The Approach of Artificial Intelligence in Neuroendocrine Carcinomas of the Breast: A Next Step towards Precision Pathology?—A Case Report and Review of the Literature
Abstract
:1. Introduction
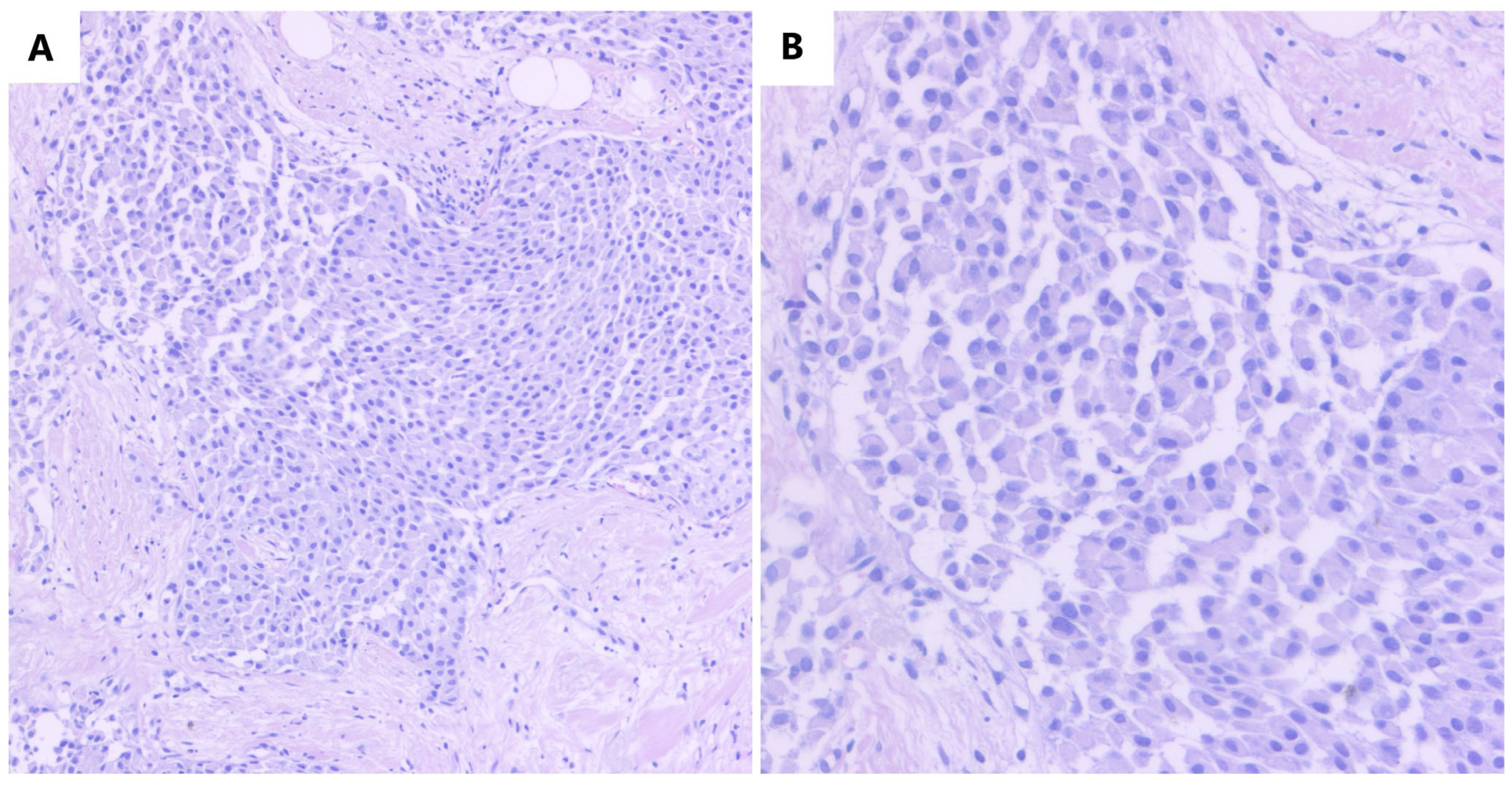
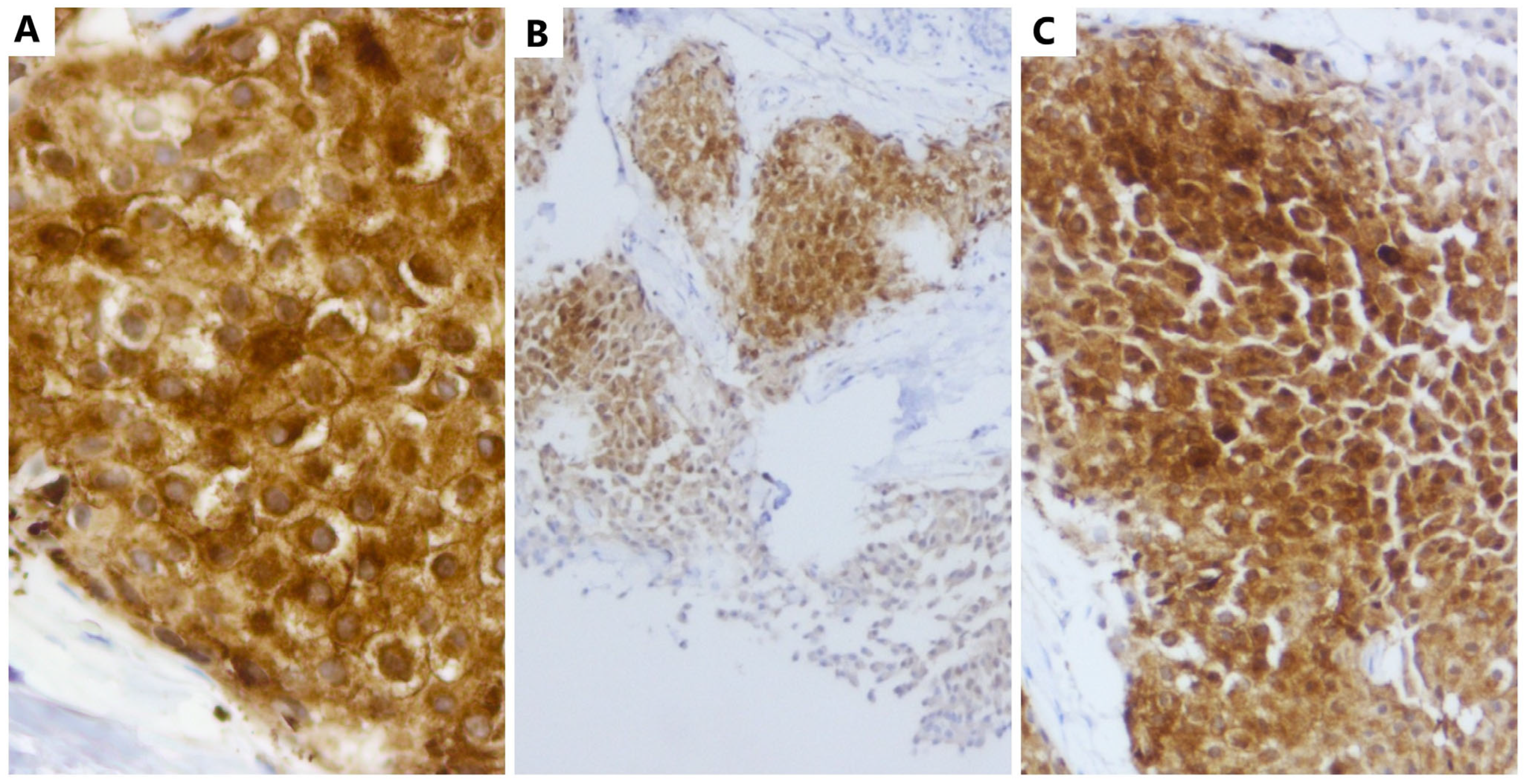
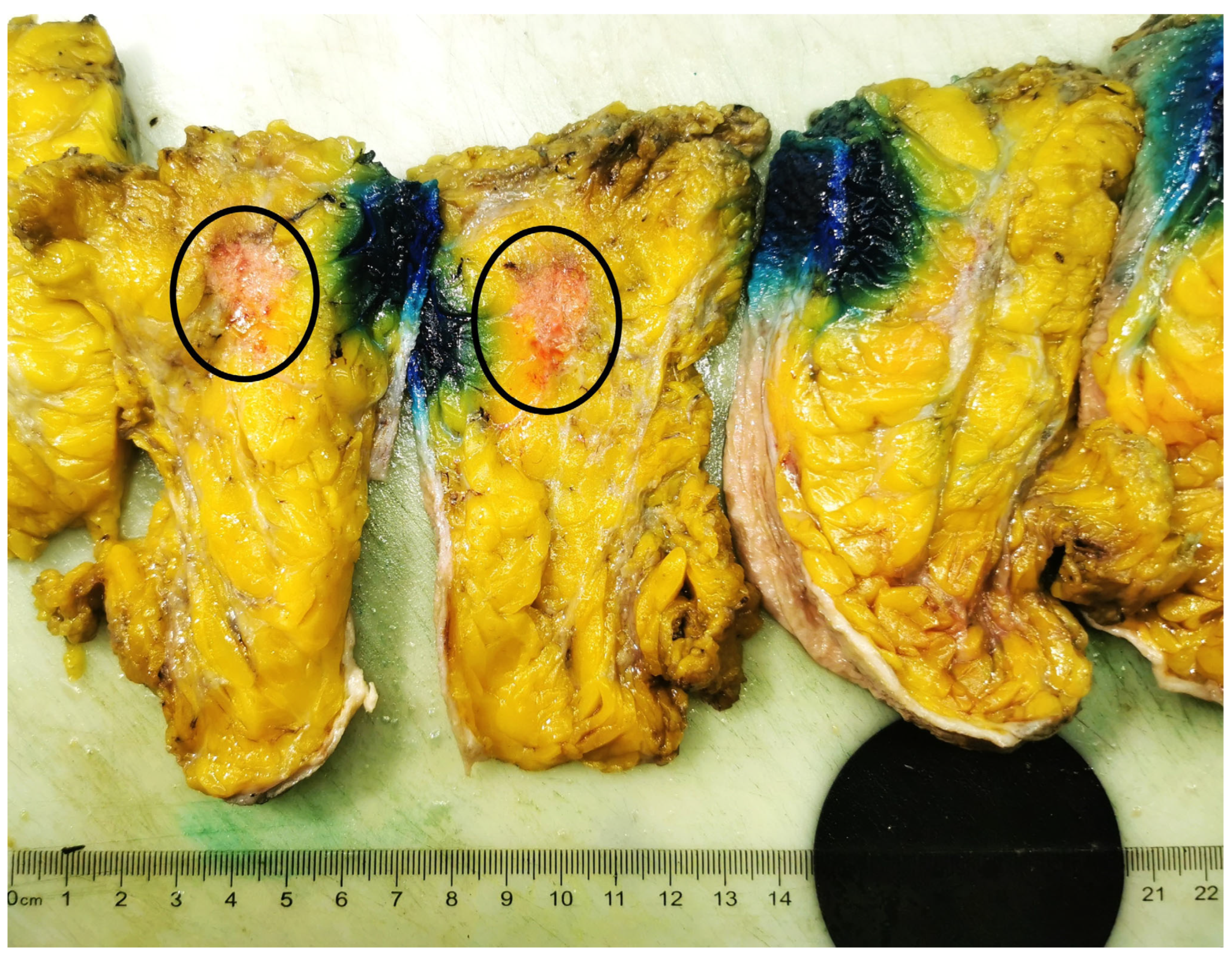
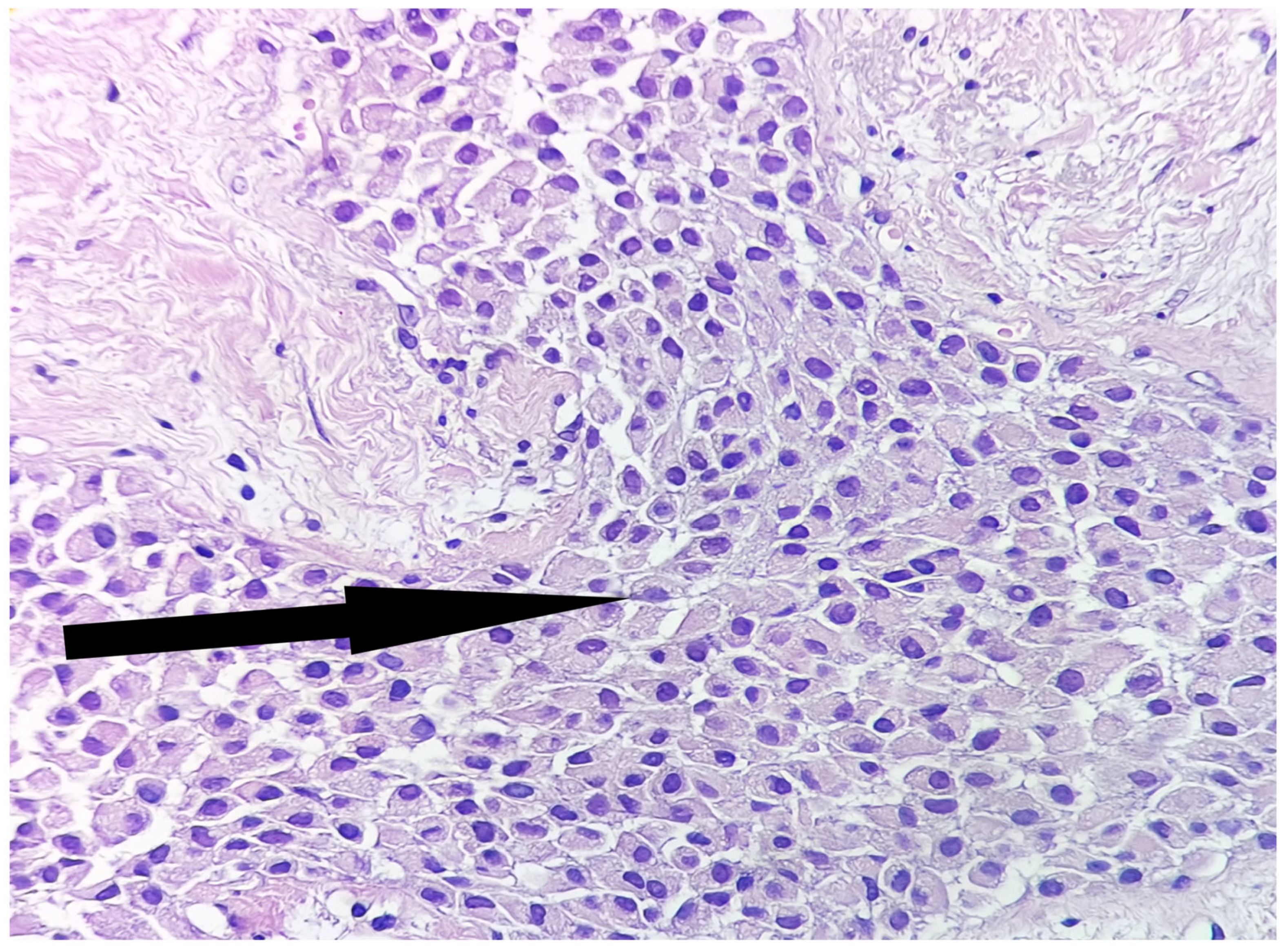
2. Case Report
2.1. Artificial Intelligence Approach and Slide Digitization
2.2. Evaluation of the MIB-1 Proliferation Index and Establishment of the Histological Grade
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosen, L.E.; Gattuso, P. Neuroendocrine Tumors of the Breast. Arch. Pathol. Lab. Med. 2017, 141, 1577–1581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angarita, F.A.; Rodríguez, J.L.; Meek, E.; Sánchez, J.O.; Tawil, M.; Torregrosa, L. Locally-advanced primary neuroendocrine carcinoma of the breast: Case report and review of the literature. World J. Surg. Oncol. 2013, 11, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, G.; Bipte, S. Updates in primary neuroendocrine breast carcinoma—A case report and review of literature. J. Cancer Res. Ther. 2020, 16, 1528–1531. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Han, D.; Yu, Y.; Li, J.; Liu, Y. Artificial intelligence-assisted interpretation of Ki-67 expression and repeatability in breast cancer. Diagn. Pathol. 2022, 17, 20. [Google Scholar] [CrossRef] [PubMed]
- Swiderska-Chadaj, Z.; Gallego, J.; Gonzalez-Lopez, L.; Bueno, G. Detection of Ki67 Hot-Spots of Invasive Breast Cancer Based on Convolutional Neural Networks Applied to Mutual Information of H&E and Ki67 Whole Slide Images. Appl. Sci. 2020, 10, 7761. [Google Scholar] [CrossRef]
