Post Treatment Sexual Function and Quality of Life of Patients Affected by Cervical Cancer: A Systematic Review
Abstract
1. Introduction
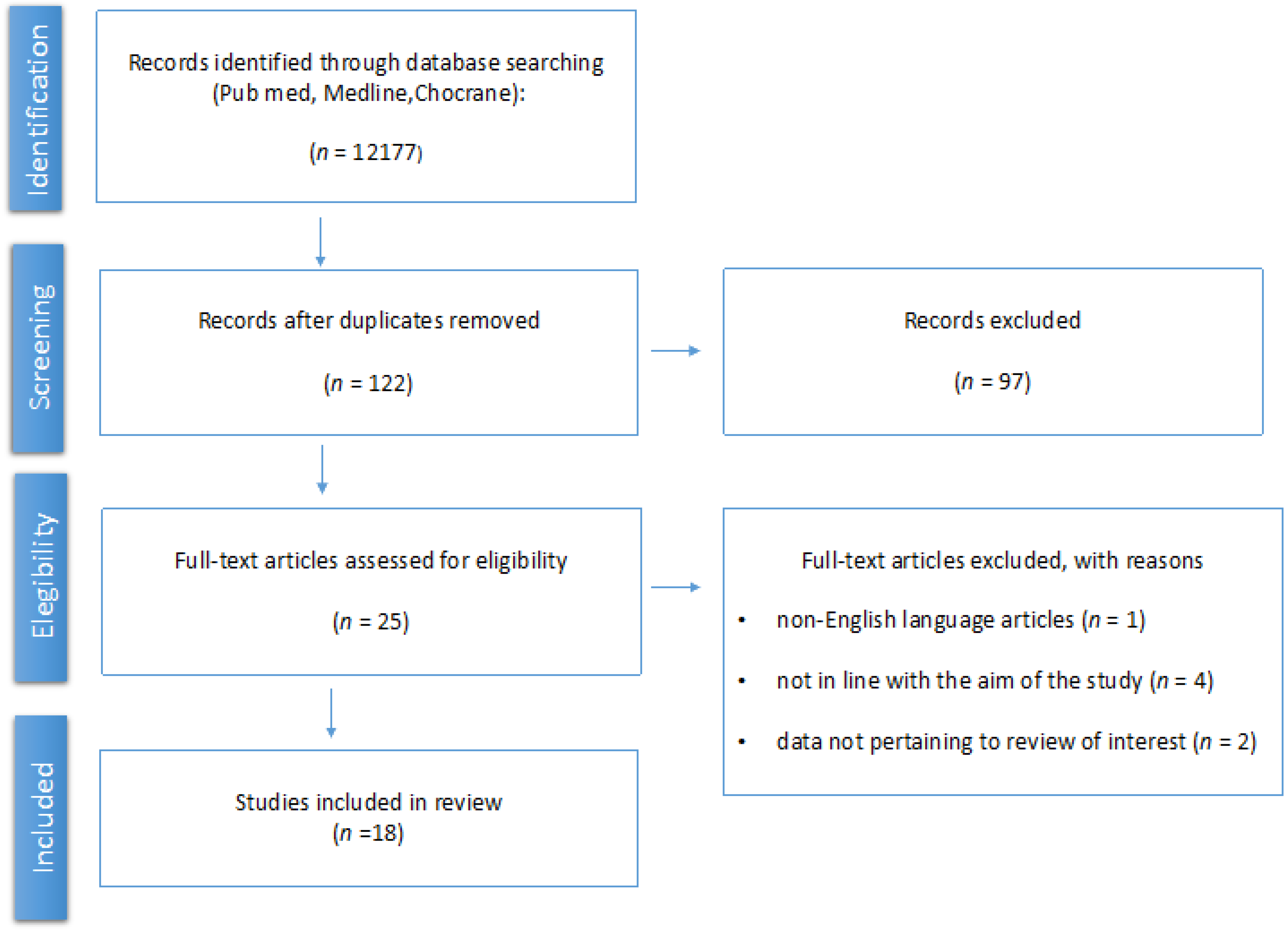
2. Materials and Methods
3. Results
3.1. Study Characteristics
3.2. Study Descriptions
3.3. Main Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lin, S.; Gao, K.; Gu, S.; You, L.; Qian, S.; Tang, M.; Wang, J.; Chen, K.; Jin, M. Worldwide trends in cervical cancer incidence and mortality, with predictions for the next 15 years. Cancer 2021, 127, 4030–4039. [Google Scholar] [CrossRef] [PubMed]
- Momenimovahed, Z.; Mazidimoradi, A.; Maroofi, P.; Allahqoli, L.; Salehiniya, H.; Alkatout, I. Global, regional and national burden, incidence, and mortality of cervical cancer. Cancer Rep. 2022, 6, e1756. [Google Scholar] [CrossRef] [PubMed]
- Arbyn, M.; Weiderpass, E.; Bruni, L.; de Sanjosé, S.; Saraiya, M.; Ferlay, J.; Bray, F. Estimates of incidence and mortality of cervical cancer in 2018: A world-wide analysis. Lancet Glob. Health 2020, 8, e191–e203. [Google Scholar] [CrossRef] [PubMed]
- Bhatla, N.; Aoki, D.; Sharma, D.N.; Sankaranarayanan, R. Cancer of the cervix uteri. Int. J. Gynecol. Obstet. 2018, 143, 22–36. [Google Scholar] [CrossRef] [PubMed]
- Vaccarella, S.; Laversanne, M.; Ferlay, J.; Bray, F. Cervical cancer in Africa, Latin America and the Caribbean and Asia: Regional inequalities and changing trends. Int. J. Cancer 2017, 141, 1997–2001. [Google Scholar] [CrossRef]
- Rossi, P.G.; Caroli, S.; Mancini, S.; Bianchi, P.S.D.; Finarelli, A.C.; Naldoni, C.; Bucchi, L.; Falcini, F.; Emilia-Romagna Cervical Cancer Screening and Pathology Registry Group. Screening history of cervical cancers in Emilia-Romagna, Italy: Defining priorities to improve cervical cancer screening. Eur. J. Cancer Prev. 2015, 24, 128–134. [Google Scholar] [CrossRef]
- Zucchetto, A.; Ronco, G.; Rossi, P.G.; Zappa, M.; Ferretti, S.; Franzo, A.; Falcini, F.; Visioli, C.B.; Zanetti, R.; Biavati, P.; et al. Screening patterns within organized programs and survival of Italian women with invasive cervical cancer. Prev. Med. 2013, 57, 220–226. [Google Scholar] [CrossRef]
- Serraino, D.; Gini, A.; Taborelli, M.; Ronco, G.; Rossi, P.G.; Zappa, M.; Crocetti, E.; Franzo, A.; Falcini, F.; Visioli, C.B.; et al. Changes in cervical cancer incidence following the introduction of organized screening in Italy. Prev. Med. 2015, 75, 56–63. [Google Scholar] [CrossRef]
- Vaccarella, S.; Lortet-Tieulent, J.; Plummer, M.; Franceschi, S.; Bray, F. Worldwide trends in cervical cancer incidence: Impact of screening against changes in disease risk factors. Eur. J. Cancer 2013, 49, 3262–3273. [Google Scholar] [CrossRef]
- Sasieni, P.; Castanon, A.; Cuzick, J. Effectiveness of cervical screening with age: Population based case-control study of prospectively recorded data. BMJ 2009, 339, b2968. [Google Scholar] [CrossRef]
- Arbyn, M.; Gultekin, M.; Morice, P.; Nieminen, P.; Cruickshank, M.; Poortmans, P.; Kelly, D.; Poljak, M.; Bergeron, C.; Ritchie, D.; et al. The European response to the WHO call to eliminate cervical cancer as a public health problem. Int. J. Cancer 2020, 148, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Gultekin, M.; Ramirez, P.T.; Broutet, N.; Hutubessy, R. World Health Organization call for action to eliminate cervical cancer globally. Int. J. Gynecol. Cancer 2020, 30, 426–427. [Google Scholar] [CrossRef] [PubMed]
- Gultekin, M.; Morice, P.; Concin, N.; Querleu, D. ESGO contribution to the WHO initiative on elimination of cervical cancer. Int. J. Gynecol. Cancer 2020, 30, 434–435. [Google Scholar] [CrossRef] [PubMed]
- Karimi-Zarchi, M.; Allahqoli, L.; Nehmati, A.; Kashi, A.M.; Taghipour-Zahir, S.; Alkatout, I. Can the prophylactic quadrivalent HPV vaccine be used as a therapeutic agent in women with CIN? A randomized trial. BMC Public Health 2020, 20, 274. [Google Scholar] [CrossRef]
- Kyrgiou, M.; Arbyn, M.; Bergeron, C.; Bosch, F.X.; Dillner, J.; Jit, M.; Kim, J.; Poljak, M.; Nieminen, P.; Sasieni, P.; et al. Cervical screening: ESGO-EFC position paper of the European Society of Gynaecologic Oncology (ESGO) and the European Federation of Colposcopy (EFC). Br. J. Cancer 2020, 123, 510–517. [Google Scholar] [CrossRef]
- Chrysostomou, A.C.; Stylianou, D.C.; Constantinidou, A.; Kostrikis, L.G. Cervical Cancer Screening Programs in Europe: The Transition Towards HPV Vaccination and Population-Based HPV Testing. Viruses 2018, 10, 729. [Google Scholar] [CrossRef]
