Outcomes after Percutaneous Coronary Intervention in Patients with Extremely Calcified Left Main Lesions
Abstract
:1. Introduction
2. Methods
2.1. Design and Patients
2.2. Data Collection
2.3. Definitions
2.4. Statistical Analysis
3. Results
3.1. Baseline Population Characteristics
3.2. Procedural Details
3.3. In-Hospital Events
3.4. Follow-Up Clinical Events
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soleimani, A.; Abbasi, A.; Kazzazi, E.H.; Hosseini, K.; Salirifar, M.; Darabian, S.; Sadeghian, S.; Sheikhfathol-Lahi, M. Prevalence of left main coronary artery disease among patients with ischemic heart disease: Insights from the Tehran Angiography Registry. Minerva Cardioangiol. 2009, 57, 175–183. [Google Scholar] [PubMed]
- D’Ascenzo, F.; Presutti, D.G.; Picardi, E.; Moretti, C.; Omede, P.; Sciuto, F.; Novara, M.; Yan, A.T.; Goodman, S.; Mahajan, N.; et al. Prevalence and non-invasive predictors of left main or three-vessel coronary disease: Evidence from a collaborative international meta-analysis including 22 740 patients. Heart 2012, 98, 914–919. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.H.; Ahn, J.M.; Chang, M.; Baek, S.; Yoon, S.H.; Kang, S.J.; Lee, S.W.; Kim, Y.H.; Lee, C.W.; Park, S.W.; et al. Left Main Coronary Artery Disease: Secular Trends in Patient Characteristics, Treatments, and Outcomes. J. Am. Coll. Cardiol. 2016, 68, 1233–1246. [Google Scholar] [CrossRef] [PubMed]
- Levine, G.N.; Bates, E.R.; Blankenship, J.C.; Bailey, S.R.; Bittl, J.A.; Cercek, B.; Chambers, C.E.; Ellis, S.G.; Guyton, R.A.; Hollenberg, S.M.; et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J. Am. Coll. Cardiol. 2011, 58, e44–e122. [Google Scholar] [CrossRef]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef]
- Genereux, P.; Redfors, B.; Witzenbichler, B.; Arsenault, M.P.; Weisz, G.; Stuckey, T.D.; Rinaldi, M.J.; Neumann, F.J.; Christopher Metzger, D.; Henry, T.D.; et al. Two-year outcomes after percutaneous coronary intervention of calcified lesions with drug-eluting stents. Int. J. Cardiol. 2017, 231, 61–67. [Google Scholar] [CrossRef]
- Madhavan, M.V.; Tarigopula, M.; Mintz, G.S.; Maehara, A.; Stone, G.W.; Genereux, P. Coronary artery calcification: Pathogenesis and prognostic implications. J. Am. Coll. Cardiol. 2014, 63, 1703–1714. [Google Scholar] [CrossRef]
- Bourantas, C.V.; Zhang, Y.J.; Garg, S.; Iqbal, J.; Valgimigli, M.; Windecker, S.; Mohr, F.W.; Silber, S.; Vries, T.; Onuma, Y.; et al. Prognostic implications of coronary calcification in patients with obstructive coronary artery disease treated by percutaneous coronary intervention: A patient-level pooled analysis of 7 contemporary stent trials. Heart 2014, 100, 1158–1164. [Google Scholar] [CrossRef]
- Wang, X.; Matsumura, M.; Mintz, G.S.; Lee, T.; Zhang, W.; Cao, Y.; Fujino, A.; Lin, Y.; Usui, E.; Kanaji, Y.; et al. In Vivo Calcium Detection by Comparing Optical Coherence Tomography, Intravascular Ultrasound, and Angiography. JACC Cardiovasc. Imaging 2017, 10, 869–879. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; Executive Group on behalf of the Joint European Society of Cardiology/American College of Cardiology/American Heart Association/World Heart Federation Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction (2018). Glob. Heart 2018, 13, 305–338. [Google Scholar] [CrossRef]
