Effectiveness and Safety of Dabigatran Reversal with Idarucizumab in the Taiwanese Population: A Comparison Based on Eligibility for Inclusion in Clinical Trials
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Setting
2.2. Study Population, Eligibilities, and the Infusion Protocol for Idarucizumab
2.3. Data Collection and Outcomes
2.4. Statistical Analysis
3. Results
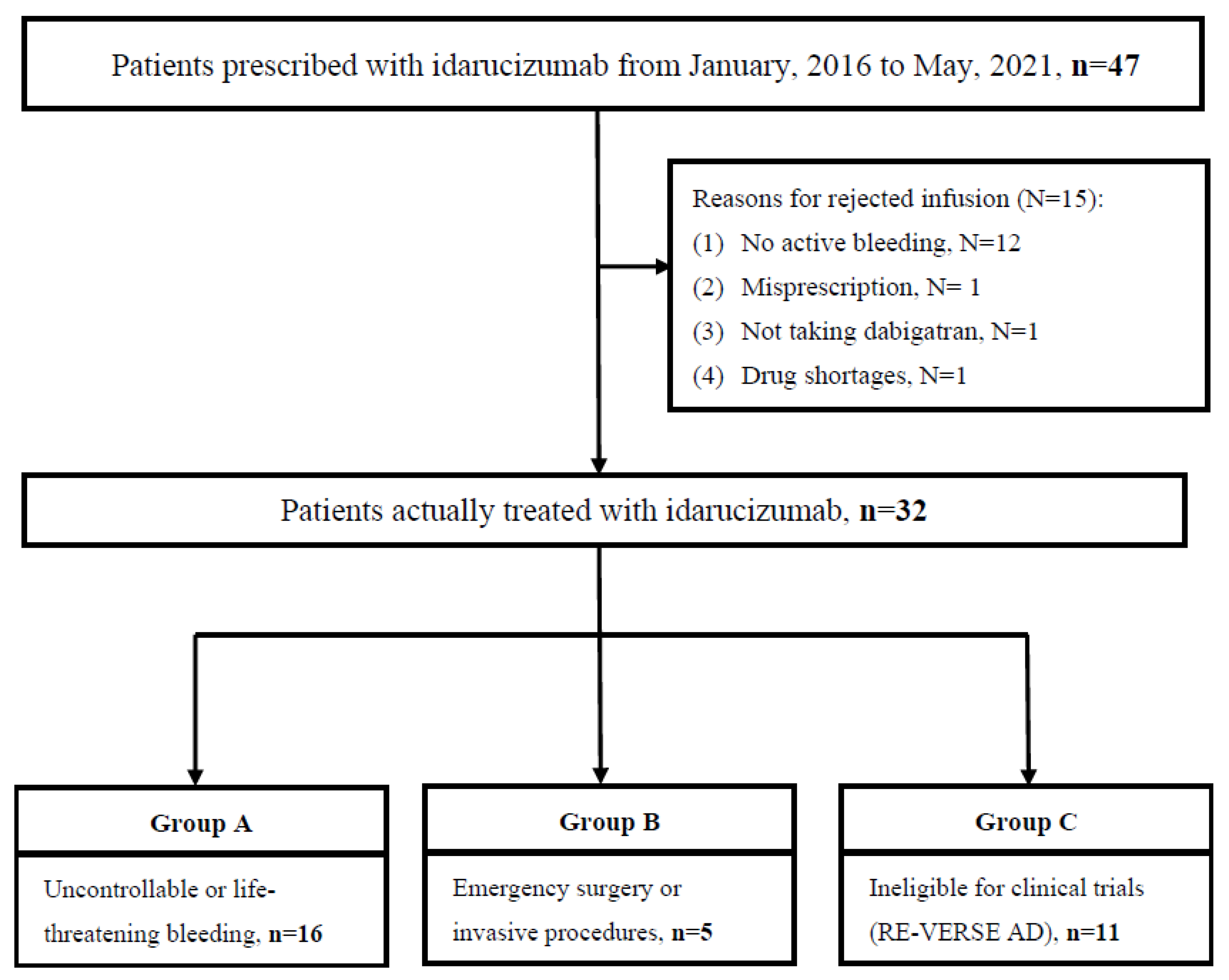
3.1. Characteristics and Eligibility of Patients
3.2. The Safety and Effectiveness Outcomes of the Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Connolly, S.J.; Ezekowitz, M.D.; Yusuf, S.; Eikelboom, J.; Oldgren, J.; Parekh, A.; Pogue, J.; Reilly, P.A.; Themeles, E.; Varrone, J.; et al. Dabigatran versus Warfarin in Patients with Atrial Fibrillation. N. Engl. J. Med. 2009, 361, 1139–1151. [Google Scholar] [CrossRef]
- Schulman, S.; Kakkar, A.K.; Goldhaber, S.Z.; Schellong, S.; Eriksson, H.; Mismetti, P.; Christiansen, A.V.; Friedman, J.; Le Maulf, F.; Peter, N.; et al. Treatment of Acute Venous Thromboembolism with Dabigatran or Warfarin and Pooled Analysis. Circulation 2014, 129, 764–772. [Google Scholar] [CrossRef]
- Van der Wall, S.J.; Lopes, R.D.; Aisenberg, J.; Reilly, P.; van Ryn, J.; Glund, S.; Elsaesser, A.; Klok, F.A.; Pollack, C.V., Jr.; Huisman, M.V.; et al. Idarucizumab for Dabigatran Reversal in the Management of Patients with Gastrointestinal Bleeding. Circulation 2019, 139, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.H.; van Ryn, J.; Sellke, F.W.; Reilly, P.A.; Elsaesser, A.; Glund, S.; Kreuzer, J.; Weitz, J.I.; Pollack, C.V., Jr. Dabigatran Reversal with Idarucizumab in Patients Requiring Urgent Surgery: A Subanalysis of the RE-VERSE AD Study. Ann. Surg. 2019, 274, e204–e211. [Google Scholar] [CrossRef]
- Vene, N.; Mavri, A.; Božič-Mijovski, M.; Gregorič, M.; Uštar, K.K.; Žerjav, U.; Gradišek, P.; Stecher, A.; Frol, S.; Nedog, V.; et al. Idarucizumab for dabigatran reversal in daily clinical practice: A case series. Eur. J. Anaesthesiol. 2020, 37, 874–878. [Google Scholar] [CrossRef] [PubMed]
- Brennan, Y.; Favaloro, E.J.; Pasalic, L.; Keenan, H.; Curnow, J. Lessons learnt from local real-life experience with idarucizumab for the reversal of dabigatran. Intern. Med. J. 2019, 49, 59–65. [Google Scholar] [CrossRef]
- Majeed, A.; Hwang, H.G.; Connolly, S.J.; Eikelboom, J.W.; Ezekowitz, M.D.; Wallentin, L.; Brueckmann, M.; Fraessdorf, M.; Yusuf, S.; Schulman, S.; et al. Management and Outcomes of Major Bleeding during Treatment with Dabigatran or Warfarin. Circulation 2013, 128, 2325–2332. [Google Scholar] [CrossRef]
