scannell, C.M.; Demir, O.M.; Ryan, M.; McConkey, H.; Ellis, H.; Masci, P.G.; Perera, D.; Chiribiri, A. High-Resolution Cardiac Magnetic Resonance Imaging Techniques for the Identification of Coronary Microvascular Dysfunction. JACC Cardiovasc. Imaging 2021, 14, 978–986. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.; Kang, N.; Chung, J.; Gupta, A.R.; Parwani, P. Evaluation of Ischemia with No Obstructive Coronary Arteries (INOCA) and Contemporary Applications of Cardiac Magnetic Resonance (CMR). Medicina 2023, 59, 1570. [Google Scholar] [CrossRef] [PubMed]
- Scarsini, R.; Shanmuganathan, M.; De Maria, G.L.; Borlotti, A.; Kotronias, R.A.; Burrage, M.K.; Terentes-Printzios, D.; Langrish, J.; Lucking, A.; Fahrni, G.; et al. Coronary Microvascular Dysfunction Assessed by Pressure Wire and CMR After STEMI Predicts Long-Term Outcomes. JACC Cardiovasc. Imaging 2021, 14, 1948–1959. [Google Scholar] [CrossRef]
- Knott, K.D.; Seraphim, A.; Augusto, J.B.; Xue, H.; Chacko, L.; Aung, N.; Petersen, S.E.; Cooper, J.A.; Manisty, C.; Bhuva, A.N.; et al. The Prognostic Significance of Quantitative Myocardial Perfusion: An Artificial Intelligence Based Approach Using Perfusion Mapping. Circulation 2020, 141, 1282–1291. [Google Scholar] [CrossRef]
- Levelt, E.; Piechnik, S.K.; Liu, A.; Wijesurendra, R.S.; Mahmod, M.; Ariga, R.; Francis, J.M.; Greiser, A.; Clarke, K.; Neubauer, S.; et al. Adenosine stress CMR T1-mapping detects early microvascular dysfunction in patients with type 2 diabetes mellitus without obstructive coronary artery disease. J. Cardiovasc. Magn. Reson. 2017, 19, 81. [Google Scholar] [CrossRef]
- Travieso, A.; Jeronimo-Baza, A.; Faria, D.; Shabbir, A.; Mejia-Rentería, H.; Escaned, J. Invasive evaluation of coronary microvascular dysfunction. J. Nucl. Cardiol. 2022, 29, 2474–2486. [Google Scholar] [CrossRef]
- Mangiacapra, F.; Viscusi, M.M.; Verolino, G.; Paolucci, L.; Nusca, A.; Melfi, R.; Ussia, G.P.; Grigioni, F. Invasive Assessment of Coronary Microvascular Function. J. Clin. Med. 2021, 11, 228. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Wu, X.; Liu, H.; Zheng, D.; Xia, L. Index of microcirculatory resistance: State-of-the-art and potential applications in computational simulation of coronary artery disease. J. Zhejiang Univ. Sci. B 2022, 23, 123–140. [Google Scholar] [CrossRef] [PubMed]
- Fearon, W.F.; Kobayashi, Y. Invasive Assessment of the Coronary Microvasculature: The Index of Microcirculatory Resistance. Circ. Cardiovasc. Interv. 2017, 10, e005361. [Google Scholar] [CrossRef] [PubMed]
- Toya, T.; Nagatomo, Y.; Ikegami, Y.; Masaki, N.; Adachi, T. Coronary microvascular dysfunction in heart failure patients. Front. Cardiovasc. Med. 2023, 10, 1153994. [Google Scholar] [CrossRef] [PubMed]
- Dryer, K.; Gajjar, M.; Narang, N.; Lee, M.; Paul, J.; Shah, A.P.; Nathan, S.; Butler, J.; Davidson, C.J.; Fearon, W.F.; et al. Coronary microvascular dysfunction in patients with heart failure with preserved ejection fraction. Am. J. Physiol. Heart Circ. Physiol. 2018, 314, H1033–H1042. [Google Scholar] [CrossRef] [PubMed]
