Weight Regain in the Second Year after Sleeve Gastrectomy Could Be a Predictor of Long-Term Outcomes?
Abstract
:1. Introduction
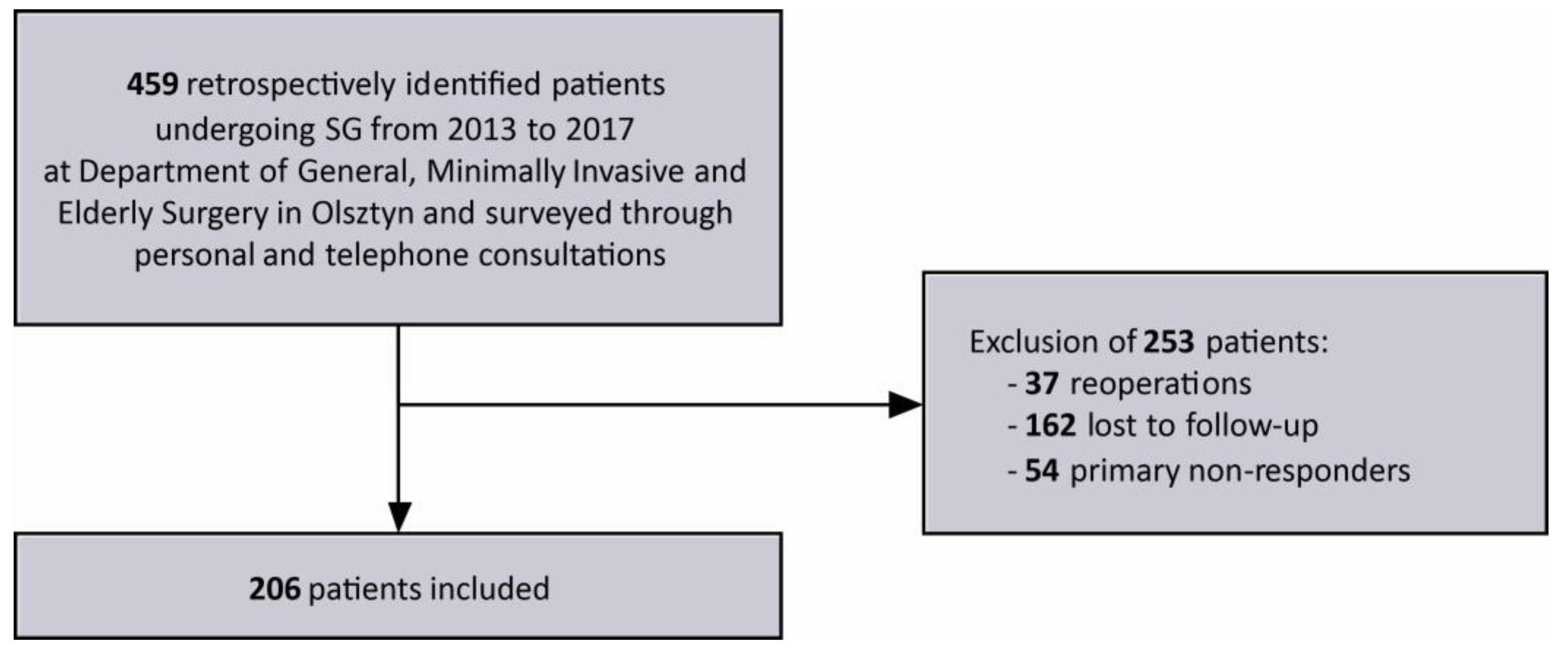
2. Materials and Methods
2.1. Outcomes Measurements
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Apovian, C.M. Obesity: Definition, comorbidities, causes, and burden. Am. J. Manag. Care 2016, 22, 176–185. [Google Scholar]
- World Health Organization. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 18 October 2022).