- Venturelli, F.; Baldacchini, F.; Campari, C.; Perilli, C.; Pascucci, M.G.; Finarelli, A.C.; Moscara, L.; Rossi, P.G. Association between mothers’ screening uptake and daughters’ HPV vaccination: A quasi-experimental study on the effect of an active invitation campaign. BMJ Open 2017, 7, e016189. [Google Scholar] [CrossRef]
- Malagón, T.; Drolet, M.; Boily, M.-C.; Laprise, J.-F.; Brisson, M. Changing Inequalities in Cervical Cancer: Modeling the Impact of Vaccine Uptake, Vaccine Herd Effects, and Cervical Cancer Screening in the Post-Vaccination Era. Cancer Epidemiol. Biomark. Prev. 2015, 24, 276–285. [Google Scholar] [CrossRef]
- Bos, A.B.; Rebolj, M.; Habbema, J.D.F.; van Ballegooijen, M. Nonattendance is still the main limitation for the effectiveness of screening for cervical cancer in the Netherlands. Int. J. Cancer 2006, 119, 2372–2375. [Google Scholar] [CrossRef]
- Andrae, B.; Kemetli, L.; Sparén, P.; Silfverdal, L.; Strander, B.; Ryd, W.; Dillner, J.; Törnberg, S. Screening-Preventable Cervical Cancer Risks: Evidence from a Nationwide Audit in Sweden. Gynecol. Oncol. 2008, 100, 622–629. [Google Scholar] [CrossRef]
- Rossi, P.G.; Baldacchini, F.; Ronco, G. The Possible Effects on Socio-Economic Inequalities of Introducing HPV Testing as Primary Test in Cervical Cancer Screening Programs. Front. Oncol. 2014, 4, 20. [Google Scholar] [CrossRef]
- Drolet, M.; Bénard, É.; Pérez, N.; Brisson, M.; HPV Vaccination Impact Study Group. Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: Updated systematic review and meta-analysis. Lancet 2019, 394, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Cameron, R.L.; Kavanagh, K.; Pan, J.; Love, J.; Cuschieri, K.; Robertson, C.; Ahmed, S.; Palmer, T.; Pollock, K.G. Human Papillomavirus Prevalence and Herd Immunity after Introduction of Vaccination Program, Scotland, 2009–2013. Emerg. Infect. Dis. 2016, 22, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Koh, W.J.; Abu-Rustum, N.R.; Bean, S. NCCN guidelines version 5.2019 in cervical cancer. J. Natl. Compr. Cancer Netw. 2019, 17, 64–84. [Google Scholar] [CrossRef] [PubMed]
- Zullo, M.A.; Manci, N.; Angioli, R.; Muzii, L.; Panici, P.B. Vesical dysfunctions after radical hysterectomy for cervical cancer: A critical review. Crit. Rev. Oncol. 2003, 48, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Sakuragi, N.; Murakami, G.; Konno, Y.; Kaneuchi, M.; Watari, H. Nerve-sparing radical hysterectomy in the precision surgery for cervical cancer. J. Gynecol. Oncol. 2020, 31, e49. [Google Scholar] [CrossRef] [PubMed]
- Querleu, D.; Morrow, C.P. Classification of radical hysterectomy. Lancet Oncol. 2008, 9, 297–303. [Google Scholar] [CrossRef]
- Stabile, C.; Goldfarb, S.; Baser, R.E.; Goldfrank, D.J.; Abu-Rustum, N.R.; Barakat, R.R.; Dickler, M.N.; Carter, J. Sexual health needs and educational intervention preferences for women with cancer. Breast Cancer Res. Treat. 2017, 165, 77–84. [Google Scholar] [CrossRef]
- Bober, S.L.; Reese, J.B.; Barbera, L.; Bradford, A.; Carpenter, K.M.; Goldfarb, S.; Carter, J. How to ask and what to do: A guide for clinical inquiry and intervention regarding female sexual health after cancer. Curr. Opin. Support. Palliat. Care 2016, 10, 44–54. [Google Scholar] [CrossRef]
- Vitale, S.G.; Caruso, S.; Rapisarda, A.M.C.; Cianci, S.; Cianci, A. Isoflavones, calcium, vitamin D and inulin improve quality of life, sexual function, body composition and metabolic parameters in menopausal women: Result from a prospective, randomized, placebo-controlled, parallel-group study. Menopausal Rev. 2018, 17, 32–38. [Google Scholar] [CrossRef]
- Caruso, S.; Cianci, S.; Fava, V.; Rapisarda, A.M.C.; Cutello, S.; Cianci, A. Vaginal health of postmenopausal women on nutraceutical containing equol. Menopause 2018, 25, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Caruso, S.; Cianci, S.; Cariola, M.; Fava, V.; Rapisarda, A.M.; Cianci, A. Effects of nutraceuticals on quality of life and sexual function of perimenopausal women. J. Endocrinol. Investig. 2017, 40, 27–32, Erratum in: J. Endocrinol. Investig. 2017, 40, 339; Erratum in: J. Endocrinol. Investig. 2017, 40, 341. [Google Scholar] [CrossRef] [PubMed]
- Caruso, S.; Rapisarda, A.M.; Cianci, S. Sexuality in menopausal women. Curr. Opin. Psychiatry 2016, 29, 323–330. [Google Scholar] [CrossRef]
- Caruso, S.; Cianci, A.; Sarpietro, G.; Matarazzo, M.G.; Panella, M.; Cianci, S. Ultralow 0.03 mg vaginal estriol in postmenopausal women who underwent surgical treatment for stress urinary incontinence: Effects on quality of life and sexual function. Menopause 2019, 27, 162–169. [Google Scholar] [CrossRef]