- Polley, M.Y.; Leung, S.C.; McShane, L.M.; Gao, D.; Hugh, J.C.; Mastropasqua, M.G.; Viale, G.; Zabaglo, L.A.; Penault-Llorca, F.; Bartlett, J.M.; et al. An international Ki67 reproducibility study. J. Natl. Cancer Inst. 2013, 105, 1897–1906. [Google Scholar] [CrossRef] [Green Version]
- Polley, M.Y.; Leung, S.C.; Gao, D.; Mastropasqua, M.G.; Zabaglo, L.A.; Bartlett, J.M.; McShane, L.M.; Enos, R.A.; Badve, S.S.; Bane, A.L.; et al. An international study to increase concordance in Ki67 scoring. Mod. Pathol. 2015, 28, 778–786. [Google Scholar] [CrossRef] [Green Version]
- Gudlaugsson, E.; Skaland, I.; Janssen, E.A.; Smaaland, R.; Shao, Z.; Malpica, A.; Voorhorst, F.; Baak, J.P. Comparison of the effect of different techniques for measurement of Ki67 proliferation on reproducibility and prognosis prediction accuracy in breast cancer. Histopathology 2012, 61, 1134–1144. [Google Scholar] [CrossRef]
- Yamamoto, S.; Chishima, T.; Mastubara, Y.; Adachi, S.; Harada, F.; Toda, Y.; Arioka, H.; Hasegawa, N.; Kakuta, Y.; Sakamaki, K. Variability in measuring the Ki-67 labeling index in patients with breast cancer. Clin. Breast Cancer 2015, 15, e35–e39. [Google Scholar] [CrossRef]
- Sakamoto, T.; Furukawa, T.; Lami, K.; Pham, H.H.N.; Uegami, W.; Kuroda, K.; Kawai, M.; Sakanashi, H.; Cooper, L.A.D.; Bychkov, A.; et al. A narrative review of digital pathology and artificial intelligence: Focusing on lung cancer. Transl. Lung Cancer Res. 2020, 9, 2255–2276. [Google Scholar] [CrossRef]