- Chirinos, J.A.; Mitchell, G.F.; Parise, H.; Benjamin, E.J.; Larson, M.G.; Keyes, M.J.; Vita, J.A.; Vasan, R.S.; Levy, D.; Hashimoto, J.; et al. Large Artery Stiffness, Microvascular Function, and Cardiovascular Risk. Circ. Cardiovasc. Imaging 2016, 9, e005903. [Google Scholar] [CrossRef] [PubMed]
- Ikonomidis, I.; Lekakis, J.; Papadopoulos, C.; Triantafyllidi, H.; Paraskevaidis, I.; Georgoula, G.; Tzortzis, S.; Revela, I.; Kremastinos, D.T. Incremental value of pulse wave velocity in the determination of coronary microcirculatory dysfunction in never-treated patients with essential hypertension. Am. J. Hypertens. 2008, 21, 806–813. [Google Scholar] [CrossRef]
- Sakalidis, A.; Dimitriadis, K.; Leontsinis, I.; Dri, E.; Mantzouranis, E.; Bora, M.; E Karanikola, A.; Iliakis, P.; Vlachakis, P.; Siafi, E.; et al. Increased arterial stiffness in patients with ischemia and no obstructive coronary artery disease. Eur. Heart J. 2023, 44, ehad655.2139. [Google Scholar] [CrossRef]
- Aursulesei Onofrei, V.; Ceasovschih, A.; Anghel, R.C.; Roca, M.; Marcu, D.T.M.; Adam, C.A.; Mitu, O.; Cumpat, C.; Mitu, F.; Crisan, A.; et al. Subendocardial Viability Ratio Predictive Value for Cardiovascular Risk in Hypertensive Patients. Medicina 2022, 59, 24. [Google Scholar] [CrossRef]
- Lin, X.; Wu, G.; Wang, S.; Huang, J. The prevalence of coronary microvascular dysfunction (CMD) in heart failure with pre-served ejection fraction (HFpEF): A systematic review and meta-analysis. Heart Fail Rev. 2023. [Google Scholar] [CrossRef]
- D’amario, D.; Migliaro, S.; Borovac, J.A.; Restivo, A.; Vergallo, R.; Galli, M.; Leone, A.M.; Montone, R.A.; Niccoli, G.; Aspromonte, N.; et al. Microvascular Dysfunction in Heart Failure with Preserved Ejection Fraction. Front. Physiol. 2019, 10, 1347. [Google Scholar] [CrossRef] [PubMed]
- Paulus, W.J.; Zile, M.R. From Systemic Inflammation to Myocardial Fibrosis: The Heart Failure with Preserved Ejection Fraction Paradigm Revisited. Circ. Res. 2021, 128, 1451–1467. [Google Scholar] [CrossRef]
- Sagris, M.; Theofilis, P.; Antonopoulos, A.S.; Oikonomou, E.; Paschaliori, C.; Galiatsatos, N.; Tsioufis, K.; Tousoulis, D. Inflammation in Coronary Microvascular Dysfunction. Int. J. Mol. Sci. 2021, 22, 13471. [Google Scholar] [CrossRef] [PubMed]
- Kanagala, P.; Arnold, J.R.; Singh, A.; Chan, D.C.S.; Cheng, A.S.H.; Khan, J.N.; Gulsin, G.S.; Yang, J.; Zhao, L.; Gupta, P.; et al. Characterizing heart failure with preserved and reduced ejection fraction: An imaging and plasma biomarker approach. PLoS ONE 2020, 15, e0232280. [Google Scholar] [CrossRef] [PubMed]
- Cornuault, L.; Rouault, P.; Duplàa, C.; Couffinhal, T.; Renault, M.-A. Endothelial Dysfunction in Heart Failure with Preserved Ejection Fraction: What are the Experimental Proofs? Front. Physiol. 2022, 13, 906272. [Google Scholar] [CrossRef]
- Tam, M.C.; Lee, R.; Cascino, T.M.; Konerman, M.C.; Hummel, S.L. Current Perspectives on Systemic Hypertension in Heart Failure with Preserved Ejection Fraction. Curr. Hypertens. Rep. 2017, 19, 12. [Google Scholar] [CrossRef]
