The Prognostic Significance of Uric Acid/Albumin Ratio in Patients with Aortic Stenosis Following Transcatheter Aortic Valve Implantation for Major Adverse Cardiac and Cerebral Events
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Population
2.2. Uric Acid/Albumin Ratio
2.3. Definitions
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
6. Study Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Supino, P.G.; Borer, J.S.; Preibisz, J.; Bornstein, A. The Epidemiology of Valvular Heart Disease: A Growing Public Health Problem. Heart Fail. Clin. 2006, 2, 379–393. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.M.; Kuusisto, J.; Reichenbach, D.D.; Gown, A.M.; O’Brien, K.D. Characterization of the early lesion of ‘degenerative’ valvular aortic stenosis. Histological and immunohistochemical studies. Circulation 1994, 90, 844–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aronow, W.S.; Ahn, C.; Kronzon, I.; Goldman, M.E. Association of coronary risk factors and use of statins with progression of mild valvular aortic stenosis in older persons. Am. J. Cardiol. 2001, 88, 693–695. [Google Scholar] [CrossRef]
- Masson, J.-B.; Kovac, J.; Schuler, G.; Ye, J.; Cheung, A.; Kapadia, S.; Tuzcu, M.E.; Kodali, S.; Leon, M.B.; Webb, J.G. Transcatheter Aortic Valve Implantation: Review of the Nature, Management, and Avoidance of Procedural Complications. JACC Cardiovasc. Interv. 2009, 2, 811–820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hioki, H.; Watanabe, Y.; Kozuma, K.; Yamamoto, M.; Naganuma, T.; Araki, M.; Tada, N.; Shirai, S.; Yamanaka, F.; Higashimori, A.; et al. Effect of Serum C-Reactive Protein Level on Admission to Predict Mortality After Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2018, 122, 294–301. [Google Scholar] [CrossRef]
- Ames, B.N.; Cathcart, R.; Schwiers, E.; Hochstein, P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: A hypothesis. Proc. Natl. Acad. Sci. USA 1981, 78, 6858–6862. [Google Scholar] [CrossRef] [Green Version]
- Ekaidem, I.S.; Usoh, I.F.; Akpanabiat, M.I.; Uboh, F.; Akpan, H.D. Urate Synthesis and Oxidative Stress in Phenytoin Hepatotoxicity: The Role of Antioxidant Vitamins. Pak. J. Biol. Sci. 2014, 17, 1179–1184. [Google Scholar] [CrossRef] [Green Version]
- Ya, B.-L.; Liu, Q.; Li, H.-F.; Cheng, H.-J.; Yu, T.; Chen, L.; Wang, Y.; Yuan, L.-L.; Li, W.-J.; Liu, W.-Y.; et al. Uric Acid Protects against Focal Cerebral Ischemia/Reperfusion-Induced Oxidative Stress via Activating Nrf2 and Regulating Neurotrophic Factor Expression. Oxidative Med. Cell. Longev. 2018, 2018, 6069150. [Google Scholar] [CrossRef] [Green Version]