- Vergani, A.; Regis, B.; Jocollé, G.; Patetta, R.; Rossi, G. Noninferiority Diagnostic Value, but Also Economic and Turnaround Time Advantages From Digital Pathology. Am. J. Surg. Pathol. 2018, 42, 841–842. [Google Scholar] [CrossRef] [PubMed]
- Inno, A.; Bogina, G.; Turazza, M.; Bortesi, L.; Duranti, S.; Massocco, A.; Zamboni, G.; Carbognin, G.; Alongi, F.; Salgarello, M.; et al. Neuroendocrine Carcinoma of the Breast: Current Evidence and Future Perspectives. Oncologist 2016, 21, 28–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arslan, E.; Çermik, T.F.; Didem Can Trabulus, F.; Canan Kelten Talu, E.; Başaran, Ş. Diagnostic impact of 18F-FDG PET/CT on the management of rare breast carcinomas: Apocrine and neuroendocrine carcinomas. Rev. Esp. Med. Nucl. Imagen Mol. (Engl. Ed.) 2019, 38, 147–153. [Google Scholar] [CrossRef]
- Vranic, S.; Palazzo, J.; Sanati, S.; Florento, E.; Contreras, E.; Xiu, J.; Swensen, J.; Gatalica, Z. Potential Novel Therapy Targets in Neuroendocrine Carcinomas of the Breast. Clin. Breast Cancer 2019, 19, 131–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bera, K.; Schalper, K.A.; Rimm, D.L.; Velcheti, V.; Madabhushi, A. Artificial intelligence in digital pathology—New tools for diagnosis and precision oncology. Nat. Rev. Clin. Oncol. 2019, 16, 703–715. [Google Scholar] [CrossRef]
- Bui, M.M.; Riben, M.W.; Allison, K.H.; Chlipala, E.; Colasacco, C.; Kahn, A.G.; Lacchetti, C.; Madabhushi, A.; Pantanowitz, L.; Salama, M.E.; et al. Quantitative Image Analysis of Human Epidermal Growth Factor Receptor 2 Immunohistochemistry for Breast Cancer: Guideline From the College of American