Resting-State EEG Connectivity at High-Frequency Bands and Attentional Performance Dysfunction in Stabilized Schizophrenia Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Continuous Performance Test and Other Cognitive Tests
2.3. Clinical Measures
2.4. Estimations for EEG Source Localization
2.5. Whole-Brain Electrical Source-Based Functional Connectivity
2.6. Statistical Analyses
3. Results
3.1. Sociodemographic and Clinical Characteristics of the Participants
3.2. Correlation Analyses
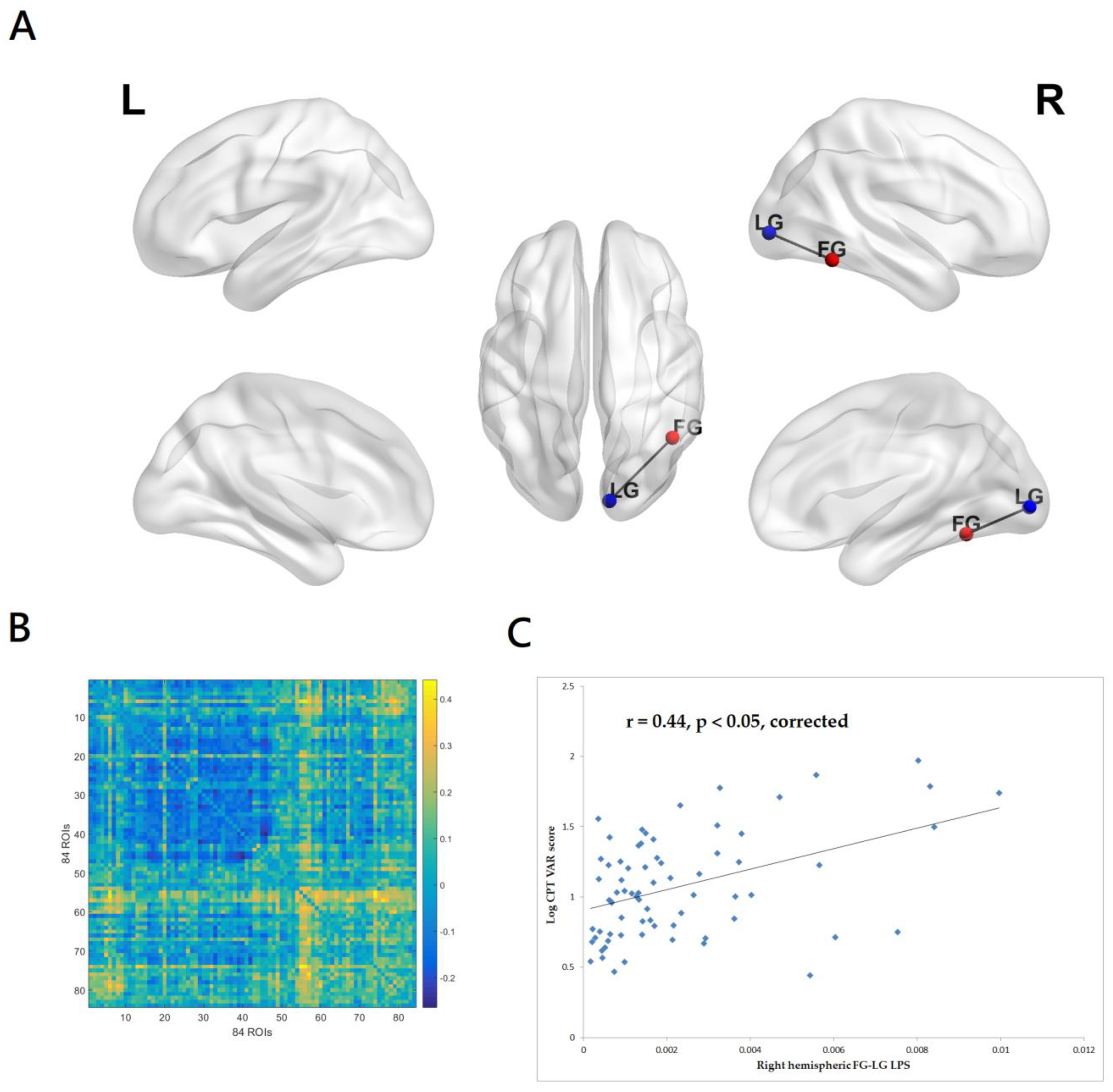
3.2.1. Correlations between CPT-II VAR Score and EEG Source-Based Functional Connectivity
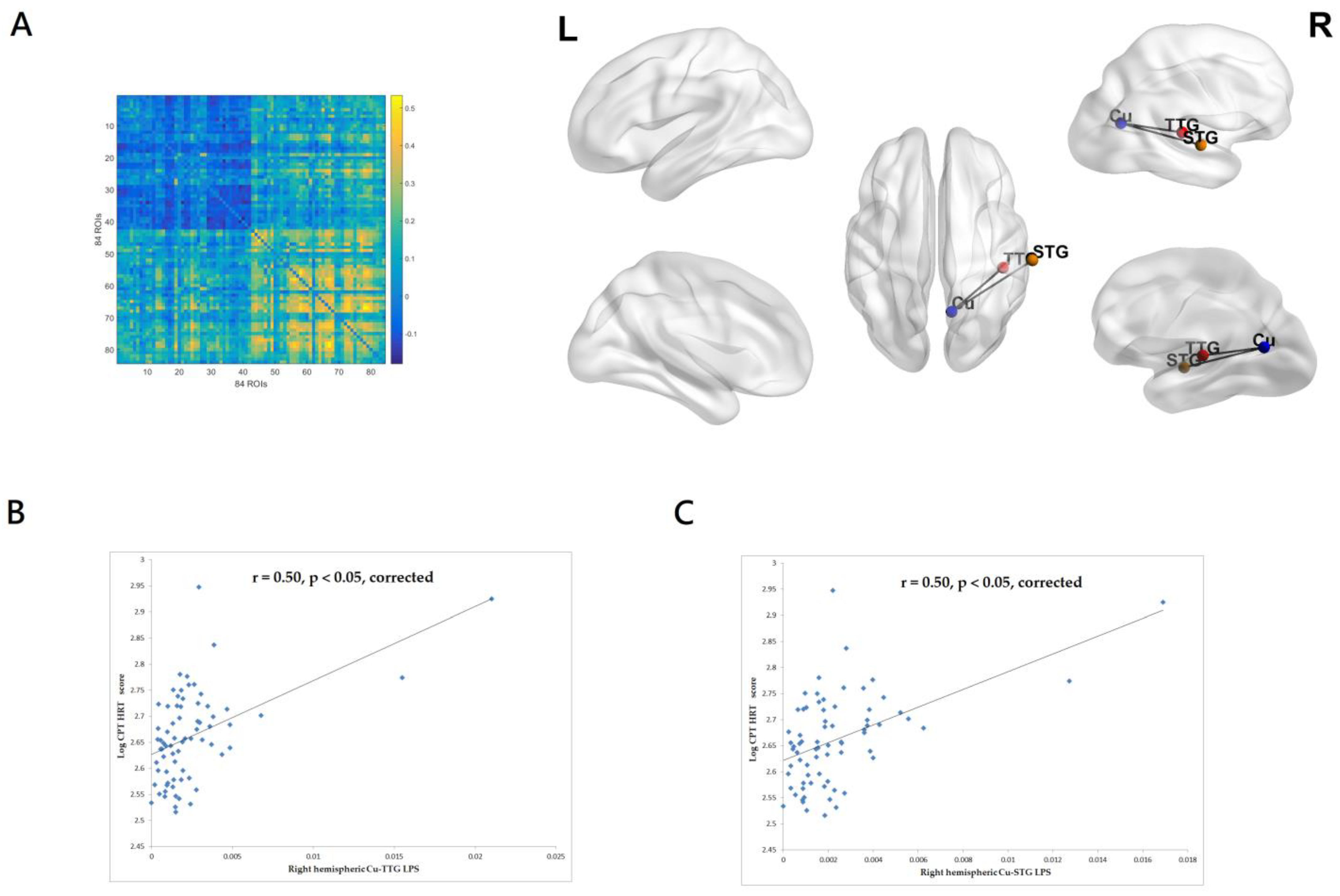
3.2.2. Correlations between CPT-II HRT Score and EEG Source-Based Functional Connectivity
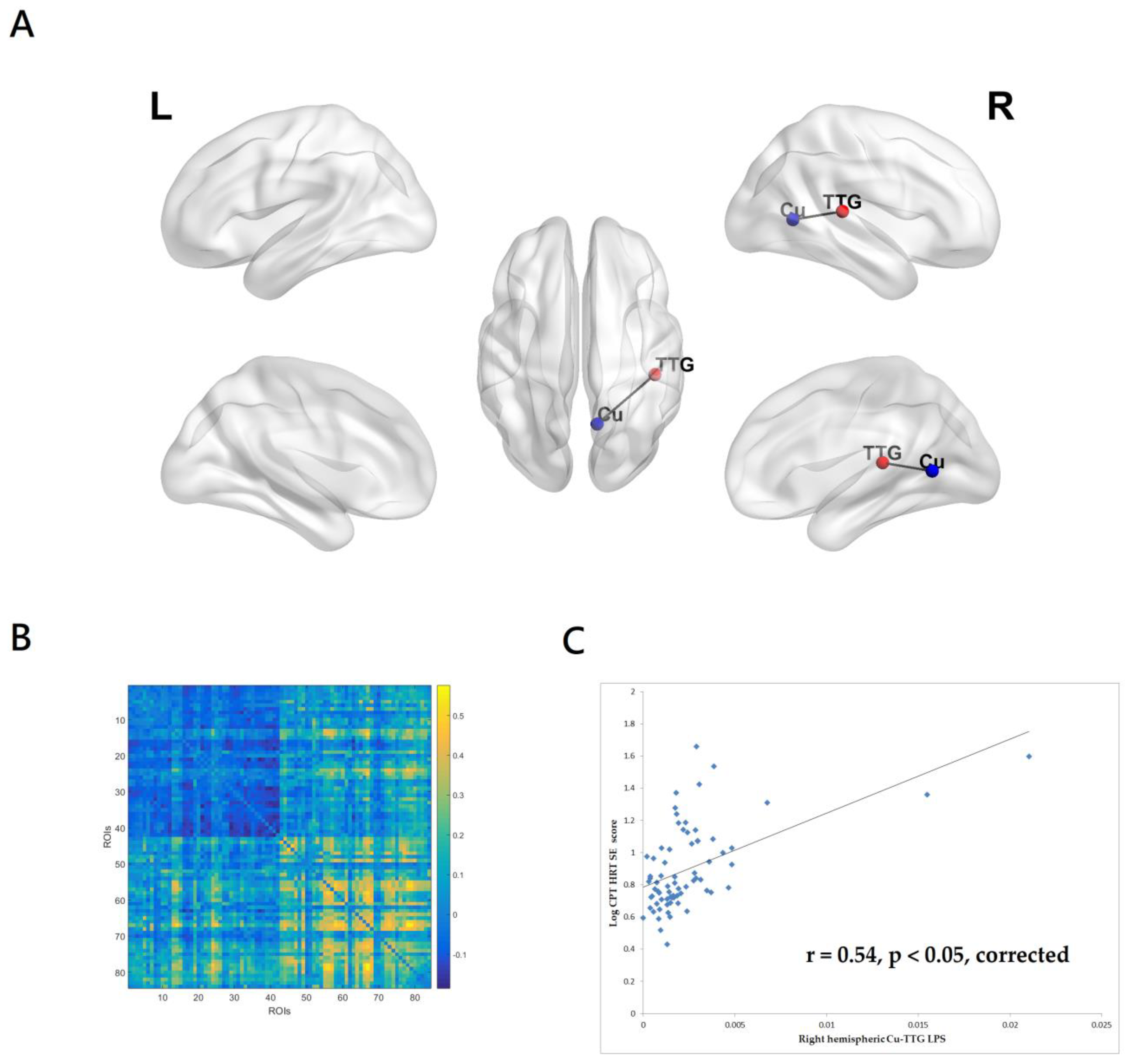
3.2.3. Correlations between CPT-II HRTSE Score and EEG Source-Based Functional Connectivity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kreyenbuhl, J.; Buchanan, R.W.; Dickerson, F.B.; Dixon, L.B. The Schizophrenia Patient Outcomes Research Team (PORT): Updated treatment recommendations 2009. Schizophr. Bull. 2010, 36, 94–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Chan, R.; Sun, J.; Yao, J.; Deng, W.; Sun, X.; Liu, X.; Sham, P.C.; Ma, X.; Meng, H.; et al. Reaction time of the Continuous Performance Test is an endophenotypic marker for schizophrenia: A study of first-episode neuroleptic-naive schizophrenia, their non-psychotic first-degree relatives and healthy population controls. Schizophr. Res 2007, 89, 293–298. [Google Scholar] [CrossRef]
- Sanz, J.C.; Gomez, V.; Vargas, M.L.; Marin, J.J. Dimensions of attention impairment and negative symptoms in schizophrenia: A multidimensional approach using the conners continuous performance test in a Spanish population. Cogn. Behav. Neurol. Off. J. Soc. Behav. Cogn. Neurol. 2012, 25, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Rekhi, G.; Saw, Y.E.; Lim, K.; Keefe, R.S.E.; Lee, J. Impact of Cognitive Impairments on Health-Related Quality of Life in Schizophrenia. Brain Sci. 2023, 13, 215. [Google Scholar] [CrossRef]
- Lopez-Luengo, B.; Gonzalez-Andrade, A.; Garcia-Cobo, M. Not All Differences between Patients with Schizophrenia and Healthy Subjects Are Pathological: Performance on the Conners’ Continuous Performance Test. Arch. Clin. Neuropsychol. Off. J. Natl. Acad. Neuropsychol. 2016, 31, 983–995. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Green, M.F.; Nuechterlein, K.H.; Swerdlow, N.R.; Greenwood, T.A.; Hellemann, G.S.; Lazzeroni, L.C.; Light, G.A.; Radant, A.D.; Seidman, L.J.; et al. The effects of age and sex on cognitive impairment in schizophrenia: Findings from the Consortium on the Genetics of Schizophrenia (COGS) study. PLoS ONE 2020, 15, e0232855. [Google Scholar] [CrossRef] [PubMed]
- Mohn, C.; Torgalsboen, A.K. Details of attention and learning change in first-episode schizophrenia. Psychiatry Res. 2018, 260, 324–330. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.H.; Hsiao, P.C.; Yeh, L.L.; Liu, C.M.; Liu, C.C.; Hwang, T.J.; Hsieh, M.H.; Chien, Y.L.; Lin, Y.T.; Chandler, S.D.; et al. Polygenic risk for schizophrenia and neurocognitive performance in patients with schizophrenia. Genes Brain Behav. 2018, 17, 49–55. [Google Scholar] [CrossRef] [Green Version]