- Cutlip, D.E.; Windecker, S.; Mehran, R.; Boam, A.; Cohen, D.J.; van Es, G.A.; Steg, P.G.; Morel, M.A.; Mauri, L.; Vranckx, P.; et al. Clinical end points in coronary stent trials: A case for standardized definitions. Circulation 2007, 115, 2344–2351. [Google Scholar] [CrossRef] [PubMed]
- Schulman, S.; Angeras, U.; Bergqvist, D.; Eriksson, B.; Lassen, M.R.; Fisher, W.; Subcommittee on Control of Anticoagulation of the Scientific; Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in surgical patients. J. Thromb. Haemost. 2010, 8, 202–204. [Google Scholar] [CrossRef] [PubMed]
- Genereux, P.; Palmerini, T.; Caixeta, A.; Cristea, E.; Mehran, R.; Sanchez, R.; Lazar, D.; Jankovic, I.; Corral, M.D.; Dressler, O.; et al. SYNTAX score reproducibility and variability between interventional cardiologists, core laboratory technicians, and quantitative coronary measurements. Circ. Cardiovasc. Interv. 2011, 4, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Medina, A.; Suarez de Lezo, J.; Pan, M. A new classification of coronary bifurcation lesions. Rev. Esp. Cardiol. 2006, 59, 183. [Google Scholar] [CrossRef] [PubMed]
- Ungureanu, C.; Gasparini, G.L.; Aminian, A.; Blaimont, M.; Dumitrascu, S.; Lepiece, C.; Colletti, G. RailTracking: A Novel Technique to Overcome Difficult Anatomy During Transradial Approach. J. Invasive Cardiol. 2022, 34, E757–E762. [Google Scholar] [PubMed]
- Darabian, S.; Blaha, M.; Whelton, S.; Homat, A.; Vahoumeni, R.; Nozari, Y.; Li, D.; Nakanishi, R.; Hamal, S.; Budoff, M.J. Abstract 15181: Left Main Calcified Lesions as a Strong Predictor of All-Cause Death. Circulation 2013, 128, A15181. [Google Scholar] [CrossRef]
- Kang, S.J.; Ahn, J.M.; Song, H.; Kim, W.J.; Lee, J.Y.; Park, D.W.; Yun, S.C.; Lee, S.W.; Kim, Y.H.; Lee, C.W.; et al. Comprehensive intravascular ultrasound assessment of stent area and its impact on restenosis and adverse cardiac events in 403 patients with unprotected left main disease. Circ. Cardiovasc. Interv. 2011, 4, 562–569. [Google Scholar] [CrossRef]
- Ielasi, A.; Kawamoto, H.; Latib, A.; Boccuzzi, G.G.; Sardella, G.; Garbo, R.; Meliga, E.; D’Ascenzo, F.; Presbitero, P.; Nakamura, S.; et al. In-Hospital and 1-Year Outcomes of Rotational Atherectomy and Stent Implantation in Patients With Severely Calcified Unprotected Left Main Narrowings (from the Multicenter ROTATE Registry). Am. J. Cardiol. 2017, 119, 1331–1337. [Google Scholar] [CrossRef]
- Chiang, M.H.; Yi, H.T.; Tsao, C.R.; Chang, W.C.; Su, C.S.; Liu, T.J.; Liang, K.W.; Ting, C.T.; Lee, W.L. Rotablation in the treatment of high-risk patients with heavily calcified left-main coronary lesions. J. Geriatr. Cardiol. 2013, 10, 217–225. [Google Scholar] [CrossRef]
- Garcia-Lara, J.; Pinar, E.; Valdesuso, R.; Lacunza, J.; Gimeno, J.R.; Hurtado, J.A.; Valdes-Chavarri, M. Percutaneous coronary intervention with rotational atherectomy for severely calcified unprotected left main: Immediate and two-years follow-up results. Catheter. Cardiovasc. Interv. 2012, 80, 215–220. [Google Scholar] [CrossRef]