Pathologists. Arch. Pathol. Lab. Med. 2019, 143, 1180–1195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffin, J.; Treanor, D. Digital pathology in clinical use: Where are we now and what is holding us back. Histopathology 2017, 70, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.J.; Lee, J.; Oien, K.A.; Treanor, D. Digital pathology access and usage in the UK: Results from a national survey on behalf of the National Cancer Research Institute’s CM-Path initiative. J. Clin. Pathol. 2018, 71, 463–466. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, M.; Howat, W.J.; Daley, F.; Zabaglo, L.; McDuffus, L.A.; Blows, F.; Coulson, P.; Raza Ali, H.; Benitez, J.; Milne, R.; et al. High-throughput automated scoring of Ki67 in breast cancer tissue microarrays from the Breast Cancer Association Consortium. J. Pathol. Clin. Res. 2016, 2, 138–153. [Google Scholar] [CrossRef] [Green Version]
- Fitzgerald, J.; Higgins, D.; Mazo Vargas, C.; Watson, W.; Mooney, C.; Rahman, A.; Aspell, N.; Connolly, A.; Aura Gonzalez, C.; Gallagher, W. Future of biomarker evaluation in the realm of artificial intelligence algorithms: Application in improved therapeutic stratification of patients with breast and prostate cancer. J. Clin. Pathol. 2021, 74, 429–434. [Google Scholar] [CrossRef]
- Lara, H.; Li, Z.; Abels, E.; Aeffner, F.; Bui, M.M.; ElGabry, E.A.; Kozlowski, C.; Montalto, M.C.; Parwani, A.V.; Zarella, M.D.; et al. Quantitative Image Analysis for Tissue Biomarker Use: A White Paper From the Digital Pathology Association. Appl. Immunohistochem. Mol. Morphol. 2021, 29, 479–493. [Google Scholar] [CrossRef] [PubMed]
