Olfactory Neuroblastoma—A Challenging Fine Line between Metastasis and Hematology
Abstract
1. Introduction
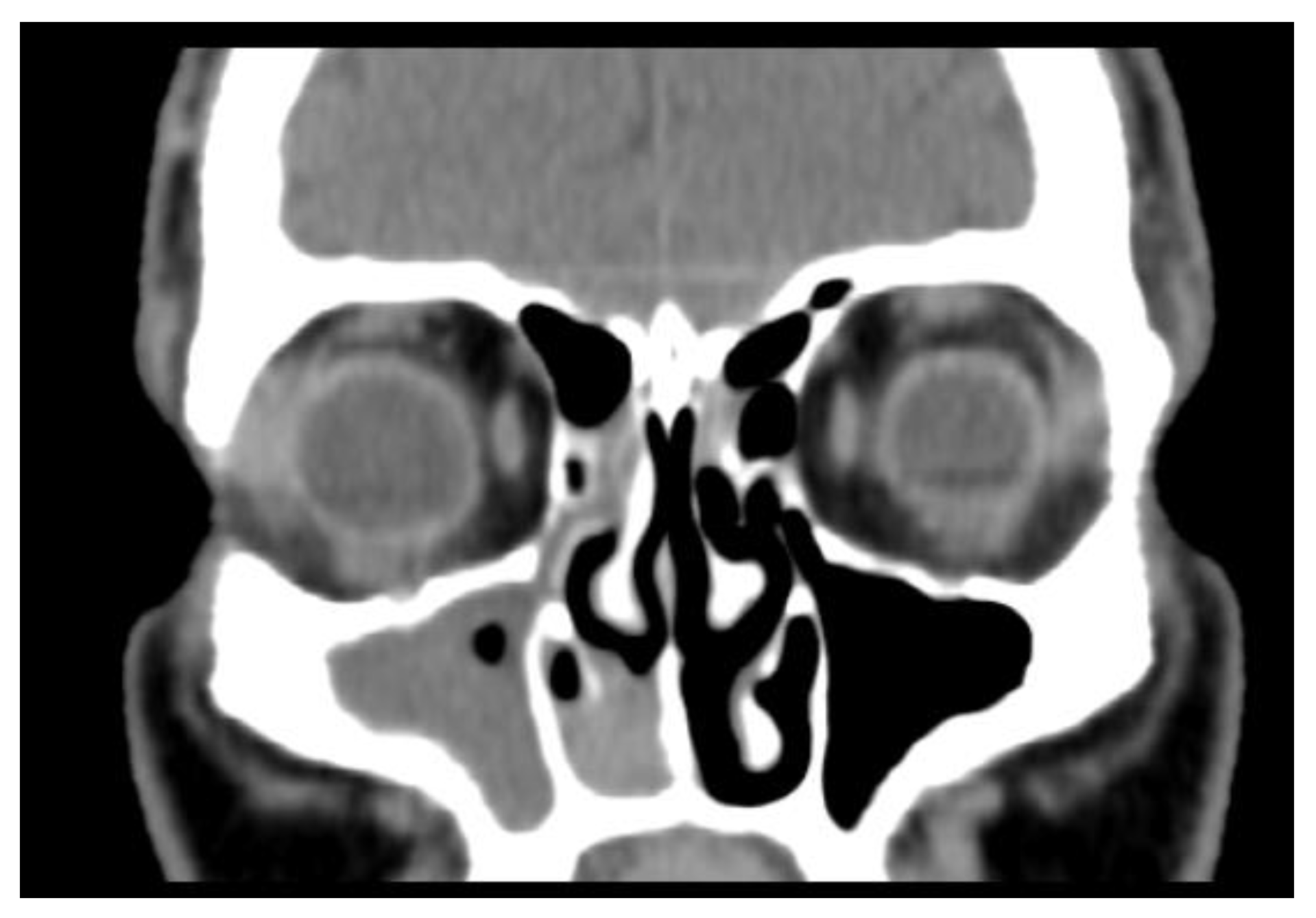
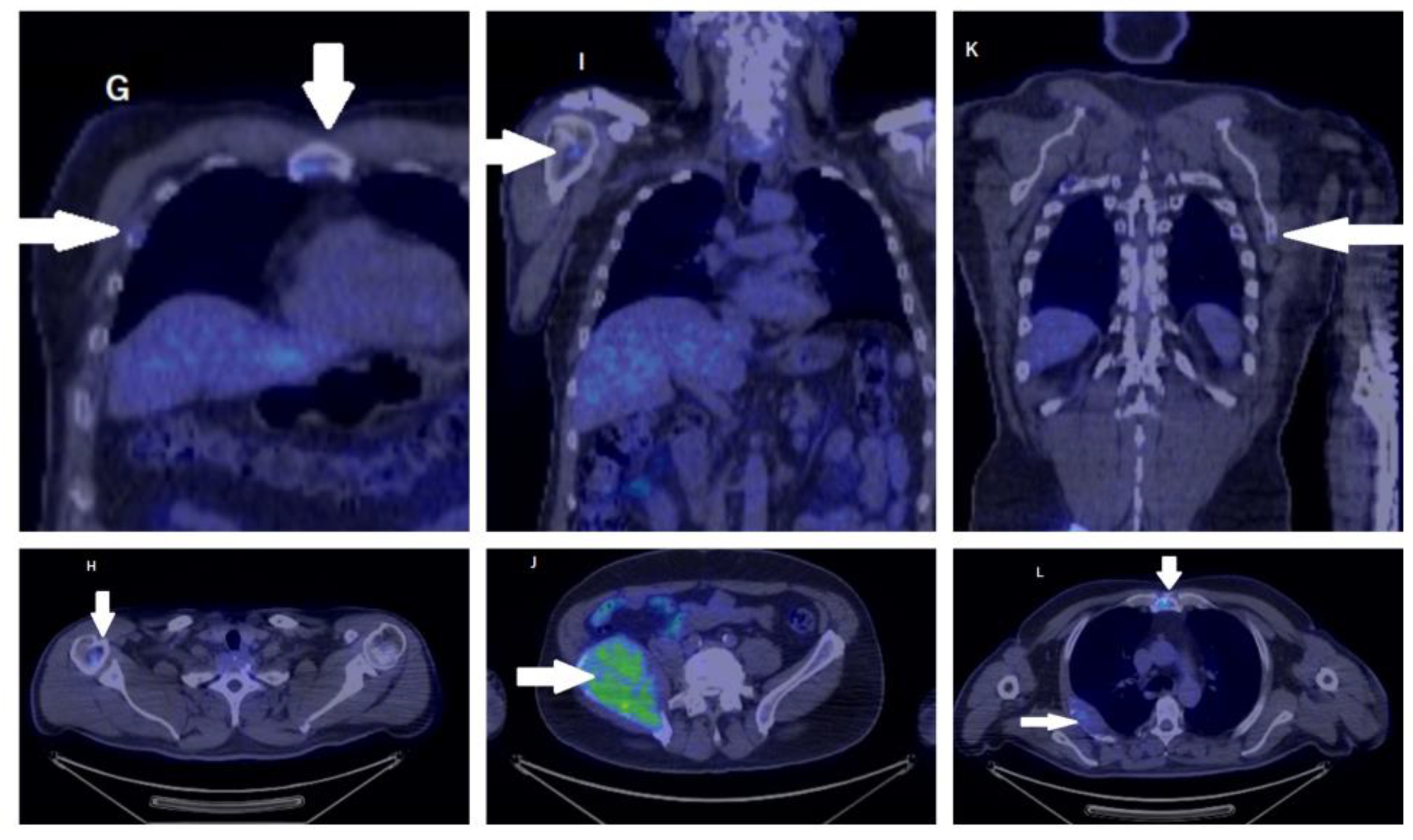
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Lund, V.J.; Clarke, P.M.; Swift, A.C.; McGarry, G.W.; Kerawala, C.; Carnell, D. Nose and paranasal sinus tumours: United Kingdom National Multidisciplinary Guidelines. J. Laryngol. Otol. 2016, 130 (Suppl. S2), S111–S118. [Google Scholar] [CrossRef]
- Faragalla, H.; Weinreb, I. Olfactory neuroblastoma: A review and update. Adv. Anat. Pathol. 2009, 16, 322–331. [Google Scholar] [CrossRef]
- Gallia, G.L.; Reh, D.D.; Salmasi, V.; Blitz, A.M.; Koch, W.; Ishii, M. Endonasal endoscopic resection of esthesioneuroblastoma: The Johns Hopkins Hospital experience and review of the literature. Neurosurg. Rev. 2011, 34, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Naples, J.G.; Spiro, J.; Tessema, B.; Kuwada, C.; Kuo, C.L.; Brown, S.M. Neck recurrence and mortality in esthesioneuroblastoma: Implications for management of the N0 neck. Laryngoscope 2016, 126, 1373–1379. [Google Scholar] [CrossRef] [PubMed]