- Schulman, S.; Kearon, C.; Kakkar, A.K.; Mismetti, P.; Schellong, S.; Eriksson, H.; Baanstra, D.; Schnee, J.; Goldhaber, S.Z. Dabigatran versus Warfarin in the Treatment of Acute Venous Thromboembolism. N. Engl. J. Med. 2009, 361, 2342–2352. [Google Scholar] [CrossRef] [PubMed]
- Reilly, P.A.; van Ryn, J.; Grottke, O.; Glund, S.; Stangier, J. Idarucizumab, a Specific Reversal Agent for Dabigatran: Mode of Action, Pharmacokinetics and Pharmacodynamics, and Safety and Efficacy in Phase 1 Subjects. Am. J. Med. 2016, 129, S64–S72. [Google Scholar] [CrossRef]
- Yasaka, M.; Ikushima, I.; Harada, A.; Imazu, S.; Taniguchi, A.; Norris, S.; Gansser, D.; Stangier, J.; Schmohl, M.; Reilly, P.A.; et al. Safety, pharmacokinetics and pharmacodynamics of idarucizumab, a specific dabigatran reversal agent in healthy Japanese volunteers: A randomized study. Res. Pr. Thromb. Haemost. 2017, 1, 202–215. [Google Scholar] [CrossRef] [PubMed]
- Pollack, C.V., Jr.; Reilly, P.A.; van Ryn, J.; Eikelboom, J.W.; Glund, S.; Bernstein, R.A.; Dubiel, R.; Huisman, M.V.; Hylek, E.M.; Kam, C.W.; et al. Idarucizumab for Dabigatran Reversal—Full Cohort Analysis. N. Engl. J. Med. 2017, 377, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Fanikos, J.; Murwin, D.; Gruenenfelder, F.; Tartakovsky, I.; França, L.R.; Reilly, P.A.; Kermer, P.; Wowern, F.V.; Lane, D.A.; Butcher, K.; et al. Global Use of Idarucizumab in Clinical Practice: Outcomes of the RE-VECTO Surveillance Program. Thromb. Haemost. 2020, 120, 27–35. [Google Scholar] [CrossRef]
- Thibault, N.; Morrill, A.M.; Willett, K.C. Idarucizumab for Reversing Dabigatran-Induced Anticoagulation: A Systematic Review. Am. J. Ther. 2018, 25, e333–e338. [Google Scholar] [CrossRef] [PubMed]
- Syed, Y.Y. Idarucizumab: A Review as a Reversal Agent for Dabigatran. Am. J. Cardiovasc. Drugs 2016, 16, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.O.; David, C.; Ferreira, J.J.; Pinto, F.J.; Costa, J.; Caldeira, D. The incidence of thrombotic events with idarucizumab and andexanet alfa: A systematic review and meta-analysis. Thromb. Res. 2020, 196, 291–296. [Google Scholar] [CrossRef]
- Haastrup, S.B.; Hellfritzsch, M.; Nybo, M.; Hvas, A.M.; Grove, E.L. Real-world experience with reversal of dabigatran by idarucizumab. Thromb. Res. 2020, 197, 179–184. [Google Scholar] [CrossRef]
- Gómez-Outes, A.; Alcubilla, P.; Calvo-Rojas, G.; Terleira-Fernández, A.I.; Suárez-Gea, M.L.; Lecumberri, R.; Vargas-Castrillón, E. Meta-Analysis of Reversal Agents for Severe Bleeding Associated with Direct Oral Anticoagulants. J. Am. Coll. Cardiol. 2021, 77, 2987–3001. [Google Scholar] [CrossRef]
- Lu, V.M.; Phan, K.; Rao, P.J.; Sharma, S.V.; Kasper, E.M. Dabigatran reversal by idarucizumab in the setting of intracranial hemorrhage: A systematic review of the literature. Clin. Neurol. Neurosurg. 2019, 181, 76–81. [Google Scholar] [CrossRef]