- Ye, S.; Yang, J.; Cao, D.; Lang, J.; Shen, K. A Systematic Review of Quality of Life and Sexual Function of Patients With Cervical Cancer After Treatment. Int. J. Gynecol. Cancer 2014, 24, 1146–1157. [Google Scholar] [CrossRef]
- Vistad, I.; Fosså, S.D.; Dahl, A.A. A critical review of patient-rated quality of life studies of long-term survivors of cervical cancer. Gynecol. Oncol. 2006, 102, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Tramacere, F.; Lancellotta, V.; Casà, C.; Fionda, B.; Cornacchione, P.; Mazzarella, C.; De Vincenzo, R.P.; Macchia, G.; Ferioli, M.; Rovirosa, A.; et al. Assessment of Sexual Dysfunction in Cervical Cancer Patients after Different Treatment Modality: A Systematic Review. Medicina 2022, 58, 1223. [Google Scholar] [CrossRef]
- Kirchheiner, K.; Smet, S.; Jürgenliemk-Schulz, I.M.; Haie-Meder, C.; Chargari, C.; Lindegaard, J.C.; Fokdal, L.U.; Spampinato, S.; Schmid, M.P.; Sturdza, A.; et al. Impact of Vaginal Symptoms and Hormonal Replacement Therapy on Sexual Outcomes After Definitive Chemoradiotherapy in Patients With Locally Advanced Cervical Cancer: Results from the EMBRACE-I Study. Int. J. Radiat. Oncol. 2021, 112, 400–413. [Google Scholar] [CrossRef]
- Novackova, M.; Pastor, Z.; Chmel, R.; Mala, I. Sexuality and quality of life after nerve-sparing radical hysterectomy for cervical cancer: A prospective study. Taiwan J. Obstet. Gynecol. 2022, 61, 641–645. [Google Scholar] [CrossRef]
- Park, S.Y.; Bae, D.-S.; Nam, J.H.; Park, C.T.; Cho, C.-H.; Lee, J.M.; Lee, M.K.; Kim, S.H.; Yun, Y.H. Quality of life and sexual problems in disease-free survivors of cervical cancer compared with the general population. Cancer 2007, 110, 2716–2725. [Google Scholar] [CrossRef]
- Jensen, P.T.; Groenvold, M.; Klee, M.C.; Thranov, I.; Petersen, M.A.; Machin, D. Early-stage cervical carcinoma, radical hysterectomy, and sexual function. A longitudinal study. Cancer 2004, 100, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Ljuca, D.; Marošević, G. Impact of chemoradiotherapy on vaginal and sexual function of patients with FIGO IIb cervical cancer. Bosn. J. Basic Med. Sci. 2011, 11, 62–64. [Google Scholar] [CrossRef] [PubMed]
- Bakker, R.; Kenter, G.; Creutzberg, C.; Stiggelbout, A.; Derks, M.; Mingelen, W.; Kroon, C.; Vermeer, W.; Ter Kuile, M. Sexual distress and associated factors among cervical cancer survivors: A cross-sectional multicenter observational study. Psychooncology 2016, 26, 1470–1477. [Google Scholar] [CrossRef] [PubMed]
- Aerts, L.; Enzlin, P.; Verhaeghe, J.; Poppe, W.; Vergote, I.; Amant, F. Long-Term Sexual Functioning in Women After Surgical Treatment of Cervical Cancer Stages IA to IB: A Prospective Controlled Study. Int. J. Gynecol. Cancer 2014, 24, 1527–1534. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, C.S.L.; Leite, I.C.G.; Andrade, A.P.S.; Ferreira, A.D.S.S.; Carvalho, S.M.; Guerra, M.R. Sexual function of women surviving cervical cancer. Arch. Gynecol. Obstet. 2015, 293, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Lim, M.C.; Kim, S.I.; Joo, J.; Lee, D.O.; Park, S.-Y. Comparison of Quality of Life and Sexuality between Cervical Cancer Survivors and Healthy Women. Cancer Res. Treat. 2016, 48, 1321–1329. [Google Scholar] [CrossRef]
- Carter, J.; Sonoda, Y.; Baser, R.E.; Raviv, L.; Chi, D.S.; Barakat, R.R.; Iasonos, A.; Brown, C.L.; Abu-Rustum, N.R. A 2-year prospective study assessing the emotional, sexual, and quality of life concerns of women undergoing radical trachelectomy versus radical hysterectomy for treatment of early-stage cervical cancer. Gynecol. Oncol. 2010, 119, 358–365. [Google Scholar] [CrossRef]
- Hofsjö, A.; Bergmark, K.; Blomgren, B.; Jahren, H.; Bohm-Starke, N. Radiotherapy for cervical cancer—Impact on the vaginal epithelium and sexual function. Acta Oncol. 2017, 57, 338–345. [Google Scholar] [CrossRef]
- De Rosa, N.; Lavitola, G.; Giampaolino, P.; Morra, I.; Nappi, C.; Bifulco, G. Impact of Ospemifene on Quality of Life and Sexual Function in Young Survivors of Cervical Cancer: A Prospective Study. BioMed Res. Int. 2017, 2017, 7513610. [Google Scholar] [CrossRef]
- Plotti, F.; Terranova, C.; Capriglione, S.; Crispino, S.; Pomi, A.L.; Nardone, C.D.C.; Montera, R.; Panici, P.B.; Angioli, R.; Scaletta, G. Assessment of Quality of Life and Urinary and Sexual Function After Radical Hysterectomy in Long-Term Cervical Cancer Survivors. Int. J. Gynecol. Cancer 2018, 28, 818–823. [Google Scholar] [CrossRef]
- Bae, H.; Park, H. Sexual function, depression, and quality of life in patients with cervical cancer. Support. Care Cancer 2015, 24, 1277–1283. [Google Scholar] [CrossRef] [PubMed]