- Zanation, A.M.; Ferlito, A.; Rinaldo, A.; Gore, M.R.; Lund, V.J.; McKinney, K.A.; Suárez, C.; Takes, R.P.; Devaiah, A.K. When, how and why to treat the neck in patients with esthesioneuroblastoma: A review. Eur. Arch. Otorhinolaryngol. 2010, 267, 1667–1671. [Google Scholar] [CrossRef] [PubMed]
- Kadish, S.; Goodman, M.; Wang, C.C. Olfactory neuroblastoma. A clinical analysis of 17 cases. Cancer 1976, 37, 1571–1576. [Google Scholar] [CrossRef] [PubMed]
- Dulguerov, P.; Calcaterra, T. Esthesioneuroblastom February a: The UCLA experience 1970–1990. Laryngoscope 1992, 102, 843–849. [Google Scholar] [CrossRef]
- Abdelmeguid, A.S.; Bell, D.; Roberts, D.; Ferrarotto, R.; Phan, J.; Su, S.Y.; Kupferman, M.; Raza, S.; DeMonte, F.; Hanna, E. Long-Term Outcomes of Olfactory Neuroblastoma: MD Anderson Cancer Center Experience and Review of the Literature. Laryngoscope 2022, 132, 290–297. [Google Scholar] [CrossRef]
- Dulguerov, P.; Allal, A.S.; Calcaterra, T.C. Esthesioneuroblastoma: A meta-analysis and review. Lancet Oncol. 2001, 2, 683–690. [Google Scholar] [CrossRef]