- Cuker, A.; Burnett, A.; Triller, D.; Crowther, M.; Ansell, J.; Van Cott, E.M.; Wirth, D.; Kaatz, S. Reversal of direct oral anticoagulants: Guidance from the Anticoagulation Forum. Am. J. Hematol. 2019, 94, 697–709. [Google Scholar] [CrossRef]
- Barber, P.A.; Wu, T.Y.; Ranta, A. Stroke reperfusion therapy following dabigatran reversal with idarucizumab in a national cohort. Neurology 2020, 94, e1968–e1972. [Google Scholar] [CrossRef]
- Pikija, S.; Sztriha, L.K.; Sebastian Mutzenbach, J.; Golaszewski, S.M.; Sellner, J. Idarucizumab in Dabigatran-Treated Patients with Acute Ischemic Stroke Receiving Alteplase: A Systematic Review of the Available Evidence. CNS Drugs 2017, 31, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Kermer, P.; Eschenfelder, C.C.; Diener, H.C.; Grond, M.; Abdalla, Y.; Abraham, A.; Althaus, K.; Becks, G.; Berrouschot, J.; Berthel, J.; et al. Antagonizing dabigatran by idarucizumab in cases of ischemic stroke or intracranial hemorrhage in Germany-Updated series of 120 cases. Int. J. Stroke 2020, 15, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Giannandrea, D.; Caponi, C.; Mengoni, A.; Romoli, M.; Marando, C.; Gallina, A.; Marsili, E.; Sacchini, E.; Mastrocola, S.; Padiglioni, C.; et al. Intravenous thrombolysis in stroke after dabigatran reversal with idarucizumab: Case series and systematic review. J. Neurol. Neurosurg. Psychiatry 2019, 90, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.W.; Tsai, Y.T.; Chou, P.C.; Chen, H.M.; Lu, C.M.; Tsao, C.R.; Chen, C.L.; Sun, M.C.; Shih, Y.S.; Hsieh, C.Y.; et al. Intravenous Thrombolysis in Acute Ischemic Stroke after Idarucizumab Reversal of Dabigatran Effect: Analysis of the Cases from Taiwan. J. Stroke Cerebrovasc. Dis. 2019, 28, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Shahjouei, S.; Zand, R. Response by Shahjouei and Zand to Letter Regarding Article, “Safety of Intravenous Thrombolysis among Patients Taking Direct Oral Anticoagulants: A Systematic Review and Meta-Analysis”. Stroke 2020, 51, e132–e133. [Google Scholar] [CrossRef]
- Frol, S.; Sagris, D.; Pretnar Oblak, J.; Šabovič, M.; Ntaios, G. Intravenous Thrombolysis after Dabigatran Reversal by Idarucizumab: A Systematic Review of the Literature. Front. Neurol. 2021, 12, 666086. [Google Scholar] [CrossRef]
- Shao, S.C.; Chan, Y.Y.; Kao Yang, Y.H.; Lin, S.J.; Hung, M.J.; Chien, R.N.; Lai, C.C.; Lai, E.C. The Chang Gung Research Database—A multi-institutional electronic medical records database for real-world epidemiological studies in Taiwan. Pharmacoepidemiol. Drug Saf. 2019, 28, 593–600. [Google Scholar] [CrossRef]