- Lee, P.; Lin, H.Y.; Liu, C.H.; Lu, W.S.; Hsieh, C.L. Relative and Absolute Reliabilities of the Conners’ Continuous Performance Test II in Schizophrenia. Arch. Clin. Neuropsychol. Off. J. Natl. Acad. Neuropsychol. 2016, 31, 769–779. [Google Scholar] [CrossRef] [Green Version]
- Hirano, Y.; Uhlhaas, P.J. Current findings and perspectives on aberrant neural oscillations in schizophrenia. Psychiatry Clin. Neurosci. 2021, 75, 358–368. [Google Scholar] [CrossRef]
- Perrottelli, A.; Giordano, G.M.; Brando, F.; Giuliani, L.; Pezzella, P.; Mucci, A.; Galderisi, S. Unveiling the Associations between EEG Indices and Cognitive Deficits in Schizophrenia-Spectrum Disorders: A Systematic Review. Diagnostics 2022, 12, 2193. [Google Scholar] [CrossRef] [PubMed]
- Schultheis, C.; Rosenbrock, H.; Mack, S.R.; Vinisko, R.; Schuelert, N.; Plano, A.; Sussmuth, S.D. Quantitative electroencephalography parameters as neurophysiological biomarkers of schizophrenia-related deficits: A Phase II substudy of patients treated with iclepertin (BI 425809). Transl. Psychiatry 2022, 12, 329. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, I.S.; Pokorny, V.J.; Lynn, P.A.; Klein, S.D.; Sponheim, S.R. Limited consistency and strength of neural oscillations during sustained visual attention in schizophrenia. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2023, in press. [Google Scholar] [CrossRef] [PubMed]
- Young, J.W.; Bismark, A.W.; Sun, Y.; Zhang, W.; McIlwain, M.; Grootendorst, I.; Light, G.A. Neurophysiological Characterization of Attentional Performance Dysfunction in Schizophrenia Patients in a Reverse-Translated Task. Neuropsychopharmacology 2017, 42, 1338–1348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friston, K.; Brown, H.R.; Siemerkus, J.; Stephan, K.E. The dysconnection hypothesis (2016). Schizophr. Res. 2016, 176, 83–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Lorenzo, G.; Daverio, A.; Ferrentino, F.; Santarnecchi, E.; Ciabattini, F.; Monaco, L.; Lisi, G.; Barone, Y.; Di Lorenzo, C.; Niolu, C.; et al. Altered resting-state EEG source functional connectivity in schizophrenia: The effect of illness duration. Front. Hum. Neurosci. 2015, 9, 234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hipp, J.F.; Engel, A.K.; Siegel, M. Oscillatory synchronization in large-scale cortical networks predicts perception. Neuron 2011, 69, 387–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaytseva, Y.; Fajnerova, I.; Dvoracek, B.; Bourama, E.; Stamou, I.; Sulcova, K.; Motyl, J.; Horacek, J.; Rodriguez, M.; Spaniel, F. Theoretical Modeling of Cognitive Dysfunction in Schizophrenia by Means of Errors and Corresponding Brain Networks. Front. Psychol. 2018, 9, 1027. [Google Scholar] [CrossRef] [Green Version]
- Vignapiano, A.; Koenig, T.; Mucci, A.; Giordano, G.M.; Amodio, A.; Altamura, M.; Bellomo, A.; Brugnoli, R.; Corrivetti, G.; Di Lorenzo, G.; et al. Disorganization and cognitive impairment in schizophrenia: New insights from electrophysiological findings. Int. J. Psychophysiol. 2019, 145, 99–108. [Google Scholar] [CrossRef]