- Su, M.-Y.M.; Lin, L.-Y.; Tseng, Y.-H.E.; Chang, C.-C.; Wu, C.-K.; Lin, J.-L.; Tseng, W.-Y.I. CMR-verified diffuse myocardial fibrosis is associated with diastolic dysfunction in HFpEF. JACC Cardiovasc. Imaging 2014, 7, 991–997. [Google Scholar] [CrossRef]
- Brann, A.; Miller, J.; Eshraghian, E.; Park, J.J.; Greenberg, B. Global longitudinal strain predicts clinical outcomes in patients with heart failure with preserved ejection fraction. Eur. J. Heart Fail. 2023, 25, 1755–1765. [Google Scholar] [CrossRef]
- Ma, J.; Chen, Q.; Ma, S. Left atrial fibrosis in atrial fibrillation: Mechanisms, clinical evaluation and management. J. Cell. Mol. Med. 2021, 25, 2764–2775. [Google Scholar] [CrossRef]
- Fauchier, L.; Bisson, A.; Bodin, A. Heart failure with preserved ejection fraction and atrial fibrillation: Recent advances and open questions. BMC Med. 2023, 21, 54. [Google Scholar] [CrossRef]
- Gorter, T.M.; van Veldhuisen, D.J.; Mulder, B.A.; Artola Arita, V.A.; van Empel, V.P.M.; Manintveld, O.C.; Tieleman, R.G.; Maass, A.H.; Vernooy, K.; van Gelder, I.C.; et al. Prevalence and Incidence of Atrial Fibrillation in Heart Failure with Mildly Reduced or Preserved Ejection Fraction: (Additive) Value of Implantable Loop Recorders. J. Clin. Med. 2023, 12, 3682. [Google Scholar] [CrossRef]
- Millan-Orge, M.; Torres-Peña, J.D.; Arenas-Larriva, A.; Quintana-Navarro, G.M.; Peña-Orihuela, P.; Alcala-Diaz, J.F.; Luque, R.M.; Rodriguez-Cantalejo, F.; Katsiki, N.; Lopez-Miranda, J.; et al. Influence of dietary intervention on microvascular endothelial function in coronary patients and atherothrombotic risk of recurrence. Sci. Rep. 2021, 11, 20301. [Google Scholar] [CrossRef] [PubMed]
- Torres-Peña, J.D.; Rangel-Zuñiga, O.A.; Alcala-Diaz, J.F.; Lopez-Miranda, J.; Delgado-Lista, J. Mediterranean Diet and Endothelial Function: A Review of its Effects at Different Vascular Bed Levels. Nutrients 2020, 12, 2212. [Google Scholar] [CrossRef] [PubMed]
- Schindler, T.H.; Valenta, I. Coronary microvascular dysfunction and prognostication in diabetes mellitus. Eur. Heart J. Cardiovasc. Imaging 2023, 24, 572–573. [Google Scholar] [CrossRef] [PubMed]
- Ong, P.; Athanasiadis, A.; Sechtem, U. Pharmacotherapy for coronary microvascular dysfunction. Eur. Heart J. Cardiovasc. Pharmacother. 2015, 1, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Michelsen, M.M.; Rask, A.B.; Suhrs, E.; Raft, K.F.; Høst, N.; Prescott, E. Effect of ACE-inhibition on coronary microvascular function and symptoms in normotensive women with microvascular angina: A randomized placebo-controlled trial. PLoS ONE 2018, 13, e0196962. [Google Scholar] [CrossRef] [PubMed]
- Soleymani, M.; Masoudkabir, F.; Shabani, M.; Vasheghani-Farahani, A.; Behnoush, A.H.; Khalaji, A. Updates on Pharmacologic Management of Microvascular Angina. Cardiovasc. Ther. 2022, 2022, 6080258. [Google Scholar] [CrossRef]
- Weber, K.T.; Sun, Y.; Gerling, I.C.; Guntaka, R.V. Regression of Established Cardiac Fibrosis in Hypertensive Heart Disease. Am. J. Hypertens. 2017, 30, 1049–1052. [Google Scholar] [CrossRef]