- Peng, F.; Yang, Y.; Liu, J.; Jiang, Y.; Zhu, C.; Deng, X.; Hu, X.; Chen, X.; Zhong, X. Low antioxidant status of serum uric acid, bilirubin and albumin in patients with neuromyelitis optica. Eur. J. Neurol. 2012, 19, 277–283. [Google Scholar] [CrossRef]
- Yang, D.; Su, Z.; Wu, S.; Bi, Y.; Li, X.; Li, J.; Lou, K.; Zhang, H.; Zhang, X. Low antioxidant status of serum bilirubin, uric acid, albumin and creatinine in patients with myasthenia gravis. Int. J. Neurosci. 2016, 126, 1120–1126. [Google Scholar] [CrossRef]
- Peters, T., Jr. Serum Albumin. Adv. Protein Chem. 1985, 37, 161–245. [Google Scholar] [CrossRef]
- Kappetein, A.P.; Head, S.J.; Généreux, P.; Piazza, N.; Van Mieghem, N.M.; Blackstone, E.H.; Brott, T.G.; Cohen, D.J.; Cutlip, D.E.; Van Es, G.-A.; et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: The Valve Academic Research Consortium-2 consensus document. Eur. Heart J. 2012, 33, 2403–2418. [Google Scholar] [CrossRef]
- Rutkovskiy, A.; Malashicheva, A.; Sullivan, G.; Bogdanova, M.; Kostareva, A.; Stensløkken, K.; Fiane, A.; Vaage, J. Valve Interstitial Cells: The Key to Understanding the Pathophysiology of Heart Valve Calcification. J. Am. Heart Assoc. 2017, 6, e006339. [Google Scholar] [CrossRef]
- Katkat, F.; Kalyoncuoglu, M.; Ozcan, S.; Tugrul, S.; Abanus, H.; Ince, O.; Balli, M.; Sahin, I.; Okuyan, E. C-Reactive Protein to Albumin Ratio as A Novel Inflammatory-Based Marker for 30-Day Mortality in Patients Undergoing Transcatheter Aortic Valve Replacement. Rev. Bras. Cir. Cardiovasc. 2022, 37, 292–300. [Google Scholar] [CrossRef]
- Cybularz, M.; Wydra, S.; Berndt, K.; Poitz, D.M.; Barthel, P.; Alkouri, A.; Heidrich, F.M.; Ibrahim, K.; Jellinghaus, S.; Speiser, U.; et al. Frailty is associated with chronic inflammation and pro-inflammatory monocyte subpopulations. Exp. Gerontol. 2021, 149, 111317. [Google Scholar] [CrossRef]
- Hu, B.; Yang, X.-R.; Xu, Y.; Sun, Y.-F.; Sun, C.; Guo, W.; Zhang, X.; Wang, W.-M.; Qiu, S.-J.; Zhou, J.; et al. Systemic Immune-Inflammation Index Predicts Prognosis of Patients after Curative Resection for Hepatocellular Carcinoma. Clin. Cancer Res. 2014, 20, 6212–6222. [Google Scholar] [CrossRef] [Green Version]
- Xiong, Z.; Zhu, C.; Qian, X.; Zhu, J.; Wu, Z.; Chen, L. Predictors of clinical SYNTAX score in coronary artery disease: Serum uric acid, smoking, and Framingham risk stratification. J. Invasive Cardiol. 2011, 23, 501–504. [Google Scholar]
- Lin, G.-M.; Li, Y.-H.; Zheng, N.-C.; Lai, C.-P.; Lin, C.-L.; Wang, J.-H.; Jaiteh, L.E.; Han, C.-L. Serum uric acid as an independent predictor of mortality in high-risk patients with obstructive coronary artery disease: A prospective observational cohort study from the ET-CHD registry, 1997–2003. J. Cardiol. 2013, 61, 122–127. [Google Scholar] [CrossRef] [Green Version]