- Brennan, A.M.; Williams, L.M.; Harris, A.W.F. Intrinsic, task-evoked and absolute gamma synchrony during cognitive processing in first onset schizophrenia. J. Psychiatr. Res. 2018, 99, 10–21. [Google Scholar] [CrossRef]
- Engel, A.K.; Gerloff, C.; Hilgetag, C.C.; Nolte, G. Intrinsic coupling modes: Multiscale interactions in ongoing brain activity. Neuron 2013, 80, 867–886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gandal, M.J.; Edgar, J.C.; Klook, K.; Siegel, S.J. Gamma synchrony: Towards a translational biomarker for the treatment-resistant symptoms of schizophrenia. Neuropharmacology 2012, 62, 1504–1518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krukow, P.; Jonak, K.; Grochowski, C.; Plechawska-Wojcik, M.; Karakula-Juchnowicz, H. Resting-state hyperconnectivity within the default mode network impedes the ability to initiate cognitive performance in first-episode schizophrenia patients. Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 102, 109959. [Google Scholar] [CrossRef] [PubMed]
- Curic, S.; Andreou, C.; Nolte, G.; Steinmann, S.; Thiebes, S.; Polomac, N.; Haaf, M.; Rauh, J.; Leicht, G.; Mulert, C. Ketamine Alters Functional Gamma and Theta Resting-State Connectivity in Healthy Humans: Implications for Schizophrenia Treatment Targeting the Glutamate System. Front. Psychiatry 2021, 12, 671007. [Google Scholar] [CrossRef]
- Chang, C.C.; Lin, Y.Y.; Tzeng, N.S.; Kao, Y.C.; Chang, H.A. Adjunct high-frequency transcranial random noise stimulation over the lateral prefrontal cortex improves negative symptoms of schizophrenia: A randomized, double-blind, sham-controlled pilot study. J. Psychiatr. Res. 2021, 132, 151–160. [Google Scholar] [CrossRef]
- Chang, C.C.; Huang, C.C.; Chung, Y.A.; Im, J.J.; Lin, Y.Y.; Ma, C.C.; Tzeng, N.S.; Chang, H.A. Online Left-Hemispheric In-Phase Frontoparietal Theta tACS for the Treatment of Negative Symptoms of Schizophrenia. J. Pers. Med. 2021, 11, 1114. [Google Scholar] [CrossRef]
- Maj, M.; D’Elia, L.; Satz, P.; Janssen, R.; Zaudig, M.; Uchiyama, C.; Starace, F.; Galderisi, S.; Chervinsky, A.; World Health Organization, Division of Mental Health/Global Programme on AIDS. Evaluation of two new neuropsychological tests designed to minimize cultural bias in the assessment of HIV-1 seropositive persons: A WHO study. Arch. Clin. Neuropsychol. 1993, 8, 123–135. [Google Scholar] [CrossRef]
- Heaton, R.K.; Chelune, G.J.; Talley, J.L. Manual, Wisconsin Card Sorting Test; Psychological Assessment Resources: Odessa, FL, USA, 1993. [Google Scholar]
- Garcia-Alba, J.; Esteba-Castillo, S.; Castellanos Lopez, M.A.; Rodriguez Hidalgo, E.; Ribas Vidal, N.; Moldenhauer Diaz, F.; Novell-Alsina, R. Validation and Normalization of the Tower of London-Drexel University Test 2nd Edition in an Adult Population with Intellectual Disability. Span. J. Psychol. 2017, 20, E32. [Google Scholar] [CrossRef]
- Scarpina, F.; Tagini, S. The Stroop Color and Word Test. Front. Psychol. 2017, 8, 557. [Google Scholar] [CrossRef] [Green Version]
- Yeh, T.C.; Huang, C.C.; Chung, Y.A.; Im, J.J.; Lin, Y.Y.; Ma, C.C.; Tzeng, N.S.; Chang, C.C.; Chang, H.A. High-Frequency Transcranial Random Noise Stimulation over the Left Prefrontal Cortex Increases Resting-State EEG Frontal Alpha Asymmetry in Patients with Schizophrenia. J. Pers. Med. 2022, 12, 1667. [Google Scholar] [CrossRef]