- Yabushita, H.; Takagi, K.; Tahara, S.; Fujino, Y.; Warisawa, T.; Kawamoto, H.; Watanabe, Y.; Mitomo, S.; Karube, K.; Matsumoto, T.; et al. Impact of rotational atherectomy on heavily calcified, unprotected left main disease. Circ. J. 2014, 78, 1867–1872. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, A.S.; Narayanan, M.R.; Tun, H.; Hindoyan, A.; Matthews, R.; Mehra, A.; Shavelle, D.M.; Clavijo, L.C. In-Hospital Outcomes of Rotational Atherectomy in High-Risk Patients With Severely Calcified Left Main Coronary Artery Disease: A Single-Center Experience. J. Invasive Cardiol. 2019, 31, 101–106. [Google Scholar] [PubMed]
- Abdel-Wahab, M.; Baev, R.; Dieker, P.; Kassner, G.; Khattab, A.A.; Toelg, R.; Sulimov, D.; Geist, V.; Richardt, G. Long-term clinical outcome of rotational atherectomy followed by drug-eluting stent implantation in complex calcified coronary lesions. Catheter. Cardiovasc. Interv. 2013, 81, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Dahdouh, Z.; Roule, V.; Dugue, A.E.; Sabatier, R.; Lognone, T.; Grollier, G. Rotational atherectomy for left main coronary artery disease in octogenarians: Transradial approach in a tertiary center and literature review. J. Interv. Cardiol. 2013, 26, 173–182. [Google Scholar] [CrossRef]
- Sulimov, D.S.; Abdel-Wahab, M.; Toelg, R.; Kassner, G.; Geist, V.; Richardt, G. High-speed rotational atherectomy of the left main coronary artery: A single-center experience in 50 high-risk patients. Cardiovasc. Revasc. Med. 2015, 16, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Beohar, N.; Chen, S.; Lembo, N.J.; Banning, A.P.; Serruys, P.W.; Leon, M.B.; Morice, M.C.; Genereux, P.; Kandzari, D.E.; Kappetein, A.P.; et al. Impact of lesion preparation strategies on outcomes of left main PCI: The EXCEL trial. Catheter. Cardiovasc. Interv. 2021, 98, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.; Hansen, S.; Meincke, F.; Frerker, C.; Kuck, K.H.; Bergmann, M.W. Safety and efficacy of lesion preparation with the AngioSculpt Scoring Balloon in left main interventions: The ALSTER Left Main registry. EuroIntervention 2016, 11, 1346–1354. [Google Scholar] [CrossRef]
- Nassar, H.; Gotsman, I.; Gerganski, P.; Moseri, M.; Lotan, C.; Gotsman, M. Cutting balloon angioplasty and stent implantation for aorto-ostial lesions: Clinical outcome and 1-year follow-up. Clin. Cardiol. 2009, 32, 183–186. [Google Scholar] [CrossRef]
- Cimci, M.; Iglesias, J.F.; Huber, C.; Mach, F.; Roffi, M. Intravascular lithotripsy to treat an ostial left main coronary artery stenosis due to porcelain aorta in a patient with congenital high-density lipoprotein deficiency. Anatol. J. Cardiol. 2020, 24, 345–346. [Google Scholar] [CrossRef]
- Agrawal, Y.; Zoltowska, D.; Nazroo, J.R.; Halabi, A.R. Impella-Assisted Intracoronary Lithotripsy of Severely Calcified Left Main Coronary Artery Bifurcation for NSTEMI With Cardiogenic Shock. Cureus 2021, 13, e14772. [Google Scholar] [CrossRef]