- Serati, M.; Salvatore, S.; Uccella, S.; Laterza, R.M.; Cromi, A.; Ghezzi, F.; Bolis, P. Sexual function after radical hysterectomy for early-stage cervical cancer: Is there a difference between laparoscopy and laparotomy? J. Sex. Med. 2009, 6, 2516–2522. [Google Scholar] [CrossRef]
- Shi, Y.; Cai, J.; Wu, Z.; Jiang, L.; Xiong, G.; Gan, X.; Wang, X. Effects of a nurse-led positive psychology intervention on sexual function, depression and subjective well-being in postoperative patients with early-stage cervical cancer: A randomized controlled trial. Int. J. Nurs. Stud. 2020, 111, 103768. [Google Scholar] [CrossRef] [PubMed]
- Cerentini, T.M.; Schlöttgen, J.; Viana da Rosa, P.; La Rosa, V.L.; Vitale, S.G.; Giampaolino, P.; Valenti, G.; Cianci, S.; Macagnan, F.E. Clinical and Psychological Outcomes of the Use of Vaginal Dilators After Gynaecological Brachytherapy: A Randomized Clinical Trial. Adv. Ther. 2019, 36, 1936–1949. [Google Scholar] [CrossRef] [PubMed]
- Stanca, M.; Căpîlna, D.M.; Trâmbițaș, C.; Căpîlna, M.E. The Overall Quality of Life and Oncological Outcomes Following Radical Hysterectomy in Cervical Cancer Survivors Results from a Large Long-Term Single-Institution Study. Cancers 2022, 14, 317. [Google Scholar] [CrossRef] [PubMed]
- Hanprasertpong, J.; Geater, A.; Jiamset, I.; Padungkul, L.; Hirunkajonpan, P.; Songhong, N. Fear of cancer recurrence and its predictors among cervical cancer survivors. J. Gynecol. Oncol. 2017, 28, e72. [Google Scholar] [CrossRef]
- Karawekpanyawong, N.; Kaewkitikul, K.; Maneeton, B.; Maneeton, N.; Siriaree, S. The prevalence of depressive disorder and its association in Thai cervical cancer patients. PLoS ONE 2021, 16, e0252779. [Google Scholar] [CrossRef]
- Cull, A.; Cowie, V.; Farquharson, D.; Livingstone, J.; Smart, G.; Elton, R. Early stage cervical cancer: Psychosocial and sexual outcomes of treatment. Br. J. Cancer 1993, 68, 1216–1220. [Google Scholar] [CrossRef]
- Ramirez, P.T.; Frumovitz, M.; Pareja, R.; Lopez, A.; Vieira, M.; Ribeiro, M.; Buda, A.; Yan, X.; Shuzhong, Y.; Chetty, N.; et al. Minimally Invasive versus Abdominal Radical Hysterectomy for Cervical Cancer. N. Engl. J. Med. 2018, 379, 1895–1904. [Google Scholar] [CrossRef]
- Uccella, S.; Capozzi, V.A.; Ricco’, M.; Perrone, E.; Zanello, M.; Ferrari, S.; Zorzato, P.C.; Seracchioli, R.; Cromi, A.; Serati, M.; et al. Sexual Function following Laparoscopic versus Transvaginal Closure of the Vaginal Vault after Laparoscopic Hysterectomy: Secondary Analysis of a Randomized Trial by the Italian Society of Gynecological Endoscopy Using a Validated Questionnaire. J. Minim. Invasive Gynecol. 2019, 27, 186–194. [Google Scholar] [CrossRef]
- Alletti, S.G.; Vizzielli, G.; Lafuenti, L.; Costantini, B.; Fagotti, A.; Fedele, C.; Cianci, S.; Perrone, E.; Gallotta, V.; Rossitto, C.; et al. Single-Institution Propensity-Matched Study to Evaluate the Psychological Effect of Minimally Invasive Interval Debulking Surgery Versus Standard Laparotomic Treatment: From Body to Mind and Back. J. Minim. Invasive Gynecol. 2018, 25, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Alletti, S.G.; Perrone, E.; Cretì, A.; Cianci, S.; Uccella, S.; Fedele, C.; Fanfani, F.; Palmieri, S.; Fagotti, A.; Scambia, G.; et al. Feasibility and perioperative outcomes of percutaneous-assisted laparoscopic hysterectomy: A multicentric Italian experience. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 245, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Cianci, S.; Rosati, A.; Rumolo, V.; Alletti, S.G.; Gallotta, V.; Turco, L.C.; Corrado, G.; Vizzielli, G.; Fagotti, A.; Fanfani, F.; et al. Robotic Single-Port Platform in General, Urologic, and Gynecologic Surgeries: A Systematic Review of the Literature and Meta-analysis. World J. Surg. 2019, 43, 2401–2419. [Google Scholar] [CrossRef] [PubMed]
- Alletti, S.G.; Cianci, S.; Perrone, E.; Fanfani, F.; Vascone, C.; Uccella, S.; Gallotta, V.; Vizzielli, G.; Fagotti, A.; Monterossi, G.; et al. Technological innovation and personalized surgical treatment for early-stage endometrial cancer patients: A prospective multicenter Italian experience to evaluate the novel percutaneous approach. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 234, 218–222. [Google Scholar] [CrossRef]
- Cianci, S.; Rosati, A.; Vargiu, V.; Capozzi, V.A.; Sozzi, G.; Gioè, A.; Alletti, S.G.; Ercoli, A.; Cosentino, F.; Berretta, R.; et al. Sentinel Lymph Node in Aged Endometrial Cancer Patients “The SAGE Study”: A Multicenter Experience. Front. Oncol. 2021, 11, 737096. [Google Scholar] [CrossRef]