- Yeh, T.C.; Huang, C.C.; Chung, Y.A.; Im, J.J.; Lin, Y.Y.; Ma, C.C.; Tzeng, N.S.; Chang, H.A. High-Frequency Transcranial Random Noise Stimulation Modulates Gamma-Band EEG Source-Based Large-Scale Functional Network Connectivity in Patients with Schizophrenia: A Randomized, Double-Blind, Sham-Controlled Clinical Trial. J. Pers. Med. 2022, 12, 1617. [Google Scholar] [CrossRef]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.Y.; Hsu, S.H.; Pion-Tonachini, L.; Jung, T.P. Evaluation of Artifact Subspace Reconstruction for Automatic Artifact Components Removal in Multi-Channel EEG Recordings. IEEE Trans Biomed. Eng. 2020, 67, 1114–1121. [Google Scholar] [CrossRef]
- Pion-Tonachini, L.; Kreutz-Delgado, K.; Makeig, S. ICLabel: An automated electroencephalographic independent component classifier, dataset, and website. Neuroimage 2019, 198, 181–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pascual-Marqui, R.D.; Lehmann, D.; Koukkou, M.; Kochi, K.; Anderer, P.; Saletu, B.; Tanaka, H.; Hirata, K.; John, E.R.; Prichep, L.; et al. Assessing interactions in the brain with exact low-resolution electromagnetic tomography. Philos. Trans. A Math. Phys. Eng. Sci. 2011, 369, 3768–3784. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, M.; Kastner, J.; Wagner, M.; Hawes, S.; Ebersole, J.S. A standardized boundary element method volume conductor model. Clin. Neurophysiol. 2002, 113, 702–712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazziotta, J.; Toga, A.; Evans, A.; Fox, P.; Lancaster, J.; Zilles, K.; Woods, R.; Paus, T.; Simpson, G.; Pike, B.; et al. A probabilistic atlas and reference system for the human brain: International Consortium for Brain Mapping (ICBM). Philos. Trans. R. Soc. Lond. B Biol. Sci. 2001, 356, 1293–1322. [Google Scholar] [CrossRef]
- Lancaster, J.L.; Woldorff, M.G.; Parsons, L.M.; Liotti, M.; Freitas, C.S.; Rainey, L.; Kochunov, P.V.; Nickerson, D.; Mikiten, S.A.; Fox, P.T. Automated Talairach atlas labels for functional brain mapping. Hum. Brain Mapp. 2000, 10, 120–131. [Google Scholar] [CrossRef]
- Olbrich, S.; Mulert, C.; Karch, S.; Trenner, M.; Leicht, G.; Pogarell, O.; Hegerl, U. EEG-vigilance and BOLD effect during simultaneous EEG/fMRI measurement. Neuroimage 2009, 45, 319–332. [Google Scholar] [CrossRef]
- Cole, M.W.; Bassett, D.S.; Power, J.D.; Braver, T.S.; Petersen, S.E. Intrinsic and task-evoked network architectures of the human brain. Neuron 2014, 83, 238–251. [Google Scholar] [CrossRef] [Green Version]
- Fox, M.D.; Raichle, M.E. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 2007, 8, 700–711. [Google Scholar] [CrossRef] [PubMed]