- Rola, P.; Wlodarczak, A.; Kulczycki, J.J.; Barycki, M.; Furtan, L.; Pecherzewski, M.; Szudrowicz, M.; Wlodarczak, S.; Doroszko, A.; Lesiak, M. Efficacy and safety of shockwave intravascular lithotripsy (S-IVL) in calcified unprotected left main percutaneous coronary intervention—Short-term outcomes. Postepy Kardiol. Interwencyjnej 2021, 17, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, C.S.; Wilson, S.J.; Bogle, R.; Hanratty, C.G.; Williams, R.; Walsh, S.J.; McEntegart, M.; Spratt, J.C. Intravascular lithotripsy for lesion preparation in patients with calcific distal left main disease. EuroIntervention 2020, 16, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Rola, P.; Kulczycki, J.J.; Wlodarczak, A.; Barycki, M.; Wlodarczak, S.; Szudrowicz, M.; Furtan, L.; Jastrzebski, A.; Pecherzewski, M.; Lesiak, M.; et al. Intravascular Lithotripsy as a Novel Treatment Method for Calcified Unprotected Left Main Diseases-Comparison to Rotational Atherectomy-Short-Term Outcomes. Int. J. Environ. Res. Public Health 2022, 19, 9011. [Google Scholar] [CrossRef] [PubMed]
- Buono, A.; Basavarajaiah, S.; Choudhury, A.; Lee, L.; Bhatia, G.; Hailan, A.; Sharma, V.; Upadhyaya, S.; Naneishvili, T.; Ielasi, A. “RotaTripsy” for Severe Calcified Coronary Artery Lesions: Insights From a Real-World Multicenter Cohort. Cardiovasc. Revasc Med. 2022, 37, 78–81. [Google Scholar] [CrossRef]
- Tamburino, C.; Capranzano, P.; Capodanno, D.; Tagliareni, F.; Biondi-Zoccai, G.; Sanfilippo, A.; Caggegi, A.; Barrano, G.; Monaco, S.; Tomasello, S.D.; et al. Plaque distribution patterns in distal left main coronary artery to predict outcomes after stent implantation. JACC Cardiovasc. Interv. 2010, 3, 624–631. [Google Scholar] [CrossRef]
- Stone, G.W.; Kappetein, A.P.; Sabik, J.F.; Pocock, S.J.; Morice, M.C.; Puskas, J.; Kandzari, D.E.; Karmpaliotis, D.; Brown, W.M., 3rd; Lembo, N.J.; et al. Five-Year Outcomes after PCI or CABG for Left Main Coronary Disease. N. Engl. J. Med. 2019, 381, 1820–1830. [Google Scholar] [CrossRef]
- Holm, N.R.; Makikallio, T.; Lindsay, M.M.; Spence, M.S.; Erglis, A.; Menown, I.B.A.; Trovik, T.; Kellerth, T.; Kalinauskas, G.; Mogensen, L.J.H.; et al. Percutaneous coronary angioplasty versus coronary artery bypass grafting in the treatment of unprotected left main stenosis: Updated 5-year outcomes from the randomised, non-inferiority NOBLE trial. Lancet 2020, 395, 191–199. [Google Scholar] [CrossRef]
- D’Ascenzo, F.; De Filippo, O.; Elia, E.; Doronzo, M.P.; Omede, P.; Montefusco, A.; Pennone, M.; Salizzoni, S.; Conrotto, F.; Gallone, G.; et al. Percutaneous vs. surgical revascularization for patients with unprotected left main stenosis: A meta-analysis of 5-year follow-up randomized controlled trials. Eur. Heart J. Qual. Care Clin. Outcomes 2021, 7, 476–485. [Google Scholar] [CrossRef]
- Park, S.; Park, S.-J.; Park, D.-W. Percutaneous Coronary Intervention for Left Main Coronary Artery Disease. JACC Asia 2022, 2, 119–138. [Google Scholar] [CrossRef]
- Park, S.J.; Kim, Y.H.; Park, D.W.; Lee, S.W.; Kim, W.J.; Suh, J.; Yun, S.C.; Lee, C.W.; Hong, M.K.; Lee, J.H.; et al. Impact of intravascular ultrasound guidance on long-term mortality in stenting for unprotected left main coronary artery stenosis. Circ. Cardiovasc. Interv. 2009, 2, 167–177. [Google Scholar] [CrossRef]