- Capozzi, V.A.; Riemma, G.; Rosati, A.; Vargiu, V.; Granese, R.; Ercoli, A.; Cianci, S. Surgical complications occurring during minimally invasive sentinel lymph node detection in endometrial cancer patients. A systematic review of the literature and metanalysis. Eur. J. Surg. Oncol. (EJSO) 2021, 47, 2142–2149. [Google Scholar] [CrossRef]
- Cianci, S.; Perrone, E.; Rossitto, C.; Fanfani, F.; Tropea, A.; Biondi, A.; Scambia, G.; Alletti, S.G. Percutaneous-assisted vs mini-laparoscopic hysterectomy: Comparison of ultra-minimally invasive approaches. Updates Surg. 2020, 73, 2347–2354. [Google Scholar] [CrossRef]
- Uccella, S.; Franchi, M.P.; Cianci, S.; Zorzato, P.C.; Bertoli, F.; Alletti, S.G.; Ghezzi, F.; Scambia, G. Laparotomy vs. minimally invasive surgery for ovarian cancer recurrence: A systematic review. Gland Surg. 2020, 9, 1130–1139. [Google Scholar] [CrossRef]
- Gueli Alletti, S.; Capozzi, V.A.; Rosati, A.; De Blasis, I.; Cianci, S.; Vizzielli, G.; Uccella, S.; Gallotta, V.; Fanfani, F.; Fagotti, A.; et al. Laparoscopy vs. laparotomy for advanced ovarian cancer: A systematic review of the literature. Minerva Med. 2019, 110, 341–357. [Google Scholar] [CrossRef]
- Membrilla-Beltran, L.; Cardona, D.; Camara-Roca, L.; Aparicio-Mota, A.; Roman, P.; Rueda-Ruzafa, L. Impact of Cervical Cancer on Quality of Life and Sexuality in Female Survivors. Int. J. Environ. Res. Public Health 2023, 20, 3751. [Google Scholar] [CrossRef]
- Bergmark, K.; Åvall-Lundqvist, E.; Dickman, P.W.; Henningsohn, L.; Steineck, G. Vaginal Changes and Sexuality in Women with a History of Cervical Cancer. N. Engl. J. Med. 1999, 340, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.; Raviv, L.; Applegarth, L.; Ford, J.S.; Josephs, L.; Grill, E.; Sklar, C.; Sonoda, Y.; Baser, R.E.; Barakat, R.R. A cross-sectional study of the psychosexual impact of cancer-related infertility in women: Third-party reproductive assistance. J. Cancer Surviv. 2010, 4, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Galica, J.; Saunders, S.; Romkey-Sinasac, C.; Silva, A.; Ethier, J.-L.; Giroux, J.; Jull, J.; Maheu, C.; Ross-White, A.; Stark, D.; et al. The needs of gynecological cancer survivors at the end of primary treatment: A scoping review and proposed model to guide clinical discussions. Patient Educ. Couns. 2022, 105, 1761–1782. [Google Scholar] [CrossRef] [PubMed]
- Cianci, S.; Rumolo, V.; Rosati, A.; Scaletta, G.; Alletti, S.G.; Cerentini, T.M.; Sleiman, Z.; Lordelo, P.; Angerame, D.; Garganese, G.; et al. Sarcopenia in Ovarian Cancer Patients, Oncologic Outcomes Revealing the Importance of Clinical Nutrition: Review of Literature. Curr. Pharm. Des. 2019, 25, 2480–2490. [Google Scholar] [CrossRef] [PubMed]
- Plotti, F.; Terranova, C.; Luvero, D.; Bartolone, M.; Messina, G.; Feole, L.; Cianci, S.; Scaletta, G.; Marchetti, C.; Di Donato, V.; et al. Diet and Chemotherapy: The Effects of Fasting and Ketogenic Diet on Cancer Treatment. Chemotherapy 2020, 65, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Lammerink, E.A.; de Bock, G.H.; Pras, E.; Reyners, A.K.; Mourits, M.J. Sexual functioning of cervical cancer survivors: A review with a female perspective. Maturitas 2012, 72, 296–304. [Google Scholar] [CrossRef]
- Lundt, A.; Jentschke, E. Long-Term Changes of Symptoms of Anxiety, Depression, and Fatigue in Cancer Patients 6 Months After the End of Yoga Therapy. Integr. Cancer Ther. 2019, 18, 1534735418822096. [Google Scholar] [CrossRef]
- Taso, C.-J.; Lin, H.-S.; Lin, W.-L.; Chen, S.-M.; Huang, W.-T.; Chen, S.-W. The effect of yoga exercise on improving depression, anxiety, and fatigue in women with breast cancer: A randomized controlled trial. J. Nurs. Res. 2014, 22, 155–164. [Google Scholar] [CrossRef]
- Sacomori, C.; Lorca, L.A.; Martinez-Mardones, M.; Salas-Ocaranza, R.I.; Reyes-Reyes, G.P.; Pizarro-Hinojosa, M.N.; Plasser-Troncoso, J. A randomized clinical trial to assess the effectiveness of pre- and post-surgical pelvic floor physiotherapy for bowel symptoms, pelvic floor function, and quality of life of patients with rectal cancer: CARRET protocol. Trials 2021, 22, 448. [Google Scholar] [CrossRef]
- Alcântara-Silva, T.R.; De Freitas-Junior, R.; Freitas, N.M.A.; Junior, W.D.P.; Da Silva, D.J.; Machado, G.D.P.; Ribeiro, M.K.A.; Carneiro, J.P.; Soares, L. Music Therapy Reduces Radiotherapy-Induced Fatigue in Patients With Breast or Gynecological Cancer: A Randomized Trial. Integr. Cancer Ther. 2018, 17, 628–635. [Google Scholar] [CrossRef]
- Burish, T.G.; Jenkins, R.A. Effectiveness of biofeedback and relaxation training in reducing the side effects of cancer chemotherapy. Health Psychol. 1992, 11, 17–23. [Google Scholar] [CrossRef]