- Seok, J.H.; Park, H.J.; Lee, J.D.; Kim, H.S.; Chun, J.W.; Son, S.J.; Oh, M.K.; Ku, J.; Lee, H.; Kim, J.J. Regional cerebral blood flow changes and performance deficit during a sustained attention task in schizophrenia: (15) O-water positron emission tomography. Psychiatry Clin. Neurosci. 2012, 66, 564–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, R.; Wang, S.; Fan, Y.; Liu, X.; Wang, J.; Lv, Y.; Wang, D.; Wu, D.; Cao, W.; Zou, Q. Acute Tai Chi Chuan exercise enhances sustained attention and elicits increased cuneus/precuneus activation in young adults. Cereb. Cortex 2022, 33, 2969–2981. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, G.; Tian, Y. Increased Temporal Dynamics of Intrinsic Brain Activity in Sensory and Perceptual Network of Schizophrenia. Front. Psychiatry 2019, 10, 484. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.; Sehatpour, P.; Hoptman, M.J.; Lakatos, P.; Dias, E.C.; Kantrowitz, J.T.; Martinez, A.M.; Javitt, D.C. Neural mechanisms of mismatch negativity dysfunction in schizophrenia. Mol. Psychiatry 2017, 22, 1585–1593. [Google Scholar] [CrossRef] [Green Version]
- Thomas, M.L.; Green, M.F.; Hellemann, G.; Sugar, C.A.; Tarasenko, M.; Calkins, M.E.; Greenwood, T.A.; Gur, R.E.; Gur, R.C.; Lazzeroni, L.C.; et al. Modeling Deficits From Early Auditory Information Processing to Psychosocial Functioning in Schizophrenia. JAMA Psychiatry 2017, 74, 37–46. [Google Scholar] [CrossRef] [Green Version]
- Edgar, J.C.; Hunter, M.A.; Huang, M.; Smith, A.K.; Chen, Y.; Sadek, J.; Lu, B.Y.; Miller, G.A.; Canive, J.M. Temporal and frontal cortical thickness associations with M100 auditory activity and attention in healthy controls and individuals with schizophrenia. Schizophr. Res. 2012, 140, 250–257. [Google Scholar] [CrossRef] [Green Version]
- Alkan, E.; Davies, G.; Evans, S.L. Cognitive impairment in schizophrenia: Relationships with cortical thickness in fronto-temporal regions, and dissociability from symptom severity. NPJ Schizophr. 2021, 7, 20. [Google Scholar] [CrossRef]
- Pittman-Polletta, B.R.; Kocsis, B.; Vijayan, S.; Whittington, M.A.; Kopell, N.J. Brain rhythms connect impaired inhibition to altered cognition in schizophrenia. Biol. Psychiatry 2015, 77, 1020–1030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, T.; Suzuki, M.; Zhou, S.Y.; Tanino, R.; Hagino, H.; Niu, L.; Kawasaki, Y.; Seto, H.; Kurachi, M. Temporal lobe gray matter in schizophrenia spectrum: A volumetric MRI study of the fusiform gyrus, parahippocampal gyrus, and middle and inferior temporal gyri. Schizophr. Res. 2006, 87, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, S.M.; All, S.D.; Kasi, R.; Berten, S.; Essex, B.; Lathrop, K.L.; Little, D.M. Increased fusiform area activation in schizophrenia during processing of spatial frequency-degraded faces, as revealed by fMRI. Psychol. Med. 2010, 40, 1159–1169. [Google Scholar] [CrossRef] [Green Version]
- Nygard, M.; Eichele, T.; Loberg, E.M.; Jorgensen, H.A.; Johnsen, E.; Kroken, R.A.; Berle, J.O.; Hugdahl, K. Patients with Schizophrenia Fail to Up-Regulate Task-Positive and Down-Regulate Task-Negative Brain Networks: An fMRI Study Using an ICA Analysis Approach. Front. Hum. Neurosci. 2012, 6, 149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palaniyappan, L.; Liddle, P.F. Diagnostic discontinuity in psychosis: A combined study of cortical gyrification and functional connectivity. Schizophr. Bull. 2014, 40, 675–684. [Google Scholar] [CrossRef]
- Fan, Y.; Gao, Y.; Ma, Q.; Zhao, B.; He, X.; Zhu, F.; Wang, W.; Ma, X.; Li, Y. Grey matter volume and its association with cognitive impairment and peripheral cytokines in excited individuals with schizophrenia. Brain Imaging Behav. 2022, 16, 2618–2626. [Google Scholar] [CrossRef] [PubMed]