- De la Torre Hernandez, J.M.; Baz Alonso, J.A.; Gomez Hospital, J.A.; Alfonso Manterola, F.; Garcia Camarero, T.; Gimeno de Carlos, F.; Roura Ferrer, G.; Recalde, A.S.; Martinez-Luengas, I.L.; Gomez Lara, J.; et al. Clinical impact of intravascular ultrasound guidance in drug-eluting stent implantation for unprotected left main coronary disease: Pooled analysis at the patient-level of 4 registries. JACC Cardiovasc. Interv. 2014, 7, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Pang, S.; Chen, X.Y.; Bourantas, C.V.; Pan, D.R.; Dong, S.J.; Wu, W.; Ren, X.M.; Zhu, H.; Shi, S.Y.; et al. Comparison of intravascular ultrasound guided versus angiography guided drug eluting stent implantation: A systematic review and meta-analysis. BMC Cardiovasc. Disord. 2015, 15, 153. [Google Scholar] [CrossRef]
- Andell, P.; Karlsson, S.; Mohammad, M.A.; Gotberg, M.; James, S.; Jensen, J.; Frobert, O.; Angeras, O.; Nilsson, J.; Omerovic, E.; et al. Intravascular Ultrasound Guidance Is Associated With Better Outcome in Patients Undergoing Unprotected Left Main Coronary Artery Stenting Compared With Angiography Guidance Alone. Circ. Cardiovasc. Interv. 2017, 10, e004813. [Google Scholar] [CrossRef] [PubMed]
- Finet, G.; Gilard, M.; Perrenot, B.; Rioufol, G.; Motreff, P.; Gavit, L.; Prost, R. Fractal geometry of arterial coronary bifurcations: A quantitative coronary angiography and intravascular ultrasound analysis. EuroIntervention 2008, 3, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Genereux, P.; Palmerini, T.; Caixeta, A.; Rosner, G.; Green, P.; Dressler, O.; Xu, K.; Parise, H.; Mehran, R.; Serruys, P.W.; et al. Quantification and impact of untreated coronary artery disease after percutaneous coronary intervention: The residual SYNTAX (Synergy Between PCI with Taxus and Cardiac Surgery) score. J. Am. Coll. Cardiol. 2012, 59, 2165–2174. [Google Scholar] [CrossRef] [PubMed]
- Farooq, V.; Serruys, P.W.; Bourantas, C.V.; Zhang, Y.; Muramatsu, T.; Feldman, T.; Holmes, D.R.; Mack, M.; Morice, M.C.; Stahle, E.; et al. Quantification of incomplete revascularization and its association with five-year mortality in the synergy between percutaneous coronary intervention with taxus and cardiac surgery (SYNTAX) trial validation of the residual SYNTAX score. Circulation 2013, 128, 141–151. [Google Scholar] [CrossRef] [PubMed]
| Total n = 70 | CdD n = 22 | rLM n = 48 | p-Value | |
|---|---|---|---|---|
| Age | 66.54 ± 10.32 | 68.64 ± 7.63 | 65.58 ± 11.29 | 0.28 |
| Male sex | 50 (71.4%) | 16 (72.7%) | 34 (70.8%) | 0.87 |
| Diabetes | 26 (37.1%) | 8 (36.4%) | 18 (37.5%) | 0.92 |
| Insulin | 8 (11.4%) | 2 (9.1%) | 6 (12.5%) | 0.67 |
| BMI | 29.27 ± 5.22 | 30.68 ± 5.14 | 28.63 ± 5.19 | 0.13 |
| Hypercholesterolemia | 69 (98.6) | 21 (95.5%) | 48 (100%) | 0.13 |
| Arterial hypertension | 63 (90%) | 20 (90.9%) | 43 (89.6%) | 0.86 |
| Peripheral Arterial Disease | 9 (12.9%) | 4 (18.2%) | 5 (10.4%) | 0.36 |
| Malignancy | 8 (11.4%) | 3 (13.6%) | 5 (10.4%) | 0.69 |
| Active smoker | 16 (22.9%) | 3 (13.6%) | 13 (27.1%) | 0.21 |
| CrCl < 60 mL/min/1.73 m2 | 6 (8.6%) | 2 (9.1%) | 4 (8.3%) | 0.91 |
| Dialysis | 1 (1.4%) | 0 | 1 (2.1%) | 0.49 |
| Previous PCI | 22 (31.4%) | 10 (45.4%) | 12 (25%) | 0.30 |
| Previous CABG | 0 | 0 | 0 | |
| LVEF (%) | 49 ±7.1 | 48.41 ± 6.43 | 49.38 ± 7.55 | 0.34 |
| Clinical Presentation | 0.49 | |||