- Negahbani, F.; Sabzi, R.; Pakniyat Jahromi, B.; Firouzabadi, D.; Movahedi, F.; Kohandel Shirazi, M.; Majidi, S.; Dehghanian, A. PathoNet introduced as a deep neural network backend for evaluation of Ki-67 and tumor-infiltrating lymphocytes in breast cancer. Sci. Rep. 2021, 11, 8489. [Google Scholar] [CrossRef] [PubMed]
- Barricelli, B.R.; Casiraghi, E.; Gliozzo, J.; Huber, V.; Leone, B.E.; Rizzi, A.; Vergani, B. ki67 nuclei detection and ki67-index estimation: A novel automatic approach based on human vision modeling. BMC Bioinform. 2019, 20, 733. [Google Scholar] [CrossRef]
- Thakur, S.S.; Li, H.; Chan, A.M.Y.; Tudor, R.; Bigras, G.; Morris, D.; Enwere, E.K.; Yang, H. The use of automated Ki67 analysis to predict Oncotype DX risk-of-recurrence categories in early-stage breast cancer. PLoS ONE 2018, 13, e0188983. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Xing, F.; Shi, X.; Kong, X.; Su, H.; Yang, L. Efficient and robust cell detection: A structured regression approach. Med. Image Anal. 2018, 44, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Saha, M.; Chakraborty, C.; Arun, I.; Ahmed, R.; Chatterjee, S. An Advanced Deep Learning Approach for Ki-67 Stained Hotspot Detection and Proliferation Rate Scoring for Prognostic Evaluation of Breast Cancer. Sci. Rep. 2017, 7, 3213. [Google Scholar] [CrossRef] [Green Version]
- Yousif, M.; van Diest, P.J.; Laurinavicius, A.; Rimm, D.; van der Laak, J.; Madabhushi, A.; Schnitt, S.; Pantanowitz, L. Artificial intelligence applied to breast pathology. Virchows Arch. 2022, 480, 191–209. [Google Scholar] [CrossRef]