- Stinefelt, B.; Leonard, S.S.; Blemings, K.P.; Shi, X.; Klandorf, H. Free radical scavenging, DNA protection, and inhibition of lipid peroxidation mediated by uric acid. Ann. Clin. Lab. Sci. 2005, 35, 37–45. [Google Scholar]
- Yu, M.-A.; Sánchez-Lozada, L.G.; Johnson, R.J.; Kang, D.-H. Oxidative stress with an activation of the renin–angiotensin system in human vascular endothelial cells as a novel mechanism of uric acid-induced endothelial dysfunction. J. Hypertens. 2010, 28, 1234–1242. [Google Scholar] [CrossRef]
- Goh, S.L.; De Silva, R.P.; Dhital, K.; Gett, R.M. Is low serum albumin associated with postoperative complications in patients undergoing oesophagectomy for oesophageal malignancies? Interact. Cardiovasc. Thorac. Surg. 2014, 20, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lee, H.W. Important predictor of mortality in patients with end-stage liver disease. Clin. Mol. Hepatol. 2013, 19, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Lis, C.G. Pretreatment serum albumin as a predictor of cancer survival: A systematic review of the epidemiological literature. Nutr. J. 2010, 9, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wada, H.; Dohi, T.; Miyauchi, K.; Doi, S.; Naito, R.; Konishi, H.; Tsuboi, S.; Ogita, M.; Kasai, T.; Okazaki, S.; et al. Independent and combined effects of serum albumin and C-reactive protein on long-term outcomes of patients undergoing percutaneous coronary intervention. Circ. J. 2017, 81, 1293–1300. [Google Scholar] [CrossRef] [Green Version]
- Fairclough, E.; Cairns, E.; Hamilton, J.; Kelly, C. Evaluation of a modified early warning system for acute medical admissions and comparison with C-reactive protein/albumin ratio as a predictor of patient outcome. Clin. Med. 2009, 9, 30–33. [Google Scholar] [CrossRef]
- Okuno, T.; Koseki, K.; Nakanishi, T.; Sato, K.; Ninomiya, K.; Tomii, D.; Tanaka, T.; Sato, Y.; Horiuchi, Y.; Koike, H.; et al. Evaluation of objective nutritional indexes as predictors of one-year outcomes after transcatheter aortic valve implantation. J. Cardiol. 2019, 74, 34–39. [Google Scholar] [CrossRef]
- Özgür, Y.; Akın, S.; Yılmaz, N.G.; Gücün, M.; Keskin, Ö. Uric acid albumin ratio as a predictive marker of short-term mortality in patients with acute kidney injury. Clin. Exp. Emerg. Med. 2021, 8, 82–88. [Google Scholar] [CrossRef]
- de Melo, P.H.M.C.; Modolo, R. The Role of Inflammation in Post-TAVI Outcomes. Arq. Bras. Cardiol. 2021, 117, 1028–1029. [Google Scholar] [CrossRef]
- Çakmak, E.; Bayam, E.; Çelik, M.; Kahyaoğlu, M.; Eren, K.; Imanov, E.; Karagöz, A.; İzgi, İ.A. Uric Acid-to-Albumin Ratio: A Novel Marker for the Extent of Coronary Artery Disease in Patients with Non-ST-Elevated Myocardial Infarction. Pulse 2020, 8, 99–107. [Google Scholar] [CrossRef]