- Tsai, M.S.; Lin, M.H.; Lee, C.P.; Yang, Y.H.; Chen, W.C.; Chang, G.H.; Tsai, Y.T.; Chen, P.C.; Tsai, Y.H. Chang Gung Research Database: A multi-institutional database consisting of original medical records. Biomed. J. 2017, 40, 263–269. [Google Scholar] [CrossRef]
- Schulman, S.; Angerås, U.; Bergqvist, D.; Eriksson, B.; Lassen, M.R.; Fisher, W. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in surgical patients. J. Thromb. Haemost. 2010, 8, 202–204. [Google Scholar] [CrossRef]
- Khorsand, N.; Majeed, A.; Sarode, R.; Beyer-Westendorf, J.; Schulman, S.; Meijer, K. Assessment of effectiveness of major bleeding management: Proposed definitions for effective hemostasis: Communication from the SSC of the ISTH. J. Thromb. Haemost. 2016, 14, 211–214. [Google Scholar] [CrossRef]
- Tomaselli, G.F.; Mahaffey, K.W.; Cuker, A.; Dobesh, P.P.; Doherty, J.U.; Eikelboom, J.W.; Florido, R.; Gluckman, T.J.; Hucker, W.J.; Mehran, R.; et al. 2020 ACC Expert Consensus Decision Pathway on Management of Bleeding in Patients on Oral Anticoagulants: A Report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2020, 76, 594–622. [Google Scholar] [CrossRef]
- Yasaka, M.; Yokota, H.; Suzuki, M.; Asakura, H.; Yamane, T.; Ogi, Y.; Ochiai, K.; Nakayama, D. Idarucizumab for Emergency Reversal of Anticoagulant Effects of Dabigatran: Interim Results of a Japanese Post-Marketing Surveillance Study. Cardiol. Ther. 2020, 9, 167–188. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, R.; Singh, A.; Chaudhary, R.; Bashline, M.; Houghton, D.E.; Rabinstein, A.; Adamski, J.; Arndt, R.; Ou, N.N.; Rudis, M.I.; et al. Evaluation of Direct Oral Anticoagulant Reversal Agents in Intracranial Hemorrhage: A Systematic Review and Meta-analysis. JAMA Netw. Open 2022, 5, e2240145. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Huang, R.J.; Peterson, E.D.; Laskowitz, D.T.; Hernandez, A.F.; Federspiel, J.J.; Schwamm, L.H.; Bhatt, D.L.; Smith, E.E.; Fonarow, G.C.; et al. Intravenous tPA (Tissue-Type Plasminogen Activator) in Patients with Acute Ischemic Stroke Taking Non–Vitamin K Antagonist Oral Anticoagulants Preceding Stroke. Stroke 2018, 49, 2237–2240. [Google Scholar] [CrossRef]
- Calkins, H.; Hindricks, G.; Cappato, R.; Kim, Y.H.; Saad, E.B.; Aguinaga, L.; Akar, J.G.; Badhwar, V.; Brugada, J.; Camm, J.; et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: Executive summary. Europace 2018, 20, 157–208. [Google Scholar] [CrossRef]
- Calkins, H.; Willems, S.; Gerstenfeld, E.P.; Verma, A.; Schilling, R.; Hohnloser, S.H.; Okumura, K.; Serota, H.; Nordaby, M.; Guiver, K.; et al. Uninterrupted Dabigatran versus Warfarin for Ablation in Atrial Fibrillation. N. Engl. J. Med. 2017, 376, 1627–1636. [Google Scholar] [CrossRef]