| Stable angina | 41 (58.6%) | 15 (68.2%) | 26 (54.2%) | |
| Non-STE ACS | 17 (24.3%) | 4 (18.2%) | 13 (27.1%) | |
| STEMI | 3 (4.3%) | 0 | 3 (6.3%) | |
| Silent ischemia | 9 (12.9%) | 3 (13.6%) | 6 (12.5%) | |
| PCI in previous 30 days | 7 (10%) | 3 (13.6%) | 4 (8.3%) | 0.98 |
| Total n = 70 | CdD n = 22 | rLM n = 48 | p-Value | |
|---|---|---|---|---|
| Timing | 0.03 | |||
| Ad-hoc | 19 (27.1%) | 3 (13.6%) | 16 (33.3%) | |
| Elective | 51 (72.9%) | 19 (86.4%) | 32 (66.7%) | |
| SYNTAX Score | 28.45 ± 8.89 | 29.18 ± 9.92 | 28.11 ± 8.47 | 0.84 |
| SYNTAX Score >32 | 22 (31.4%) | 8 (36.3%) | 14 (29.1%) | 0.17 |
| Pharmacological support before procedure | 2 (2.9%) | 0 | 2 (4.2%) | 0.49 |
| MCS before procedure | 1 (1.4%) | 0 | 1 (2.1%) | 0.49 |
| Type of MCS | ECMO | n/a | ECMO | |
| Transradial | 61 (87.1%) | 19 (86.3%) | 42 (87.5%) | 0.52 |
| Distal radial | 45 (64.2%) | 16 (72.7%) | 29 (60.4%) | 0.42 |
| Sheathless system | 0.34 | |||
| Total | 47 (67.1%) | 16 (72.7%) | 31 (64.5%) | |
| 7F | 36 (51.4%) | 14 (63.6%) | 22 (45.8%) | |
| 6F | 11 (15.7%) | 2 (9.1%) | 9 (18.8%) | |
| Catheter size | 0.19 | |||
| 7F | 59 (84.3%) | 19 (86.3%) | 40 (83.3%) | |
| 8F | 1 (1.4%) | 1 (4.5%) | 0 | |
| Branching | 0.87 | |||
| Bifurcation | 67 (95.7%) | 22 (100%) | 45 (93.8%) | |
| Trifurcation | 3 (4.3%) | 0 | 3 (6.3%) | |
| LM stenosis ≥70% | 36 (51.4%) | 13 (59.1%) | 23 (47.9%) | 0.38 |
| Three-vessel disease | 25 (35.7%) | 8 (36.3%) | 17 (35.4%) | 0.94 |
| Distal bifurcation angle | 0.48 | |||
| <45° | 3 (4.3%) | 0 | 3 (6.3%) | |
| 45–70° | 27 (38.6%) | 9 (40.9%) | 18 (37.5%) | |
| >70° | 40 (57.1%) | 13 (59.1%) | 27 (56.3%) | |
| LM ostial involved | 6 (8.6%) | 2 (9.1%) | 4 (8.3%) | 0.91 |
| LM body shaft only | 5 (7.1%) | 1 (4.5%) | 4 (8.3%) | 0.56 |
| Medina Classification | 0.61 | |||
| 1,1,0 | 39 (55.7%) | 10 (45.5%) | 29 (60.4%) | |
| 1,1,1 | 17 (24.3%) | 6 (27.3%) | 11 (22.9%) | |
| 1,0,1 | 6 (8.6%) | 3 (13.6%) | 3 (6.3%) | |
| 1,0,0 | 6 (8.6%) | 3 (13.6%) | 3 (6.3%) | |
| 0,0,1 | 1 (1.4%) | 0 | 1 (2.1%) | |
| Angiographic LM calcification | 0.02 | |||
| No/mild | 30 (42.8%) | 2 (9.1%) | 28 (58.3%) | |
| Moderate | 23 (32.9%) | 7 (31.8%) | 16 (33.3%) | |
| Severe | 17 (24.2%) | 13 (59.1%) | 4 (8.3%) | |
| Eccentric calcification | 19 (27.1%) | 7 (31.8%) | 12 (25%) | 0.55 |
| Lesion thrombus | 3 (4.3%) | 0 | 3 (6.3%) | 0.23 |
| LM in-stent restenosis | 1 (4.3%) | 0 | 1 (2.1%) | 0.49 |
| Total n = 70 | CdD n = 22 | rLM n = 48 | p-Value | |
|---|---|---|---|---|
| LM only PCI | 29 (41.4%) | 9 (40.9%) | 20 (41.7%) | 0.95 |
| Stent LM towards | 0.14 | |||
| CX | 6 (8.6%) | 3 (13.6%) | 3 (6.3%) | |
| LAD | 64 (91.4%) | 19 (86.4%) | 45 (93.8%) | |
| LM pharmacological balloon | 1 (1.4%) | 0 | 1 (2.1%) | |
| LM non-bifurcation stenting | 1 (1.4%) | 0 | 1 (2.1%) | 0.49 |
| Bifurcation 1-stent | 51 (72.9%) | 16 (72.7%) | 35 (72.9%) | 0.98 |
| Bifurcation 2-stents | 17 (24.3%) | 6 (27.3%) | 11 (22.9%) | 0.69 |
| 2-stent technique | 0.54 | |||
| DK-Crush | 11 (15.7%) | 4 (18.2%) | 7 (14.6%) | |
| TAP | 3 (4.3%) | 1 (4.5%) | 2 (4.2%) | |
| Culotte | 1 (1.4%) | 0 | 1 (2.1%) | |
| Nano-Crush | 2 (2.9%) | 1 (4.5%) | 1 (2.1%) | |
| LM pre-dilation | 70 (100%) | 22 (100%) | 48 (100%) | |