- Jethanamest, D.; Morris, L.G.; Sikora, A.G.; Kutler, D.I. Esthesioneuroblastoma: A population-based analysis of survival and prognostic factors. Arch. Otolaryngol. Head Neck Surg. 2007, 133, 276–280. [Google Scholar] [CrossRef]
- Ozsahin, M.; Gruber, G.; Olszyk, O.; Karakoyun-Celik, O.; Pehlivan, B.; Azria, D.; Roelandts, M.; Kaanders, J.H.; Cengiz, M.; Krengli, M.; et al. Outcome and prognostic factors in olfactory neuroblastoma: A rare cancer network study. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 992–997. [Google Scholar] [CrossRef] [PubMed]
- Fitzmaurice, C.; Allen, C.; Barber, R.M.; Barregard, L.; Bhutta, Z.A.; Brenner, H.; Dicker, D.J.; Chimed-Orchir, O.; Dandona, R.; Satpathy, M.; et al. Global Burden of Disease Cancer Collaboration. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2017, 3, 524–548, Erratum in JAMA Oncol. 2017, 3, 418. [Google Scholar] [PubMed]
- Myeloma Canada. Available online: https://myelomacanada.ca/en/about-multiple-myeloma/what-is-myeloma/statistics (accessed on 23 February 2023).