- Longo, M.; Zatterale, F.; Naderi, J.; Parrillo, L.; Formisano, P.; Raciti, G.A.; Beguinot, F.; Miele, C. Adipose Tissue Dysfunction as Determinant of Obesity-Associated Metabolic Complications. Int. J. Mol. Sci. 2019, 20, 2358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schutz, D.D.; Busetto, L.; Dicker, D.; Farpour-Lambert, N.; Pryke, R.; Toplak, H.; Widmer, D.; Yumuk, V.; Schutz, Y. European Practical and Patient-Centred Guidelines for Adult Obesity Management in Primary Care. Obes. Facts 2019, 12, 40–66. [Google Scholar] [CrossRef]
- Flegal, K.M.; Kit, B.K.; Orpana, H.; Graubard, B.I. Association of All-Cause Mortality With Overweight and Obesity Using Standard Body Mass Index Categories: A systematic review and meta-analysis. JAMA 2013, 309, 71–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, N.T.; Varela, J.E. Bariatric surgery for obesity and metabolic disorders: State of the art. Nat. Rev. Gastroenterol. Hepatol. 2016, 14, 160–169. [Google Scholar] [CrossRef]
- Sjöström, L. Review of the key results from the Swedish Obese Subjects (SOS) trial-a prospective controlled intervention study of bariatric surgery. J. Intern. Med. 2013, 273, 219–234. [Google Scholar] [CrossRef]
- Welbourn, R.; Hollyman, M.; Kinsman, R.; Dixon, J.; Liem, R.; Ottosson, J.; Ramos, A.; Våge, V.; Al-Sabah, S.; Brown, W.; et al. Bariatric Surgery Worldwide: Baseline Demographic Description and One-Year Outcomes from the Fourth IFSO Global Registry Report 2018. Obes. Surg. 2018, 29, 782–795. [Google Scholar] [CrossRef] [Green Version]
- Brajcich, B.C.; Hungness, E.S. Sleeve Gastrectomy. JAMA 2020, 324, 908. [Google Scholar] [CrossRef] [PubMed]
- Emile, S.; Elfeki, H.; Elalfy, K.; Abdallah, E. Laparoscopic Sleeve Gastrectomy Then and Now: An Updated Systematic Review of the Progress and Short-term Outcomes Over the Last 5 Years. Surg. Laparosc. Endosc. Percutaneous Tech. 2017, 27, 307–317. [Google Scholar] [CrossRef]
- Lent, M.R.; Hu, Y.; Benotti, P.N.; Petrick, A.T.; Wood, G.C.; Still, C.D.; Kirchner, H.L. Demographic, clinical, and behavioral determinants of 7-year weight change trajectories in Roux-en-Y gastric bypass patients. Surg. Obes. Relat. Dis. 2018, 14, 1680–1685. [Google Scholar] [CrossRef]
- Voorwinde, V.; Hoekstra, T.; Monpellier, V.M.; Steenhuis, I.H.; Janssen, I.M.; van Stralen, M.M. Five-year weight loss, physical activity, and eating style trajectories after bariatric surgery. Surg. Obes. Relat. Dis. 2022, 18, 911–918. [Google Scholar] [CrossRef]
- Courcoulas, A.P.; King, W.C.; Belle, S.H.; Berk, P.; Flum, D.R.; Garcia, L.; Gourash, W.; Horlick, M.; Mitchell, J.E.; Pomp, A.; et al. Seven-Year Weight Trajectories and Health Outcomes in the Longitudinal Assessment of Bariatric Surgery (LABS) Study. JAMA Surg. 2018, 153, 427–434. [Google Scholar] [CrossRef] [Green Version]
- Lauti, M.; Kularatna, M.; Hill, A.G.; MacCormick, A.D. Weight Regain Following Sleeve Gastrectomy—A Systematic Review. Obes. Surg. 2016, 26, 1326–1334. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Klem, M.L.; Kalarchian, M.A.; Ji, M.; Burke, L.E. Predictors of weight regain after sleeve gastrectomy: An integrative review. Surg. Obes. Relat. Dis. 2019, 15, 995–1005. [Google Scholar] [CrossRef]