| Pre-dilation balloon size | 3.19 ± 0.35 | 3.09 ± 0.33 | 3.24 ± 0.35 | 0.13 |
| Pre-dilation pressure | 20.1 ± 2.2 | 21.2 ± 2.5 | 18.8 ± 1.9 | 0.08 |
| Pre-dilation SB | 17 (24.3%) | 6 (27.3%) | 11 (22.9%) | 0.74 |
| LM stent diameter | 3.58 ± 0.24 | 3.55 ± 0.21 | 3.59 ± 0.26 | 0.4 |
| SB stent diameter | 3.01 ± 0.32 | 2.96 ± 0.33 | 3.05 ± 0.33 | 0.66 |
| LM stent length | 36.48 ± 16.25 | 37.27 ± 17.54 | 36.11 ± 15.79 | 0.86 |
| POT balloon diameter | 5.25 ± 0.43 | 5.41 ± 0.36 | 5.18 ± 0.44 | 0.03 |
| Tri-kissing | 2 (2.9%) | 0 | 2 (4.2%) | 0.28 |
| SB stenting required | 1 (1.4%) | 0 | 1 (2.1%) | 0.56 |
| SB bailout stenting technique | T-stenting | n/a | T-stenting | |
| Covered LM ostium | 67 (95.7%) | 21 (95.5%) | 46 (95.8%) | 0.94 |
| Final KBD | 67 (95.7%) | 22 (100%) | 45 (93.8%) | 0.23 |
| MB KBD diameter | 3.70 ± 0.24 | 3.7 ± 0.27 | 3.70 ± 0.23 | 0.93 |
| SB KBD diameter | 3.02 ± 0.36 | 2.99 ± 0.43 | 3.04 ± 0.32 | 0.35 |
| Re-POT | 17 (24.3%) | 6 (27.3%) | 11 (22.9%) | 0.69 |
| Stent Name | 0.02 | |||
| Synergy MegatronTM (Boston Sci.) | 39 (55.7%) | 18 (81.8%) | 21 (43.8%) | |
| XienceTM (Abbott) | 29 (41.4%) | 4 (18.2%) | 25 (52.1%) | |
| Promus EliteTM (Boston Sci.) | 1 (1.4%) | 0 | 1 (2.1%) | |
| Angiographic success | 70 (100%) | 22 (100%) | 48 (100%) | |
| Complications during LM PCI | 1 (1.4%) | 0 | 1 (2.1%) | 0.56 |
| Type of complication | Stent longitudinal compression—1 (1.4%) | n/a | Stent longitudinal compression—1 (2.1%) | |
| Treatment of complication | Re-POT—1 (1.4%) | n/a | Re-POT—1 (2.1%) | |
| Pharm. support initiated during procedure | 0 | 0 | 0 | |
| MCS initiated during procedure | 0 | 0 | 0 | |
| Procedure time (s) | 76.54 ± 36.5 | 93.18 ± 42.29 | 69.92 ± 31.15 | 0.02 |
| Fluoroscopy time (s) | 1579 ± 686.9 | 1865.18 ± 699.8 | 1448.19 ± 646.65 | 0.16 |
| Total dose area product (mGy.m2) | 8.73 ± 6.32 | 8.83 ± 5.8 | 8.69 ± 6.24 | 0.5 |
| Contrast amount (mL) | 168.57 ± 60.56 | 170.14 ± 69.88 | 167.85 ± 56.57 | 0.89 |
| Guide extension | 7 (10%) | 6 (27.3%) | 1 (2.1%) | <0.001 |
| Microcatheter | 5 (7.1%) | 3 (13.6%) | 2 (4.2%) | 0.31 |
| Intravascular imaging | ||||
| Total | 14 (20%) | 5 (22.7%) | 9 (18.8%) | 0.69 |
| IVUS | 10 (14.3%) | 4 (18.2%) | 6 (12.5%) | |
| OCT | 4 (5.7%) | 1 (4.5%) | 3 (6.3%) | |
| LM MSA (mm2) | 12.4 ± 2.1 | 12.8 ± 1.7 | 11.5 ± 2.8 | 0.24 |
| Complete revascularization | 53 (75.7%) | 17 (77.3%) | 36 (75%) | 0.83 |
| No. of other significant lesions | 2 (1–2.25) | 2 (1–2) | 2 (1–3) | 0.4 |
| Any CTO | 9 (12.9%) | 4 (18.2%) | 5 (10.4%) | 0.36 |
| Planned PCI for residual lesions | 14 (20%) | 6 (27.2%) | 8 (16.6%) | 0.21 |
| Residual SYNTAX Score ≥8 | 6 (8.5%) | 1 (4.5%) | 5 (10.4%) | 0.15 |
| Total n = 70 | CdD n = 22 | rLM n = 48 | |
|---|---|---|---|
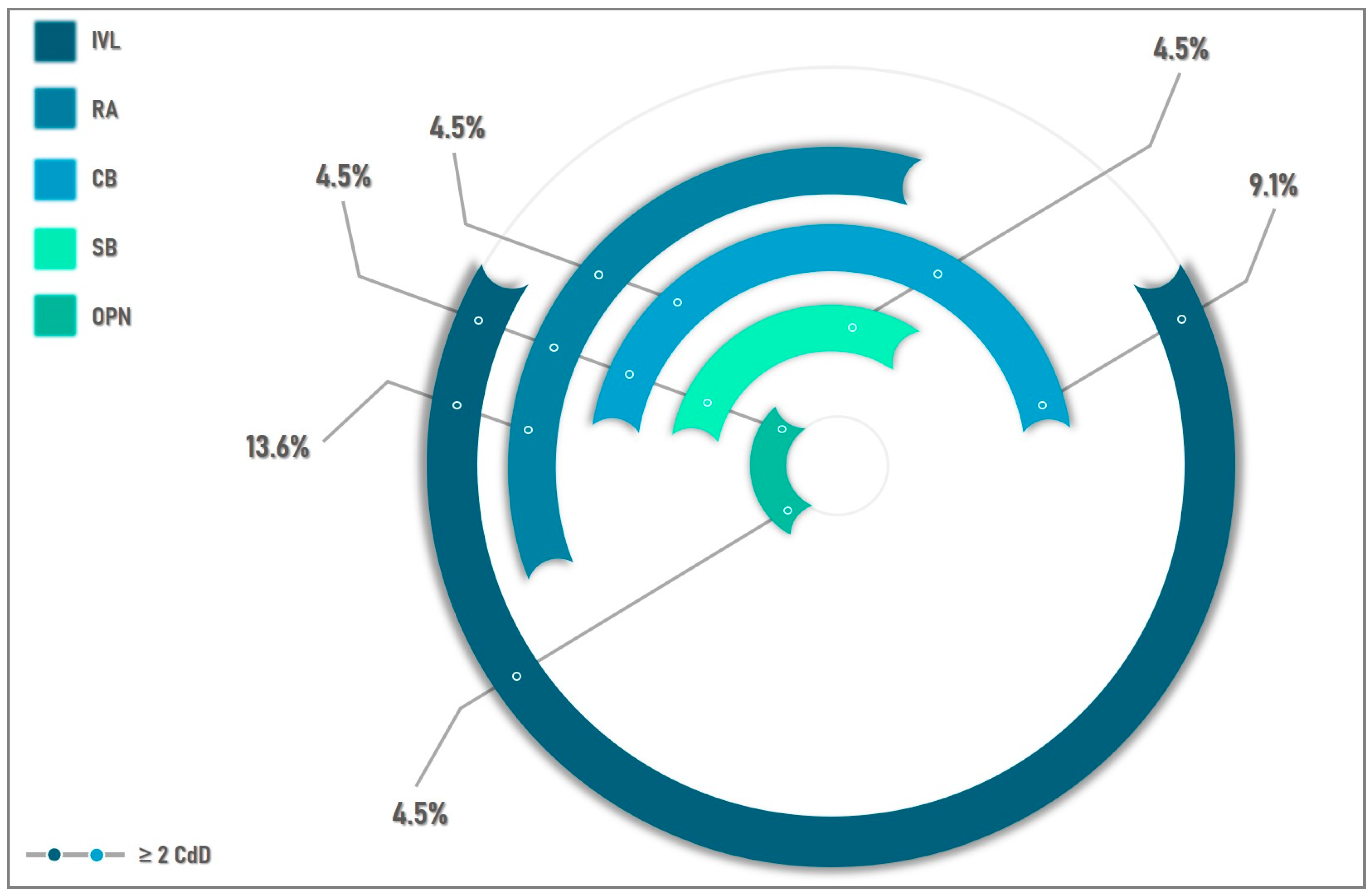
| Ca-dedicated devices | 22 (31.4%) | 22 (100%) | n/a |
| Rotational atherectomy | 9 (12.9%) | 9 (40.9%) | n/a |
| Bailout RA | 5 (7.1%) | 5 (22.7%) | n/a |
| Max. burr size | 1.75 (1.5–2) | 1.75 (1.5–2) | n/a |
| No. of burrs | |||
| 1 burr | 8 (11.4%) | 8 (36.3%) | n/a |
| 2 burrs | 1 (1.4%) | 1 (4.5%) | n/a |
| Burring LM towards | |||
| LAD | 8 (11.4%) | 8 (11.4%) | n/a |
| CX | 1 (1.4%) | 1 (1.4%) | n/a |