- Lalousis, P.A.; Malaviya, A.; Upthegrove, R.; Heinze, K.; Diukova, A.; Auer, D.; Liddle, P.; Mallikarjun, P. Trait related aberrant connectivity in clinically stable patients with schizophrenia: A seed based resting state fMRI study. Brain Imaging Behav. 2022, 16, 2705–2714. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, S.; Gallinat, J. Resting-state brain activity in schizophrenia and major depression: A quantitative meta-analysis. Schizophr. Bull. 2013, 39, 358–365. [Google Scholar] [CrossRef] [Green Version]
- Minzenberg, M.J.; Firl, A.J.; Yoon, J.H.; Gomes, G.C.; Reinking, C.; Carter, C.S. Gamma oscillatory power is impaired during cognitive control independent of medication status in first-episode schizophrenia. Neuropsychopharmacology 2010, 35, 2590–2599. [Google Scholar] [CrossRef] [Green Version]
- Sadiq, M.T.; Yu, X.; Yuan, Z.; Aziz, M.Z. Motor imagery BCI classification based on novel two-dimensional modelling in empirical wavelet transform. Electron. Lett. 2020, 56, 1367–1369. [Google Scholar] [CrossRef]
- Sadiq, M.T.; Yu, X.; Yuan, Z.; Aziz, M.Z.; Rehman, N.u.; Ding, W.; Xiao, G. Motor Imagery BCI Classification Based on Multivariate Variational Mode Decomposition. IEEE Trans. Emerg. Top. Comput. Intell. 2022, 6, 1177–1189. [Google Scholar] [CrossRef]
- Sadiq, M.T.; Yu, X.; Yuan, Z.; Zeming, F.; Rehman, A.U.; Ullah, I.; Li, G.; Xiao, G. Motor Imagery EEG Signals Decoding by Multivariate Empirical Wavelet Transform-Based Framework for Robust Brain–Computer Interfaces. IEEE Access 2019, 7, 171431–171451. [Google Scholar] [CrossRef]
- Sadiq, M.T.; Akbari, H.; Siuly, S.; Li, Y.; Wen, P. Alcoholic EEG signals recognition based on phase space dynamic and geometrical features. Chaos Solitons Fractals 2022, 158, 112036. [Google Scholar] [CrossRef]
- Akbari, H.; Sadiq, M.T.; Payan, M.; Esmaili, S.S.; Baghri, H.; Bagheri, H. Depression detection based on geometrical features extracted from SODP shape of EEG signals and binary PSO. Traitement Du Signal 2022, 38, 13–26. [Google Scholar] [CrossRef]
- Akbari, H.; Sadiq, M.T.; Jafari, N.; Too, J.; Mikaeilvand, N.; Cicone, A.; Serra-Capizzano, S. Recognizing seizure using Poincare plot of EEG signals and graphical features in DWT domain. Bratisl. Lek. Listy 2023, 124, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Sheffield, J.M.; Barch, D.M. Cognition and resting-state functional connectivity in schizophrenia. Neurosci. Biobehav. Rev. 2016, 61, 108–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Sample 1 N = 36 | Sample 2 N = 36 | Total Sample N = 72 | p Values | |
|---|---|---|---|---|
| Gender (f/m) | 15/21 | 18/18 | 33/39 | 0.48 |
| Age, years old | 43.33 ± 11.83 | 42.47 ± 9.96 | 42.90 ± 10.87 | 0.74 |
| Years of education, years | 13.06 ± 3.00 | 13.56 ± 3.06 | 13.31 ± 3.02 | 0.49 |
| Years since diagnosis, years | 18.97 ± 11.56 | 16.28 ± 10.34 | 17.44 ± 10.97 | 0.37 |
| Chlorpromazine equivalent dose, mg/day | 593.30 ± 304.15 | 613.99 ± 410.85 | 603.64 ± 359.05 | 0.81 |
| PANSS total score | 69.86 ± 9.42 | 73.28 ± 8.51 | 71.57 ± 9.08 | 0.11 |