- Greimel, E.R.; Winter, R.; Kapp, K.S.; Haas, J. Quality of life and sexual functioning after cervical cancer treatment: A long-term follow-up study. Psycho-Oncol. J. Psychol. Soc. Behav. Dimens. Cancer 2009, 18, 476–482. [Google Scholar] [CrossRef]
- Flay, L.D.; Matthews, J.H. The effects of radiotherapy and surgery on the sexual function of women treated for cervical cancer. Int. J. Radiat. Oncol. 1995, 31, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Cianci, S.; Tarascio, M.; Rosati, A.; Caruso, S.; Uccella, S.; Cosentino, F.; Scaletta, G.; Gueli Alletti, S.; Scambia, G. Sexual function and qulity of life of patients affected by ovarian cancer. Minerva Med. 2019, 110, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Cianci, S.; Rosati, A.; Capozzi, V.A.; Tarascio, M.; Uccella, S.; Palumbo, M.A.; Caruso, S. Quality of life and sexual functioning of patient affected by endometrial cancer. Minerva Med. 2021, 112, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.A.; Jhingran, A.; Oaknin, A.; Denny, L. Cervical cancer. Lancet 2019, 393, 169–182. [Google Scholar] [CrossRef]
- Johnson, C.A.; James, D.; Marzan, A.; Armaos, M. Cervical Cancer: An Overview of Pathophysiology and Management. Semin. Oncol. Nurs. 2019, 35, 166–174. [Google Scholar] [CrossRef]
- Stanley, M. Pathology and epidemiology of HPV infection in females. Gynecol. Oncol. 2010, 117, S5–S10. [Google Scholar] [CrossRef]
- Bruni, L.; Saura-Lázaro, A.; Montoliu, A.; Brotons, M.; Alemany, L.; Diallo, M.S.; Afsar, O.Z.; LaMontagne, D.S.; Mosina, L.; Contreras, M.; et al. HPV vaccination introduction worldwide and WHO and UNICEF estimates of national HPV immunization coverage 2010–2019. Prev. Med. 2020, 144, 106399. [Google Scholar] [CrossRef]
- Patel, C.; Brotherton, J.M.; Pillsbury, A.; Jayasinghe, S.; Donovan, B.; Macartney, K.; Marshall, H. The impact of 10 years of human papillomavirus (HPV) vaccination in Australia: What additional disease burden will a nonavalent vaccine prevent? Eurosurveillance 2018, 23, 1700737. [Google Scholar] [CrossRef]
- Farnsworth, A. Cervical cancer screening in Australia: Past and present. Cancer Cytopathol. 2015, 124, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Kramer, J. Eradicating cervical cancer: Lessons learned from Rwanda and Australia. Int. J. Gynecol. Obstet. 2021, 154, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Santesso, N.; Mustafa, R.A.; Schünemann, H.J.; Arbyn, M.; Blumenthal, P.D.; Cain, J.; Chirenje, M.; Denny, L.; De Vuyst, H.; Eckert, L.O.; et al. World Health Organization Guidelines for treatment of cervical intraepithelial neoplasia 2–3 and screen-and-treat strategies to prevent cervical cancer. Int. J. Gynecol. Obstet. 2015, 132, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Bhatla, N.; Aoki, D.; Sharma, D.N.; Sankaranarayanan, R. Cancer of the cervix uteri: 2021 update. Int. J. Gynecol. Obstet. 2021, 155 (Suppl. S1), 28–44. [Google Scholar] [CrossRef]
- Stefan, D.C.; Dangou, J.-M.; Barango, P.; Mahamadou, I.D.; Kapambwe, S. The World Health Organization targets for cervical cancer control by 2030: A baseline assessment in six African countries—Part I. Ecancermedicalscience 2022, 16, 1453. [Google Scholar] [CrossRef]
- Kjaer, S.K.; Dehlendorff, C.; Belmonte, F.; Baandrup, L. Real-World Effectiveness of Human Papillomavirus Vaccination Against Cervical Cancer. Gynecol. Oncol. 2021, 113, 1329–1335. [Google Scholar] [CrossRef]
- Athanasiou, S.; Pitsouni, E.; Grigoriadis, T.; Michailidis, G.; Tsiveleka, A.; Rodolakis, A.; Loutradis, D. A study protocol of vaginal laser therapy in gynecological cancer survivors. Climacteric 2020, 23, 53–58. [Google Scholar] [CrossRef]
- Hubbs, J.L.; Michelson, E.L.D.; Vogel, R.I.; Rivard, C.L.; Teoh, D.G.K.; Geller, M.A. Sexual quality of life after the treatment of gynecologic cancer: What women want. Support. Care Cancer 2019, 27, 4649–4654. [Google Scholar] [CrossRef]
- Murina, F.; Felice, R.; Di Francesco, S.; Nelvastellio, L.; Cetin, I. Ospemifene plus fractional CO2 laser: A powerful strategy to treat postmenopausal vulvar pain. Gynecol. Endocrinol. 2020, 36, 431–435. [Google Scholar] [CrossRef]
- Angioli, R.; Stefano, S.; Filippini, M.; Pieralli, A.; Montera, R.; Plotti, F.; Gatti, A.; Bartolone, M.; Luvero, D. Effectiveness of CO2 laser on urogenital syndrome in women with a previous gynecological neoplasia: A multicentric study. Int. J. Gynecol. Cancer 2020, 30, 590–595. [Google Scholar] [CrossRef]