- Bonouvrie, D.S.; Uittenbogaart, M.; Luijten, A.A.P.M.; van Dielen, F.M.H.; Leclercq, W.K.G. Lack of Standard Definitions of Primary and Secondary (Non)responders After Primary Gastric Bypass and Gastric Sleeve: A Systematic Review. Obes. Surg. 2018, 29, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Brethauer, S.A.; Kim, J.; el Chaar, M.; Papasavas, P.; Eisenberg, D.; Rogers, A.; Ballem, N.; Kligman, M.; Kothari, S. & ASMBS Clinical Issues Committee. Standardized outcomes reporting in metabolic and bariatric surgery. Surg. Obes. Relat. Dis. 2015, 11, 489–506. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, M.; The Bariatric Metabolic Surgery Standardization (BMSS) Working Group; Fobi, M.A.L.; Buchwald, J.N. Standardization of Bariatric Metabolic Procedures: World Consensus Meeting Statement. Obes. Surg. 2019, 29, 309–345. [Google Scholar] [CrossRef] [Green Version]
- Kheirvari, M.; Nikroo, N.D.; Jaafarinejad, H.; Farsimadan, M.; Eshghjoo, S.; Hosseini, S.; Anbara, T. The advantages and disadvantages of sleeve gastrectomy; clinical laboratory to bedside review. Heliyon 2020, 6, e03496. [Google Scholar] [CrossRef] [Green Version]
- Janik, M.; Rogula, T.G.; Mustafa, R.R.; Saleh, A.A.; Abbas, M.; Khaitan, L. Setting realistic expectations for weight loss after laparoscopic sleeve gastrectomy. Videosurgery Other Miniinvasive Tech. 2019, 14, 415–419. [Google Scholar] [CrossRef]
- Karpińska, I.A.; Kulawik, J.; Pisarska-Adamczyk, M.; Wysocki, M.; Pędziwiatr, M.; Major, P. Is It Possible to Predict Weight Loss After Bariatric Surgery?—External Validation of Predictive Models. Obes. Surg. 2021, 31, 1–11. [Google Scholar] [CrossRef]
- Manning, S.; Pucci, A.; Carter, N.C.; Elkalaawy, M.; Querci, G.; Magno, S.; Tamberi, A.; Finer, N.; Fiennes, A.G.; Hashemi, M.; et al. Early postoperative weight loss predicts maximal weight loss after sleeve gastrectomy and Roux-en-Y gastric bypass. Surg. Endosc. 2014, 29, 1484–1491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tettero, O.M.; Monpellier, V.M.; Janssen, I.M.C.; Steenhuis, I.H.M.; van Stralen, M.M. Early Postoperative Weight Loss Predicts Weight Loss up to 5 Years After Roux-En-Y Gastric Bypass, Banded Roux-En-Y Gastric Bypass, and Sleeve Gastrectomy. Obes. Surg. 2022, 32, 2891–2902. [Google Scholar] [CrossRef]
- Yang, P.-J.; Chen, C.-L.; Chen, C.-N.; Lin, M.-T.; Wang, W. Early weight loss as a predictor of 3-year weight loss and weight regain in patients with good compliance after sleeve gastrectomy. Surg. Obes. Relat. Dis. 2021, 17, 1418–1423. [Google Scholar] [CrossRef] [PubMed]
- Bohdjalian, A.; Langer, F.B.; Shakeri-Leidenmühler, S.; Gfrerer, L.; Ludvik, B.; Zacherl, J.; Prager, G. Sleeve Gastrectomy as Sole and Definitive Bariatric Procedure: 5-Year Results for Weight Loss and Ghrelin. Obes. Surg. 2010, 20, 535–540. [Google Scholar] [CrossRef]
- Sarela, A.I.; Dexter, S.P.; O’Kane, M.; Menon, A.; McMahon, M.J. Long-term follow-up after laparoscopic sleeve gastrectomy: 8–9-year results. Surg. Obes. Relat. Dis. 2012, 8, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Keren, D.; Matter, I.; Lavy, A. Lifestyle Modification Parallels to Sleeve Success. Obes. Surg. 2013, 24, 735–740. [Google Scholar] [CrossRef]
- Kushner, R.F.; Sorensen, K.W. Prevention of Weight Regain Following Bariatric Surgery. Curr. Obes. Rep. 2015, 4, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Weiner, R.A.; Weiner, S.; Pomhoff, I.; Jacobi, C.; Makarewicz, W.; Weigand, G. Laparoscopic Sleeve Gastrectomy—Influence of Sleeve Size and Resected Gastric Volume. Obes. Surg. 2007, 17, 1297–1305. [Google Scholar] [CrossRef]