| PANSS positive subscale | 14.36 ± 4.08 | 16.00 ± 4.46 | 15.18 ± 4.32 | 0.11 |
| PANSS negative subscale | 18.89 ± 3.21 | 19.53 ± 3.71 | 19.21 ± 3.46 | 0.44 |
| PANSS general subscale | 36.61 ± 4.66 | 37.75 ± 4.63 | 37.18 ± 4.65 | 0.30 |
| PSP global scale | 55.36 ± 10.80 | 52.89 ± 10.03 | 53.13 ± 10.42 | 0.32 |
| BCIS-R | 23.58 ± 4.55 | 23.81 ± 4.96 | 23.69 ± 4.73 | 0.96 |
| BCIS-C | 15.00 ± 3.26 | 16.17 ± 3.00 | 15.83 ± 3.13 | 0.37 |
| BCIS R-C index | 8.25 ± 4.16 | 7.64 ± 5.54 | 7.94 ± 4.87 | 0.60 |
| CPT-II | ||||
| d’ | 0.61 ± 0.49 | 0.86 ± 0.59 | 0.74 ± 0.55 | 0.05 |
| OM | 13.00 ± 21.11 | 15.67 ± 31.08 | 14.33 ± 26.41 | 0.67 |
| COM | 17.14 ± 9.69 | 12.75 ± 8.44 | 14.94 ± 9.29 | 0.04 |
| PER | 4.17 ± 7.27 | 3.42 ± 6.67 | 3.79 ± 6.94 | 0.65 |
| HRT | 455.57 ± 100.16 | 478.92 ± 105.17 | 467.25 ± 102.64 | 0.34 |
| HRTSE | 9.65 ± 8.15 | 9.82 ± 8.19 | 9.73 ± 8.11 | 0.93 |
| VAR | 17.64 ± 17.46 | 16.81 ± 18.20 | 17.23 ± 17.72 | 0.85 |
| HRTBC | 0.01 ± 0.03 | 0.01 ± 0.03 | 0.01 ± 0.03 | 0.29 |
| HRTISIC | 0.07 ± 0.04 | 0.07 ± 0.04 | 0.06 ± 0.04 | 0.72 |
| CTT1 | 60.08 ± 20.93 | 54.14 ± 25.56 | 57.11 ± 23.39 | 0.28 |
| CTT2 | 105.02 ± 28.10 | 104.96 ± 39.36 | 104.99 ± 33.96 | 0.99 |
| WCST non-perseverative error | 21.22 ± 20.45 | 15.50 ± 16.38 | 18.36 ± 18.62 | 0.52 |
| TOL accuracy | 4.53 ± 2.13 | 3.56 ± 2.32 | 4.04 ± 2.27 | 0.07 |
| TOL time | 234.58 ± 94.30 | 236.03 ± 91.23 | 235.31 ± 92.13 | 0.95 |
| Stroop Interference Test | ||||
| Naming interference tendency | 0.49 ± 0.45 | 0.33 ± 0.32 | 0.41 ± 0.40 | 0.08 |
| Reading interference tendency | 0.28 ± 0.29 | 0.28 ± 0.24 | 0.28 ± 0.26 | 0.84 |
| ROI | Structure | x | y | z | ROI | Structure | x | y | z |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Postcentral Gyrus | −55 | −25 | 50 | 43 | Postcentral Gyrus | 55 | −25 | 50 |
| 2 | Postcentral Gyrus | −45 | −30 | 45 | 44 | Inferior Parietal Lobule | 50 | −30 | 45 |
| 3 | Precentral Gyrus | −35 | −25 | 55 | 45 | Postcentral Gyrus | 40 | −25 | 50 |
| 4 | Precentral Gyrus | −35 | −20 | 50 | 46 | Postcentral Gyrus | 35 | −25 | 50 |
| 5 | Paracentral Lobule | −15 | −45 | 60 | 47 | Paracentral Lobule | 15 | −45 | 60 |
| 6 | Middle Frontal Gyrus | −30 | −5 | 55 | 48 | Middle Frontal Gyrus | 30 | −5 | 55 |
| 7 | Precuneus | −20 | −65 | 50 | 49 | Precuneus | 15 | −65 | 50 |
| 8 | Superior Frontal Gyrus | −20 | 30 | 50 | 50 | Superior Frontal Gyrus | 20 | 25 | 50 |
| 9 | Middle Frontal Gyrus | −30 | 30 | 35 | 51 | Middle Frontal Gyrus | 30 | 30 | 35 |
| 10 | Superior Frontal Gyrus | −25 | 55 | 5 | 52 | Superior Frontal Gyrus | 25 | 55 | 5 |
| 11 | Middle Frontal Gyrus | −20 | 40 | −15 | 53 | Superior Frontal Gyrus | 20 | 45 | −20 |
| 12 | Insula | −40 | −10 | 10 | 54 | Insula | 40 | −5 | 10 |
| 13 | Lingual Gyrus | −10 | −90 | 0 | 55 | Lingual Gyrus | 10 | −90 | 0 |
| 14 | Lingual Gyrus | −15 | −85 | 0 | 56 | Lingual Gyrus | 15 | −85 | 0 |
| 15 | Cuneus | −25 | −75 | 10 | 57 | Cuneus | 25 | −75 | 10 |
| 16 | Fusiform Gyrus | −45 | −20 | −30 | 58 | Fusiform Gyrus | 45 | −20 | −30 |
| 17 | Middle Temporal Gyrus | −60 | −20 | −15 | 59 | Middle Temporal Gyrus | 60 | −15 | −15 |
| 18 | Superior Temporal Gyrus | −55 | −25 | 5 | 60 | Superior Temporal Gyrus | 55 | −20 | 5 |