- Kosmala, A.; Bley, T.; Petritsch, B. Imaging of Multiple Myeloma. Rofo 2019, 191, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Wallington-Beddoe, C.T.; Mynott, R.L. Prognostic and predictive biomarker developments in multiple myeloma. J. Hematol. Oncol. 2021, 14, 151. [Google Scholar] [CrossRef] [PubMed]
- Czapiewski, P.; Kunc, M.; Haybaeck, J. Genetic and molecular alterations in olfactory neuroblastoma: Implications for pathogenesis, prognosis and treatment. Oncotarget 2016, 7, 52584–52596. [Google Scholar] [CrossRef]
- Kirn, J.W.; Kong, G.; Lee, C.H.; Kim, D.Y.; Rhee, C.S.; Min, Y.G.; Kim, C.W.; Chung, J.H. Expression of Bcl-2 in olfactory neuroblastoma and its association with chemotherapy and survival. Otolaryngol. Head Neck Surg. 2008, 139, 708–712. [Google Scholar]
- Castelnuovo, P.; Turri-Zanoni, M.; Battaglia, P.; Antognoni, P.; Bossi, P.; Locatelli, D. Sinonasal Malignancies of Anterior Skull Base: Histology-driven Treatment Strategies. Otolaryngol. Clin. N. Am. 2016, 49, 183–200. [Google Scholar] [CrossRef]
- Castelnuovo, P.; Bignami, M.; Delù, G.; Battaglia, P.; Bignardi, M.; Dallan, I. Endonasal endoscopic resection and radiotherapy in olfactory neuroblastoma: Our experience. Head Neck 2007, 29, 845–850. [Google Scholar] [CrossRef]
- Morita, A.; Ebersold, M.J.; Olsen, K.D.; Foote, R.L.; Lewis, J.E.; Quast, L.M. Esthesioneuroblastoma: Prognosis and management. Neurosurgery 1993, 32, 706–714, discussion 714–715. [Google Scholar] [CrossRef]
- Arnold, M.A.; Farnoosh, S.; Gore, M.R. Comparing Kadish and Modified Dulguerov Staging Systems for Olfactory Neuroblastoma: An Individual Participant Data Meta-analysis. Otolaryngol. Head Neck Surg. 2020, 163, 418–427. [Google Scholar] [CrossRef]
- Montava, M.; Verillaud, B.; Kania, R.; Sauvaget, E.; Bresson, D.; Mancini, J.; Froelich, S.; Herman, P. Critical analysis of recurrences of esthesioneuroblastomas: Can we prevent them? Eur. Arch. Oto-Rhino-Laryngol. 2014, 271, 3215–3222. [Google Scholar] [CrossRef] [PubMed]
- Balica, N.; Cotulbea, S.; Poenaru, M.; Marin, A.H.; Doros, C.; Lupescu, S.; Boia, E.R.; Stefanescu, H. Olfactory neuroblastoma-Case report. Front. ORLQ. Mag. Med. Cl. 2014, V, 20–24. [Google Scholar]
- Abdelmeguid, A.S. Olfactory Neuroblastoma. Curr. Oncol. Rep. 2018, 20, 7. [Google Scholar] [CrossRef] [PubMed]
- Bartel, R.; Gonzalez-Compta, X.; Cisa, E.; Cruellas, F.; Torres, A.; Rovira, A.; Manos, M. Importance of neoadjuvant chemotherapy in olfactory neuroblastoma treatment: Series report and literature review. Acta Otorrinolaringol. Esp. 2018, 69, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Modesto, A.; Blanchard, P.; Tao, Y.G.; Rives, M.; Janot, F.; Serrano, E.; Benlyazid, A.; Guigay, J.; Ferrand, F.R.; Delord, J.P.; et al. Multimodal treatment and long-term outcome of patients with esthesioneuroblastoma. Oral. Oncol. 2013, 49, 830–834. [Google Scholar] [CrossRef] [PubMed]