- Kreipe, H. [Ki67: Biological intertumor variance versus variance of assay]. Pathologe 2018, 39, 272–277. [Google Scholar] [CrossRef]
- Cornish, T.C. Clinical Application of Image Analysis in Pathology. Adv. Anat. Pathol. 2020, 27, 227–235. [Google Scholar] [CrossRef]
- Kreipe, H.; Harbeck, N.; Christgen, M. Clinical validity and clinical utility of Ki67 in early breast cancer. Ther. Adv. Med. Oncol. 2022, 14, 17588359221122725. [Google Scholar] [CrossRef]
- Bodén, A.C.S.; Molin, J.; Garvin, S.; West, R.A.; Lundström, C.; Treanor, D. The human-in-the-loop: An evaluation of pathologists’ interaction with artificial intelligence in clinical practice. Histopathology 2021, 79, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.; Acs, B.; Lippert, M.; Hartman, J. Prognostic potential of automated Ki67 evaluation in breast cancer: Different hot spot definitions versus true global score. Breast Cancer Res. Treat. 2020, 183, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Larsen, M.; Aglen, C.F.; Hoff, S.R.; Lund-Hanssen, H.; Hofvind, S. Possible strategies for use of artificial intelligence in screen-reading of mammograms, based on retrospective data from 122,969 screening examinations. Eur. Radiol. 2022, 32, 8238–8246. [Google Scholar] [CrossRef] [PubMed]
- Moser, E.C.; Narayan, G. Improving breast cancer care coordination and symptom management by using AI driven predictive toolkits. Breast 2020, 50, 25–29. [Google Scholar] [CrossRef] [Green Version]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiorean, D.M.; Mitranovici, M.-I.; Mureșan, M.C.; Buicu, C.