| 19 | Posterior Cingulate | −5 | −40 | 25 | 61 | Posterior Cingulate | 5 | −45 | 25 |
| 20 | Cingulate Gyrus | −5 | 0 | 35 | 62 | Cingulate Gyrus | 5 | 0 | 35 |
| 21 | Medial Frontal Gyrus | −10 | 20 | −15 | 63 | Subcallosal Gyrus | 5 | 15 | −15 |
| 22 | Parahippocampal Gyrus | −20 | −35 | −5 | 64 | Parahippocampal Gyrus | 20 | −35 | −5 |
| 23 | Parahippocampal Gyrus | −20 | −10 | −25 | 65 | Parahippocampal Gyrus | 20 | −10 | −25 |
| 24 | Posterior Cingulate | −5 | −50 | 5 | 66 | Posterior Cingulate | 5 | −50 | 5 |
| 25 | Posterior Cingulate | −15 | −60 | 5 | 67 | Cuneus | 10 | −60 | 5 |
| 26 | Precuneus | −10 | −50 | 30 | 68 | Precuneus | 10 | −50 | 35 |
| 27 | Anterior Cingulate | −5 | 30 | 20 | 69 | Anterior Cingulate | 5 | 30 | 20 |
| 28 | Anterior Cingulate | −5 | 20 | 20 | 70 | Anterior Cingulate | 0 | 20 | 20 |
| 29 | Parahippocampal Gyrus | −15 | 0 | −20 | 71 | Parahippocampal Gyrus | 15 | 0 | −20 |
| 30 | Parahippocampal Gyrus | −20 | −25 | −20 | 72 | Parahippocampal Gyrus | 25 | −25 | −20 |
| 31 | Parahippocampal Gyrus | −30 | −30 | −25 | 73 | Parahippocampal Gyrus | 30 | −25 | −25 |
| 32 | Fusiform Gyrus | −45 | −55 | −15 | 74 | Fusiform Gyrus | 45 | −55 | −15 |
| 33 | Superior Temporal Gyrus | −40 | 15 | −30 | 75 | Superior Temporal Gyrus | 40 | 15 | −30 |
| 34 | Middle Temporal Gyrus | −45 | −65 | 25 | 76 | Middle Temporal Gyrus | 45 | −65 | 25 |
| 35 | Inferior Parietal Lobule | −50 | −40 | 40 | 77 | Inferior Parietal Lobule | 50 | −45 | 45 |
| 36 | Transverse Temporal Gyrus | −45 | −30 | 10 | 78 | Transverse Temporal Gyrus | 45 | −30 | 10 |
| 37 | Superior Temporal Gyrus | −60 | −25 | 10 | 79 | Superior Temporal Gyrus | 65 | −25 | 10 |
| 38 | Transverse Temporal Gyrus | −60 | −10 | 15 | 80 | Transverse Temporal Gyrus | 60 | −10 | 15 |
| 39 | Precentral Gyrus | −50 | 10 | 15 | 81 | Precentral Gyrus | 55 | 10 | 15 |
| 40 | Inferior Frontal Gyrus | −50 | 20 | 15 | 82 | Inferior Frontal Gyrus | 50 | 20 | 15 |
| 41 | Middle Frontal Gyrus | −45 | 35 | 20 | 83 | Middle Frontal Gyrus | 45 | 35 | 20 |
| 42 | Inferior Frontal Gyrus | −30 | 25 | −15 | 84 | Inferior Frontal Gyrus | 30 | 25 | −15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeh, T.-C.; Huang, C.C.-Y.; Chung, Y.-A.; Park, S.Y.; Im, J.J.; Lin, Y.-Y.; Ma, C.-C.; Tzeng, N.-S.; Chang, H.-A. Resting-State EEG Connectivity at High-Frequency Bands and Attentional Performance Dysfunction in Stabilized Schizophrenia Patients. Medicina 2023, 59, 737. https://doi.org/10.3390/medicina59040737
Yeh T-C, Huang CC-Y, Chung Y-A, Park SY, Im JJ, Lin Y-Y, Ma C-C, Tzeng N-S, Chang H-A. Resting-State EEG Connectivity at High-Frequency Bands and Attentional Performance Dysfunction in Stabilized Schizophrenia Patients. Medicina. 2023; 59(4):737. https://doi.org/10.3390/medicina59040737
Chicago/Turabian StyleYeh, Ta-Chuan, Cathy Chia-Yu Huang, Yong-An Chung, Sonya Youngju Park, Jooyeon Jamie Im, Yen-Yue Lin, Chin-Chao Ma, Nian-Sheng Tzeng, and Hsin-An Chang. 2023. "Resting-State EEG Connectivity at High-Frequency Bands and Attentional Performance Dysfunction in Stabilized Schizophrenia Patients" Medicina 59, no. 4: 737. https://doi.org/10.3390/medicina59040737