- Li, S.; Chen, H.; Zhou, L.; Cui, H.; Liang, S.; Li, H. The uric acid to albumin ratio: A novel predictor of long-term cardiac mortality in patients with unstable angina pectoris after percutaneous coronary intervention. Scand. J. Clin. Lab. Investig. 2022, 82, 304–310. [Google Scholar] [CrossRef]
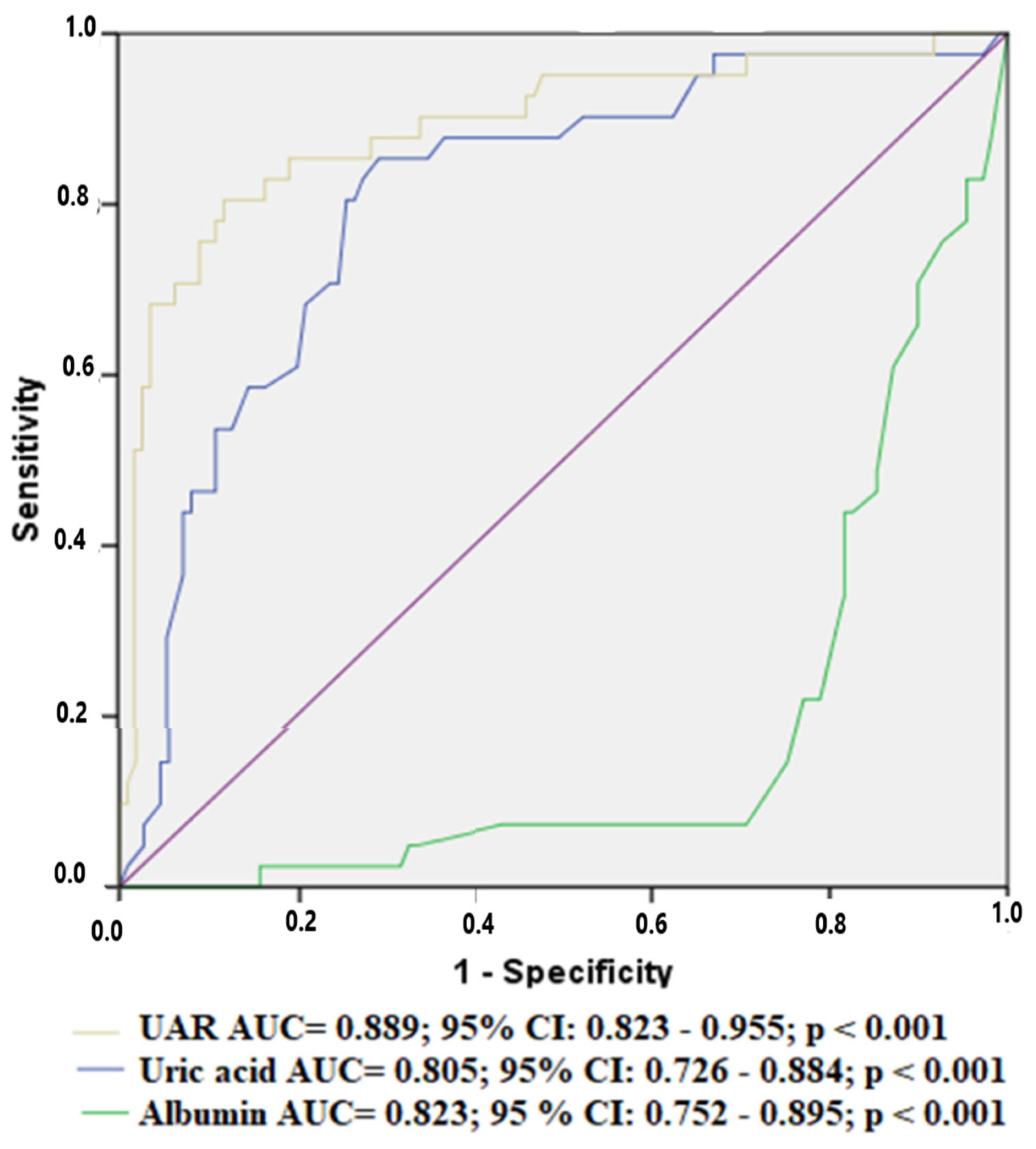
| Variables | All Population | No MACCEs (n = 109) | MACCEs (n = 41) | p |
|---|---|---|---|---|
| Male gender, n % | 84 (56) | 57 (52.2) | 27 (65.8) | 0.136 |
| Age | 77 ± 5 | 77 ± 5 | 77.1 ± 4 | 0.903 |
| BMI (kg/m2) | 26.8 ± 4.2 | 26.7 ± 4.0 | 27.0 ± 4.8 | 0.673 |
| Hypertension, n (%) | 90 (60) | 66 (60.5) | 24 (58.5) | 0.820 |
| Diabetes mellitus, n (%) | 47 (31.3) | 30 (27.5) | 17 (41.5) | 0.101 |
| Vascular disease history, n (%) | 41 (27.3) | 20 (19.8) | 21 (42.9) | 0.003 |
| Chronic renal failure, n (%) | 25 (16.7) | 17 (15.6) | 8 (19.5) | 0.566 |
| COPD, n (%) | 56 (37.3) | 39 (35.8) | 17 (41.5) | 0.521 |
| Smoking, n (%) | 20 (13.3) | 15 (13.8) | 5 (12.2) | 0.801 |
| CVA history, n (%) | 11 (7.3) | 6 (5.5) | 5 (12.2) | 0.161 |
| NYHA Class III-IV, n (%) | 10 (6.7) | 4 (3.7) | 6 (14.6) | 0.016 |
| Atrial fibrillation, n (%) | 18 (12.0) | 13 (11.9) | 5 (12.2) | 0.964 |
| Presence of RBBB or LBBB, n (%) | 21 (14) | 14 (12.8) | 7 (17.1) | 0.506 |
| Aortic valve area, cm2 | 0.81 ± 0.10 | 0.82 ± 0.1 | 0.78 ± 0.1 | 0.022 |
| Mean Aortic valve gradient, mmHg | 47.9 ± 4.2 | 47.3 ± 3.6 | 49.6 ± 5.0 | 0.002 |
| Left ventricular ejection fraction, % | 54.8 ± 8.5 | 55.3 ± 7.6 | 53.4 ± 10.6 | 0.223 |
| Fasting glucose, mg/dL | 123.8 ± 39.4 | 124.2 ± 40 | 122.7 ± 38.4 | 0.832 |
| Creatinine, median, mg/dL, [IQR] | 0.90 [0.80–1.50] | 0.90 [0.70–1.50] | 0.90 [0.80–1.50] | 0.948 |
| TC, mg/dL | 209 ± 39 | 208.7 ± 43.7 | 208.6 ± 34 | 0.984 |
| Triglyceride, mg/dL, median, [IQR] | 175 [150–195] | 175 [148–195] | 175 [153–195] | 0.432 |
| WBC, cells/mcL | 6.9 ± 1.7 | 6.4 ± 1.7 | 7.9 ± 1.1 | <0.001 |
| Hgb, g/dL | 15.2 ± 3.8 | 15.3 ± 4.3 | 14.9 ± 1.0 | 0.504 |
| Platelet, cells/mcL | 263 ± 71 | 260 ± 68 | 269 ± 79 | 0.499 |
| CRP, median, mg/L, [IQR] | 3.50 [1.28–6.30] | 3.2 [1.0–4.9] | 5.7 [2.9–10.0] | 0.001 |
| Uric acid, mg/dL | 6.0 ± 2.1 | 5.4 ± 1.9 | 7.5 ± 1.9 | <0.001 |
| Albumin, g/dL | 3.4 ± 0.79 | 3.7 ± 0.6 | 2.7 ± 0.8 | <0.001 |
| UAR | 1.92 ± 0.96 | 1.51 ± 0.58 | 2.99 ± 0.92 | <0.001 |
| Variables | All Population | No MACCEs (n = 109) | MACCEs (n = 41) | p |
|---|---|---|---|---|
| Conscious sedation, n % | 85 (56.7) | 65 (59.6) | 20 (48.8) | 0.232 |
| Type of Valve, n (%) | 0.309 | |||
| SEV | 74 (49.3) | 51 (46.8) | 23 (56.1) | |
| BEV | 76 (50.7) | 58 (53.2) | 18 (43.9) | |
| Predilatation, n (%) | 46 (30.7) | 34 (31.2) | 12 (29.3) | 0.820 |