- Vargiu, V.; Amar, I.D.; Rosati, A.; Dinoi, G.; Turco, L.C.; Capozzi, V.A.; Scambia, G.; Villa, P. Hormone replacement therapy and cervical cancer: A systematic review of the literature. Climacteric 2021, 24, 120–127. [Google Scholar] [CrossRef] [PubMed]
- DiNicola, S.; Pasta, V.; Costantino, D.; Guaraldi, C.; Bizzarri, M. Hyaluronic acid and vitamins are effective in reducing vaginal atrophy in women receiving radiotherapy. Minerva Obstet. Gynecol. 2015, 67, 523–531. [Google Scholar]
- D’Oria, O.; Giannini, A.; Buzzaccarini, G.; Tinelli, A.; Corrado, G.; Frega, A.; Vizza, E.; Caserta, D. Fractional Co2 laser for vulvo-vaginal atrophy in gynecologic cancer patients: A valid therapeutic choice? A systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2022, 277, 84–89. [Google Scholar] [CrossRef]
- dos Santos, C.C.M.; Uggioni, M.L.R.; Colonetti, T.; Colonetti, L.; Grande, A.J.; Da Rosa, M.I. Hyaluronic Acid in Postmenopause Vaginal Atrophy: A Systematic Review. J. Sex. Med. 2021, 18, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Santen, R.J.; Stuenkel, C.A.; Davis, S.R.; Pinkerton, J.V.; Gompel, A.; Lumsden, M.A. Managing Menopausal Symptoms and Associated Clinical Issues in Breast Cancer Survivors. J. Clin. Endocrinol. Metab. 2017, 102, 3647–3661. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.; Vaz, A.F.; Grion, R.C.; Costa-Paiva, L.; Baccaro, L.F. Topical estrogen, testosterone, and vaginal dilator in the prevention of vaginal stenosis after radiotherapy in women with cervical cancer: A randomized clinical trial. BMC Cancer 2021, 21, 682. [Google Scholar] [CrossRef]
- Logan, S.; Anazodo, A. The psychological importance of fertility preservation counseling and support for cancer patients. Acta Obstet. Gynecol. Scand. 2019, 98, 583–597. [Google Scholar] [CrossRef]
- Schultz, W.C.M.W.; Van de Wiel, H.B.M.; Hahn, D.E.E.; Bouma, J. Psychosexual functioning after treatment for gynecologi-cal cancer: An integrative model, review of determinant factors and clinical guidelines. Int. J. Gynecol. Cancer 1992, 2, 281–290. [Google Scholar] [CrossRef]
- Plotti, F.; Nelaj, E.; Sansone, M.; Antonelli, E.; Altavilla, T.; Angioli, R.; Panici, P.B. Sexual Function after Modified Radical Hysterectomy (Piver II/Type B) vs. Classic Radical Hysterectomy (Piver III/Type C2) for Early Stage Cervical Cancer. A Prospective Study. J. Sex. Med. 2012, 9, 909–917. [Google Scholar] [CrossRef]
- Plotti, F.; Sansone, M.; Di Donato, V.; Antonelli, E.; Altavilla, T.; Angioli, R.; Panici, P.B. Quality of life and sexual function after type C2/type III radical hysterectomy for locally advanced cervical cancer: A prospective study. J. Sex. Med. 2011, 8, 894–904. [Google Scholar] [CrossRef]
| Author | Study Design | Number of Patients and Features | Purpose | Questionnaire | Study Findings |
|---|---|---|---|---|---|
| Kirchheiner [38] 2021 | Prospective study | 1045 pts | SF in CC | EORTC QLQ-CX 24 | 60% were sexually active after treatment; Vaginal symptoms were negatively correlated with sexual enjoyment (p ≤ 0.001). |
| Novackova [39] 2022 | Prospective study | 36 pts | SF in early stage CC with nerve sparing radical hysterctomy therapy | FSFI EORTC QLQ-CX 24 EORTC QLQ-C30 | Decreased sexual functioning after surgery, but no changes in sexual activity, enjoyment of worry |
| Park [40] 2007 | Retrospective case-control study | 860 pts 494 CG | SF in CC | EORTC QLQ-C30 | CC pts had more sexual disfunction compared to CG. CC pts who received both surgery and adjuvant therapy than surgery alone reported significantly worse sexual or vaginal problems |
| Jensen [41] 2003 | Multicenter prospective case-control study | 173 pts 328 CG | SF after RH in CC | SVQ EORTC | CC had more vaginal symptoms, 91% were sexually active before and after surgery, but with a decrease of frequency |
| Ljuca [42] 2010 | Retrospective-prospective study | 35 pts | SF before and after chemoradiotherapy in CC | EORTC QLQ-CX 24 | Improvement of vaginal function after chemoradiotherapy, but no difference in SF before and after therapy |
| Bakker [43] 2011 | Cross-sectional study | 252 pts | SF in CC | FSDS EORTC QLQ-CX24 HADS MMQ | 38% CC had sexual distress; it was associated with vaginal symptoms and body imagine concerns |
| Aerts [44] 2014 | Prospective case-control study | 31 pts 93 CG 93 TLH | Compare SF in CC who only underwent surgery RH vs. healthy control group and womaen who underwent TLH for benign gynecological disease | SSFS SSPQ | The CC compared to the healthy group had a high risk of sexual disfunction; No difference in SF for CC compared to women with benign conditions |