- Su, S.Y.; Bell, D.; Ferrarotto, R.; Phan, J.; Roberts, D.; Kupferman, M.E.; Frank, S.J.; Fuller, C.D.; Gunn, G.B.; Kies, M.S.; et al. Outcomes for olfactory neuroblastoma treated with induction chemotherapy. Head Neck 2017, 39, 1671–1679. [Google Scholar] [CrossRef]
- Thompson, L.D. Olfactory neuroblastoma. Head Neck Pathol. 2009, 3, 252–259. [Google Scholar] [CrossRef]
- Loy, A.H.; Reibel, J.F.; Read, P.W.; Thomas, C.Y.; Newman, S.A.; Jane, J.A.; Levine, P.A. Esthesioneuroblastoma: Continued follow-up of a single institution’s experience. Arch. Otolaryngol. Head Neck Surg. 2006, 132, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Morrison, T.; Booth, R.A.; Hauff, K.; Berardi, P.; Visram, A. Laboratory assessment of multiple myeloma. Adv. Clin. Chem. 2019, 89, 1–58. [Google Scholar] [CrossRef]



| Kadish System (1976) | Kadish Modified System | TNM Staging System for Olfactory Neuroblastoma from the Dulguerov Modified Version | |||
|---|---|---|---|---|---|
| Group A | Tumor confined to nasal cavity | Group A | Tumor confined to nasal cavity | T1 | Tumor involving the nasal cavity and/or paranasal sinuses (excluding sphenoid), sparing the most superior ethmoidal cells |
| Group B | Tumor involved nasal cavity and paranasal sinuses | Group B | Tumor involved nasal cavity and paranasal sinuses | T2 | Tumor involving the nasal cavity and/or paranasal sinuses (including the sphenoid) with extension to or erosion of the cribriform plate |
| Group C | Tumor spread beyond the nasal cavity and paranasal sinuses | Group C | Tumor extent beyond nasal cavity and paranasal sinuses, including involvement of the cribriform plate, base of the skull, orbit or intracranial cavity | T3 | Tumor extending into the orbit or protruding into the anterior cranial fossa, without dural invasion |
| Group D | Tumor with metastasis to cervical lymph nodes or distant sites | T4 | Tumor involving the brain | ||
| N0 | No cervical lymph-node metastasis | ||||
| N1 | Any form of cervical lymph-node metastasis | ||||
| M0 | No metastases | ||||
| M1 | Distant metastasis | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marina, T.C.; Constantin, B.N.; Flavia, B.; Silvana, S.O.; Marioara, P.; Sarau, C.A. Olfactory Neuroblastoma—A Challenging Fine Line between Metastasis and Hematology. Medicina 2023, 59, 731. https://doi.org/10.3390/medicina59040731
Marina TC, Constantin BN, Flavia B, Silvana SO, Marioara P, Sarau CA. Olfactory Neuroblastoma—A Challenging Fine Line between Metastasis and Hematology. Medicina. 2023; 59(4):731. https://doi.org/10.3390/medicina59040731
Chicago/Turabian StyleMarina, Trandafir Cornelia, Balica Nicolae Constantin, Baderca Flavia, Sarau Oana Silvana, Poenaru Marioara, and Cristian Andrei Sarau. 2023. "Olfactory Neuroblastoma—A Challenging Fine Line between Metastasis and Hematology" Medicina 59, no. 4: 731. https://doi.org/10.3390/medicina59040731
APA StyleMarina, T. C., Constantin, B. N., Flavia, B., Silvana, S. O., Marioara, P., & Sarau, C. A. (2023). Olfactory Neuroblastoma—A Challenging Fine Line between Metastasis and Hematology. Medicina, 59(4), 731. https://doi.org/10.3390/medicina59040731