| Postdilatation, n (%) | 21 (14) | 16 (14.7) | 5 (12.2) | 0.696 |
| Implantation depth, mm | 5.2 ± 0.8 | 5.2 ± 0.7 | 5.3 ± 1.0 | 0.445 |
| Paravalvular leakage (>2+), n (%) | 11 (7.3) | 6 (5.5) | 5 (12.2) | 0.161 |
| Major vascular complications, n (%) | 7 (4.7) | 0 (0) | 7 (17.1) | <0.001 |
| Bleeding complications, n (%) | 9 (6) | 0 (0) | 9 (22) | <0.001 |
| Pericardial Tamponade, n (%) | 5 (3.3) | 3 (2.8) | 2 (4.9) | 0.518 |
| Acute renal failure, n (%) | 13 (8.7) | 9 (8.3) | 4 (9.8) | 0.771 |
| Permanent pacemaker, n (%) | 7 (4.7) | 0 (0) | 7 (17.1) | <0.001 |
| Postprocedural IS or TIA, n (%) | 5 (3.3) | 0 (0) | 5 (12.2) | <0.001 |
| Rehospitalization, n (%) (cardiovascular-caused) | 12 (8) | 0 (0) | 12 (29.3) | <0.001 |
| Sepsis with worsening of heart function, n (%) | 0 | 0 | 0 | 0 |
| Poor positioning of the prosthesis/thrombosis, n (%) | 6 (4) | 3 (2.8) | 3 (7.3) | 0.204 |
| Myocardial infarction, n (%) | 0 | 0 | 0 | 0 |
| Infective endocarditis, n (%) | 0 | 0 | 0 | 0 |
| Death (all-cause), n (%) | 10 (6.7) | 0 (0) | 10 (24.4) | <0.001 |
| Variables | Univariate HR (95% CI) | p | Model 1 Multivariate * HR (95% CI) | p | Model 2 Multivariate * HR (95% CI) | p |
|---|---|---|---|---|---|---|
| VDH | 0.422 (0.187–0.953) | 0.038 | 1.793 (0.923–3.485) | 0.085 | 1.838 (0.984–3.545) | 0.056 |
| AVA | 0.054 (0.003–0.993) | 0.049 | 0.189 (0.008–4.549) | 0.305 | 0.205 (0.009–4.520) | 0.315 |
| MVC | 2.368 (1.050–5.341) | 0.038 | 1.310 (0.562–3.051) | 0.532 | 1.398 (0.600–3.261) | 0.437 |
| CRP | 1.133 (1.063–1.208) | <0.001 | 1.087 (1.016–1.162) | 0.015 | 1.096 (1.026–1.172) | 0.007 |
| Uric acid | 1.393 (1.216–1.597) | <0.001 | 1.337 (1.142–1.565) | <0.001 | - | - |
| Albumin | 0.377 (0.254–0.558) | <0.001 | 0.396 (0.251–0.623) | <0.001 | - | - |
| UAR | 2.724 (1.999–3.713) | <0.001 | - | - | 2.478 (1.779–3.453) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biter, H.I.; Tosu, A.R. The Prognostic Significance of Uric Acid/Albumin Ratio in Patients with Aortic Stenosis Following Transcatheter Aortic Valve Implantation for Major Adverse Cardiac and Cerebral Events. Medicina 2023, 59, 686. https://doi.org/10.3390/medicina59040686
Biter HI, Tosu AR. The Prognostic Significance of Uric Acid/Albumin Ratio in Patients with Aortic Stenosis Following Transcatheter Aortic Valve Implantation for Major Adverse Cardiac and Cerebral Events. Medicina. 2023; 59(4):686. https://doi.org/10.3390/medicina59040686
Chicago/Turabian StyleBiter, Halil Ibrahim, and Aydin Rodi Tosu. 2023. "The Prognostic Significance of Uric Acid/Albumin Ratio in Patients with Aortic Stenosis Following Transcatheter Aortic Valve Implantation for Major Adverse Cardiac and Cerebral Events" Medicina 59, no. 4: 686. https://doi.org/10.3390/medicina59040686