| Correa [45] 2015 | Retrospective case control study | 37 pts 37 CG | SF in CC | FSFI | CC decreased SF by 64.9%, vaginal stenosis by 59.5%, not sexually active was at 80%, and who were sexually active had sexual dysfunction |
| Lee [46] 2016 | Cross-sectional, case-control study | 104 pts CG 104 | compare SF between sexually active CC and healthy women. | EORTC QLQ-C30 EORTC QLQ-CX24 FSFI | No difference in SF |
| Carter [47] 2010 | Prospective study | 52 pts: 33 RT + 19 RH | SF and QOL in early-stage cervical cancer undergoing RT or RH | FSFI | FSFI mean scores were below 26.55—the clinical cut-off; No difference in SF between group with RH and RT |
| Hofsjö [48] 2017 | Retrospective case-control study | 34 pts 37 CG | SF in CC | a questionnaire designed to assess sexual function | Reduced satisfaction in sex, lubrification, elasticity and vaginal length; No differences in interest in sex between the pts and the CG in physical and psychological wellbeing and level of anxiety and depression |
| De Rosa [49] 2017 | Prospective study | 52 pts | Evaluates the effect on VHI, QoL, and SF of ospemifene in CC survivors. | EORTC QLQ-C30 | Improvement of body image, sexual enjoyment and SF |
| Plotti [50] 2017 | Retrospective study | 90 pts | SF in long-term CC survivor affected by LACC and treated with type C2/type III RH | EORTC QLQ-CX24 EORTC QLQ-C30 | Good level of sexual enjoyment with a slight worsening of sexual activity |
| Bae [51] 2015 | Cross-sectional study | 137 Pts | To examine the level of sexual function, depression and quality of life in CC pts | FSFI HADS FACT-G | CC pts had low sexual functionand about 45.4% of them experienced more than a moderate level of depression. Also, pts with lower sexual function had lower QoL and higher levels of depression |
| Serati [52] 2009 | Prospective case-control study | 38 pts (20 laparoscopic RH, 18 laparotomic RH) 35 CG | SF after RH vs. a control group of healthy women SF after laparoscopic RH vs. laparotomic RH | FSFI | RH worsens SF, without any significative difference between laparoscopy and laparotomy |
| Shi [53] 2020 | RCT | 91 pts RH: intervention group n = 46 control group n = 45 | To assess the efficacy of a nurse-led positive psychology intervention on sexual function, depression and subjective well-being among postop pts with early CC | FSFI | Sexual function, depression and subjective well-being were significantly improved in the intervention group |
| Cerentini [54] 2019 | Prospective case-control study | 88 pts | use of vaginal dilators after brachytherapy in CC. | EORTC QLQ-C30 | vaginal dilators did not increase the dimension of thevagina |
| Mihai Stanca [55] 2022 | Retrospective observational study | 430 pts | QoL and SF in CC | EORTC QLQ-CX 24 QLQ-C30 | Decreased sexual function, activity and enjoyment |
| No interest in sex [39,40,41,42,43,44,46,49] |
| Physical problem (depression, anxiety, surgical scar) [40,43,44,46,47,51,52,54] |
| No partner [39,41,43,44,45] |
| Vaginal dryness [38,39,40,41,48,49,50,52,54] |
| Dyspareunia and pain [39,40,41,42,43,47,49,53] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cianci, S.; Tarascio, M.; Arcieri, M.; La Verde, M.; Martinelli, C.; Capozzi, V.A.; Palmara, V.; Gulino, F.; Gueli Alletti, S.; Caruso, G.; et al. Post Treatment Sexual Function and Quality of Life of Patients Affected by Cervical Cancer: A Systematic Review. Medicina 2023, 59, 704. https://doi.org/10.3390/medicina59040704
Cianci S, Tarascio M, Arcieri M, La Verde M, Martinelli C, Capozzi VA, Palmara V, Gulino F, Gueli Alletti S, Caruso G, et al. Post Treatment Sexual Function and Quality of Life of Patients Affected by Cervical Cancer: A Systematic Review. Medicina. 2023; 59(4):704. https://doi.org/10.3390/medicina59040704
Chicago/Turabian StyleCianci, Stefano, Mattia Tarascio, Martina Arcieri, Marco La Verde, Canio Martinelli, Vito Andrea Capozzi, Vittorio Palmara, Ferdinando Gulino, Salvatore Gueli Alletti, Giuseppe Caruso, and et al. 2023. "Post Treatment Sexual Function and Quality of Life of Patients Affected by Cervical Cancer: A Systematic Review" Medicina 59, no. 4: 704. https://doi.org/10.3390/medicina59040704
APA StyleCianci, S., Tarascio, M., Arcieri, M., La Verde, M., Martinelli, C., Capozzi, V. A., Palmara, V., Gulino, F., Gueli Alletti, S., Caruso, G., Restaino, S., Vizzielli, G., Conte, C., Palumbo, M., & Ercoli, A. (2023). Post Treatment Sexual Function and Quality of Life of Patients Affected by Cervical Cancer: A Systematic Review. Medicina, 59(4), 704. https://doi.org/10.3390/medicina59040704