- Hood, M.M.; Corsica, J.; Bradley, L.; Wilson, R.; Chirinos, D.A.; Vivo, A. Managing severe obesity: Understanding and improving treatment adherence in bariatric surgery. J. Behav. Med. 2016, 39, 1092–1103. [Google Scholar] [CrossRef]
- Ünal, Ş.; Sevinçer, G.M.; Maner, A.F. Bariatrik Cerrahi Sonrası Kilo Geri Alımının; Gece Yeme, Duygusal Yeme, Yeme Endişesi, Depresyon ve Demografik Özellikler Tarafından Yordanması [Prediction of Weight Regain After Bariatric Surgery by Night Eating, Emotional Eating, Eating Concerns, Depression and Demographic Characteristics]. Turk. J. Psychiatry 2019, 30, 31–41. [Google Scholar]
- Bach, P.; Grosshans, M.; Koopmann, A.; Pfeifer, A.-M.; Vollstädt-Klein, S.; Otto, M.; Kienle, P.; Bumb, J.M.; Kiefer, F. Predictors of weight loss in participants with obesity following bariatric surgery–A prospective longitudinal fMRI study. Appetite 2021, 163, 105237. [Google Scholar] [CrossRef]
- Hood, M.M.; Kelly, M.C.; Feig, E.H.; Webb, V.; Bradley, L.E.; Corsica, J. Measurement of adherence in bariatric surgery: A systematic review. Surg. Obes. Relat. Dis. 2018, 14, 1192–1201. [Google Scholar] [CrossRef]
- Obando, D.C.; Ramírez-Rentería, C.; Ferreira-Hermosillo, A.; Albarrán-Sanchez, A.; Sosa-Eroza, E.; Molina-Ayala, M.; Espinosa-Cárdenas, E. Are there really any predictive factors for a successful weight loss after bariatric surgery? BMC Endocr. Disord. 2020, 20, 20. [Google Scholar] [CrossRef]
- Spaniolas, K.; Kasten, K.R.; Celio, A.; Burruss, M.B.; Pories, W.J. Postoperative Follow-up After Bariatric Surgery: Effect on Weight Loss. Obes. Surg. 2016, 26, 900–903. [Google Scholar] [CrossRef]
- Gould, J.C.; Beverstein, G.; Reinhardt, S.; Garren, M.J. Impact of routine and long-term follow-up on weight loss after laparoscopic gastric bypass. Surg. Obes. Relat. Dis. 2007, 3, 627–630. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, H.J.; Nedrebø, B.G.; Fosså, A.; Andersen, J.R.; Assmus, J.; Dagsland, V.H.; Dankel, S.N.; Gudbrandsen, O.A.; Fernø, J.; Hjellestad, I.; et al. Seven-year trajectories of body weight, quality of life and comorbidities following Roux-en-Y gastric bypass and sleeve gastrectomy. Int. J. Obes. 2022, 46, 739–749. [Google Scholar] [CrossRef]
- Flølo, T.N.; Tell, G.S.; Kolotkin, R.L.; Aasprang, A.; Norekvål, T.M.; Våge, V.; Hufthammer, K.O.; Andersen, J.R. Changes in quality of life 5 years after sleeve gastrectomy: A prospective cohort study. BMJ Open 2019, 9, e031170. [Google Scholar] [CrossRef] [PubMed]
- Felsenreich, D.M.; Langer, F.B.; Kefurt, R.; Panhofer, T.P.; Schermann, M.; Beckerhinn, P.; Sperker, C.; Prager, G. Weight loss, weight regain, and conversions to Roux-en-Y gastric bypass: 10-year results of laparoscopic sleeve gastrectomy. Surg. Obes. Relat. Dis. 2016, 12, 1655–1662. [Google Scholar] [CrossRef]
- Karmali, S.; Brar, B.; Shi, X.; Sharma, A.M.; De Gara, C.; Birch, D.W. Weight Recidivism Post-Bariatric Surgery: A Systematic Review. Obes. Surg. 2013, 23, 1922–1933. [Google Scholar] [CrossRef]
- Coughlin, J.W.; Guarda, A.S.; Clark, J.M.; Furtado, M.M.; Steele, K.E.; Heinberg, L.J. A Screening Tool to Assess and Manage Behavioral Risk in the Postoperative Bariatric Surgery Patient: The WATCH. J. Clin. Psychol. Med. Settings 2013, 20, 456–463. [Google Scholar] [CrossRef] [PubMed]