| IVL | 13 (18.5%) | 13 (59.1%) | n/a |
| IVL balloon size | 3.41 ± 0.49 | 3.41 ± 0.49 | n/a |
| No. of pulses on LM | 56.92 (30–80) | 56.92 (30–80) | n/a |
| IVL device crossing success | n/a | 100% | n/a |
| IVL used in >1 segment | 10 (14.2%) | 10 (45.4%) | n/a |
| Cutting Balloons | 7 (10%) | 7 (31.8%) | n/a |
| Scoring Balloon | 2 (2.8%) | 2 (9%) | n/a |
| OPN Balloon | 2 (2.8%) | 2 (9%) | n/a |
| OPN/IVL for stent under-expansion | 0 | 0 | n/a |
| Total n = 70 | CdD n = 22 | rLM n = 48 | p-Value | |
|---|---|---|---|---|
| In-hospital | ||||
| Procedural success | 70 (100%) | 22 (100%) | 48 (100%) | n/a |
| Clinical success | 70 (100%) | 22 (100%) | 48 (100%) | n/a |
| Death before discharge | 0 | 0 | 0 | n/a |
| Peri-procedural MI | 0 | 0 | 0 | n/a |
| In-hospital morbidity | 2 (2.8%) | 1 (4.5%) | 1 (2.1%) | 0.87 |
| Bleeding | 1 (1.4%) | 1 (4.5%) | 0 | |
| Access site related complication | 0 | 0 | 0 | |
| Contrast induced-nephropathy | 1 (1.4%) | 0 | 1 (2.1%) | 0.566 |
| Complication treatment | Conservative | Conservative | Conservative | |
| MACCE | 0 | 0 | 0 | n/a |
| At-discharge | ||||
| Aspirin | 70 (100%) | 22 (100%) | 48 (100%) | n/a |
| Thienopyridine | 70 (100%) | 22 (100%) | 48 (100%) | n/a |
| Clopidogrel | 42 (60%) | 14 (63.6%) | 28 (58.3%) | |
| Ticagrelor | 27 (38.6%) | 8 (36.4%) | 19 (39.6%) | |
| Prasugrel | 1 (1.4%) | 0 | 1 (2.1%) | |
| Beta-blocker | 45 (64.3%) | 10 (45.5%) | 35 (72.9%) | 0.02 |
| ACEi | 58 (82.9%) | 17 (77.3%) | 41 (85.4%) | 0.40 |
| Statins | 67 (95.7%) | 20 (90.9%) | 47 (97.9%) | 0.17 |
| Total n = 70 | CdD n = 22 | rLM n = 48 | p-Value | |
|---|---|---|---|---|
| MACCE | 3 (4.2%) | 0 | 3 (6.2%) | 0.23 |
| Cardiac death | 1 (1.4%) | 0 | 1 (2.1%) | 0.49 |
| Non-procedural MI | 0 | 0 | 0 | n/a |
| TLR | 2 (2.8%) | 0 | 2 (4.1%) | 0.33 |
| Stroke | 0 | 0 | 0 | n/a |
| All-cause death | 1 (1.4%) | 0 | 1 (2.1%) | 0.49 |
| Survival at 1-year | 69 (98.5%) | 22 (100%) | 47 (97.9%) | 0.49 |
| MB in-stent restenosis | 0 | 0 | 0 | n/a |
| SB restenosis | 2 (2.8%) | 0 | 2 (4.1%) | 0.33 |
| Possible ST | 1 (1.4%) | 0 | 1 (2.1%) | 0.49 |
| Hospitalization for HF | 1 (1.4%) | 0 | 1 (1.4%) | 0.49 |
| Bleeding | 4 (5.7%) | 2 (9%) | 2 (4.1%) | 0.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dumitrascu, S.; Bartos, D.; Ungureanu, C. Outcomes after Percutaneous Coronary Intervention in Patients with Extremely Calcified Left Main Lesions. Medicina 2023, 59, 825. https://doi.org/10.3390/medicina59050825
Dumitrascu S, Bartos D, Ungureanu C. Outcomes after Percutaneous Coronary Intervention in Patients with Extremely Calcified Left Main Lesions. Medicina. 2023; 59(5):825. https://doi.org/10.3390/medicina59050825
Chicago/Turabian StyleDumitrascu, Silviu, Daniela Bartos, and Claudiu Ungureanu. 2023. "Outcomes after Percutaneous Coronary Intervention in Patients with Extremely Calcified Left Main Lesions" Medicina 59, no. 5: 825. https://doi.org/10.3390/medicina59050825
APA StyleDumitrascu, S., Bartos, D., & Ungureanu, C. (2023). Outcomes after Percutaneous Coronary Intervention in Patients with Extremely Calcified Left Main Lesions. Medicina, 59(5), 825. https://doi.org/10.3390/medicina59050825