- Engholm, M.; Bertelsen, J.B.; Mathiassen, O.N.; Bøtker, H.E.; Vase, H.; Peters, C.D.; Bech, J.N.; Buus, N.H.; Schroeder, A.P.; Rickers, H.; et al. Effects of renal denervation on coronary flow reserve and forearm dilation capacity in patients with treatment-resistant hypertension. A randomized, double-blinded, sham-controlled clinical trial. Int. J. Cardiol. 2018, 250, 29–34. [Google Scholar] [CrossRef]
- Ullrich, H.; Hammer, P.; Olschewski, M.; Münzel, T.; Escaned, J.; Gori, T. Coronary Venous Pressure and Microvascular Hemodynamics in Patients with Microvascular Angina: A Randomized Clinical Trial. JAMA Cardiol. 2023, 8, 979–983. [Google Scholar] [CrossRef]
- Kelshiker, M.A.; Seligman, H.; Howard, J.P.; Rahman, H.; Foley, M.; Nowbar, A.N.; A Rajkumar, C.; Shun-Shin, M.J.; Ahmad, Y.; Sen, S.; et al. Coronary flow reserve and cardiovascular outcomes: A systematic review and meta-analysis. Eur. Heart J. 2022, 43, 1582–1593. [Google Scholar] [CrossRef] [PubMed]
- Dorbala, S.; Di Carli, M.F. Cardiac PET perfusion: Prognosis, risk stratification, and clinical management. Semin. Nucl. Med. 2014, 44, 344–357. [Google Scholar] [CrossRef] [PubMed]
- Murthy, V.L.; Naya, M.; Foster, C.R.; Hainer, J.; Gaber, M.; Di Carli, G.; Blankstein, R.; Dorbala, S.; Sitek, A.; Pencina, M.J.; et al. Improved cardiac risk assessment with noninvasive measures of coronary flow reserve. Circulation 2011, 124, 2215–2224. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Lee, J.C.Y.; Leung, S.T.; Lai, A.; Lee, T.-F.; Chiang, J.B.; Cheng, Y.W.; Chan, H.-L.; Yiu, K.-H.; Goh, V.K.-M.; et al. Long-Term Prognosis of Patients with Coronary Microvascular Disease Using Stress Perfusion Cardiac Magnetic Resonance. JACC Cardiovasc. Imaging 2021, 14, 602–611. [Google Scholar] [CrossRef]
| Diagnostic Modality | Parameter | Advantages | Disadvantages |
|---|---|---|---|
| Echocardiography | CFRV |
|
|
| CT coronary angiography and cardiac perfusion | MBF |
|
|
| PET | MPR, MBF |
|
|
| CMR | MBF, MPR, MPRI |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zdravkovic, M.; Popadic, V.; Klasnja, S.; Klasnja, A.; Ivankovic, T.; Lasica, R.; Lovic, D.; Gostiljac, D.; Vasiljevic, Z. Coronary Microvascular Dysfunction and Hypertension: A Bond More Important than We Think. Medicina 2023, 59, 2149. https://doi.org/10.3390/medicina59122149
Zdravkovic M, Popadic V, Klasnja S, Klasnja A, Ivankovic T, Lasica R, Lovic D, Gostiljac D, Vasiljevic Z. Coronary Microvascular Dysfunction and Hypertension: A Bond More Important than We Think. Medicina. 2023; 59(12):2149. https://doi.org/10.3390/medicina59122149
Chicago/Turabian StyleZdravkovic, Marija, Viseslav Popadic, Slobodan Klasnja, Andrea Klasnja, Tatjana Ivankovic, Ratko Lasica, Dragan Lovic, Drasko Gostiljac, and Zorana Vasiljevic. 2023. "Coronary Microvascular Dysfunction and Hypertension: A Bond More Important than We Think" Medicina 59, no. 12: 2149. https://doi.org/10.3390/medicina59122149
APA StyleZdravkovic, M., Popadic, V., Klasnja, S., Klasnja, A., Ivankovic, T., Lasica, R., Lovic, D., Gostiljac, D., & Vasiljevic, Z. (2023). Coronary Microvascular Dysfunction and Hypertension: A Bond More Important than We Think. Medicina, 59(12), 2149. https://doi.org/10.3390/medicina59122149