| All | WG Group | WM Group | p-Value * | |
|---|---|---|---|---|
| N | 206 | 69 (33.5%) | 137 (66.5%) | |
| Gender | 0.283 | |||
| Female (%) | 164 (80%) | 52 (75%) | 112 (82%) | |
| Male (%) | 42 (20%) | 17 (25%) | 25 (18%) | |
| Mean age (±SD) [years] | 39.03 (10.20) | 39.25 (10.52) | 38.25 (10.08) | 0.877 |
| Mean IBMI (±SD) [kg/m2] | 43.38 (5.99) | 42.97 (5.37) | 43.58 (6.28) | 0.627 |
| T2D | 0.714 | |||
| Yes (%) | 36 (17%) | 13 (19%) | 23 (17%) | |
| No (%) | 170 (83%) | 56 (81%) | 114 (83%) | |
| AH | 0.870 | |||
| Yes (%) | 79 (38%) | 42 (61%) | 85 (62%) | |
| No (%) | 127 (62%) | 27 (39%) | 52 (38%) | |
| Mean operation time (±SD) [min] | 71.93 (26.07) | 73.84 (26.18) | 70.90 (26.05) | 0.355 |
| Mean length of hospital stay (±SD) [days] | 3.30 (1.88) | 3.26 (1.21) | 3.32 (2.16) | 0.902 |
| Domicile size (number of inhabitants) | 0.222 | |||
| <25,000 (%) | 93 (45%) | 37 (54%) | 56 (41%) | |
| 25,000–100,000 (%) | 32 (16%) | 9 (13%) | 23 (17%) | |
| >100,000 (%) | 81 (39%) | 23 (33%) | 58 (42%) | |
| All | WG Group | WM Group | p-Value * | |
|---|---|---|---|---|
| Mean %EBMIL 5 years after SG (±SD) | 90.23 (33.72) | 77.18 (34.88) | 96.80 (31.23) | <0.005 |
| Mean %EWL 5 years after SG (±SD) | 58.23 (21.41) | 50.00 (22.74) | 62.38 (19.51) | <0.005 |
| Mean %TWL 5 years after SG (±SD) | 29.02 (10.84) | 24.62 (11.07) | 31.24 (10.05) | <0.005 |
| Mean change in %EBMIL between 1st and 5th year after SG (±SD) | 19.32 (27.54) | 34.96 (26.26) | 11.44 (24.73) | <0.005 |
| Mean change in %EWL between 1st and 5th year after SG (±SD) | 12.58 (17.78) | 22.78 (17.11) | 7.45 (15.83) | <0.005 |
| Mean change in %TWL between 1st and 5th year after SG (±SD) | 6.27 (9.22) | 11.29 (8.68) | 3.74 (8.43) | <0.005 |
| ≥50% EBMIL 5 years after SG | 0.003 | |||
| Yes (%) | 181 (88%) | 54 (78%) | 127 (93%) | |
| No (%) | 25 (12%) | 15 (22%) | 10 (7%) | |
| ≥50% EWL 5 years after SG | <0.005 | |||
| Yes (%) | 138 (67%) | 37 (54%) | 106 (77%) | |
| No (%) | 68 (33%) | 32 (46%) | 31 (23%) | |
| ≥25% TWL 5 years after SG | <0.005 | |||
| Yes (%) | 132 (64%) | 29 (42%) | 103 (75%) | |
| No (%) | 74 (36%) | 40 (58%) | 34 (25%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kapała, J.; Maroszczuk, T.; Lewandowska, J.; Lech, P.; Dowgiałło-Gornowicz, N. Weight Regain in the Second Year after Sleeve Gastrectomy Could Be a Predictor of Long-Term Outcomes? Medicina 2023, 59, 766. https://doi.org/10.3390/medicina59040766
Kapała J, Maroszczuk T, Lewandowska J, Lech P, Dowgiałło-Gornowicz N. Weight Regain in the Second Year after Sleeve Gastrectomy Could Be a Predictor of Long-Term Outcomes? Medicina. 2023; 59(4):766. https://doi.org/10.3390/medicina59040766
Chicago/Turabian StyleKapała, Jan, Tomasz Maroszczuk, Julia Lewandowska, Paweł Lech, and Natalia Dowgiałło-Gornowicz. 2023. "Weight Regain in the Second Year after Sleeve Gastrectomy Could Be a Predictor of Long-Term Outcomes?" Medicina 59, no. 4: 766. https://doi.org/10.3390/medicina59040766
APA StyleKapała, J., Maroszczuk, T., Lewandowska, J., Lech, P., & Dowgiałło-Gornowicz, N. (2023). Weight Regain in the Second Year after Sleeve Gastrectomy Could Be a Predictor of Long-Term Outcomes? Medicina, 59(4), 766. https://doi.org/10.3390/medicina59040766