-F.; Moraru, R.; Moraru, L.; Cotoi, T.C.; Cotoi, O.S.; Apostol, A.; Turdean, S.G.; et al. The Approach of Artificial Intelligence in Neuroendocrine Carcinomas of the Breast: A Next Step towards Precision Pathology?—A Case Report and Review of the Literature. Medicina 2023, 59, 672. https://doi.org/10.3390/medicina59040672
Chiorean DM, Mitranovici M-I, Mureșan MC, Buicu C-F, Moraru R, Moraru L, Cotoi TC, Cotoi OS, Apostol A, Turdean SG, et al. The Approach of Artificial Intelligence in Neuroendocrine Carcinomas of the Breast: A Next Step towards Precision Pathology?—A Case Report and Review of the Literature. Medicina. 2023; 59(4):672. https://doi.org/10.3390/medicina59040672
Chicago/Turabian StyleChiorean, Diana Maria, Melinda-Ildiko Mitranovici, Maria Cezara Mureșan, Corneliu-Florin Buicu, Raluca Moraru, Liviu Moraru, Titiana Cornelia Cotoi, Ovidiu Simion Cotoi, Adrian Apostol, Sabin Gligore Turdean, and et al. 2023. "The Approach of Artificial Intelligence in Neuroendocrine Carcinomas of the Breast: A Next Step towards Precision Pathology?—A Case Report and Review of the Literature" Medicina 59, no. 4: 672. https://doi.org/10.3390/medicina59040672
APA StyleChiorean, D. M., Mitranovici, M.-I., Mureșan, M. C., Buicu, C.-F., Moraru, R., Moraru, L., Cotoi, T. C., Cotoi, O. S., Apostol, A., Turdean, S. G., Mărginean, C., Petre, I., Oală, I. E., Simon-Szabo, Z., Ivan, V., Roșca, A. N., & Toru, H. S. (2023). The Approach of Artificial Intelligence in Neuroendocrine Carcinomas of the Breast: A Next Step towards Precision Pathology?—A Case Report and Review of the Literature. Medicina, 59(4), 672. https://doi.org/10.3390/medicina59040672