- Di Biase, L.; Burkhardt, J.D.; Santangeli, P.; Mohanty, P.; Sanchez, J.E.; Horton, R.; Gallinghouse, G.J.; Themistoclakis, S.; Rossillo, A.; Lakkireddy, D.; et al. Periprocedural stroke and bleeding complications in patients undergoing catheter ablation of atrial fibrillation with different anticoagulation management: Results from the Role of Coumadin in Preventing Thromboembolism in Atrial Fibrillation (AF) Patients Undergoing Catheter Ablation (COMPARE) randomized trial. Circulation 2014, 129, 2638–2644. [Google Scholar]
- Zhao, X.; Chen, L.Z.; Su, X.; Long, D.Y.; Sang, C.H.; Yu, R.H.; Tang, R.B.; Bai, R.; Liu, N.; Jiang, C.X.; et al. A strategy of idarucizumab for pericardial tamponade during perioperative period of atrial fibrillation ablation. Pacing Clin. Electrophysiol. 2021, 44, 1824–1831. [Google Scholar] [CrossRef]
- Steffel, J.; Collins, R.; Antz, M.; Cornu, P.; Desteghe, L.; Haeusler, K.G.; Oldgren, J.; Reinecke, H.; Roldan-Schilling, V.; Rowell, N.; et al. 2021 European Heart Rhythm Association Practical Guide on the Use of Non-Vitamin K Antagonist Oral Anticoagulants in Patients with Atrial Fibrillation. Europace 2021, 23, 1612–1676. [Google Scholar] [CrossRef] [PubMed]
- Okishige, K.; Yamauchi, Y.; Hanaki, Y.; Inoue, K.; Tanaka, N.; Yamaji, H.; Murakami, T.; Manita, M.; Tabata, K.; Ooie, T.; et al. Clinical experience of idarucizumab use in cases of cardiac tamponade under uninterrupted anticoagulation of dabigatran during catheter ablation of atrial fibrillation. J. Thromb. Thrombolysis 2019, 47, 487–494. [Google Scholar] [CrossRef]
- Gragnano, F.; Calabrò, P.; Valgimigli, M. Is triple antithrombotic therapy, or rather its duration and composition, the true culprit for the excess of bleeding events observed in patients with atrial fibrillation undergoing coronary intervention? Eur. Heart J. 2019, 40, 216–217. [Google Scholar] [CrossRef] [PubMed]
- Mourafetis, J.; Doctor, N.; Leung, S. Treatment of gastrointestinal bleeding with idarucizumab in a patient receiving dabigatran. Am. J. Health Pharm. 2018, 75, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Kurdziel, M.; Hudzik, B.; Kazik, A.; Piegza, J.; Szkodziński, J.; Gąsior, M. Idarucizumab for dabigatran reversal in cardiac tamponade complicating percutaneous intervention in ST elevation myocardial infarction. Adv. Interv. Cardiol. 2021, 17, 129–130. [Google Scholar] [CrossRef] [PubMed]
| Variable | All n = 32 | Eligible for Trials n = 21 | Group A n = 16 | Group B n = 5 | Group C n = 11 | RE-VERSE AD, n = 503 |
|---|---|---|---|---|---|---|
| Male, n (%) | 15 (46.9%) | 10 (47.6%) | 8 (50%) | 2 (40%) | 5 (45.5%) | 274 (54.5%) |
| Age, mean (SD, years old) | 76.2 (11.5) | 79.6 (9.6) | 81.2 (9.3) | 74.6 (9.8) | 69.5 (12.5) | 78 |
| Body weight, median (SD, kg) | 63.6 (14.2) | 60.2 (11.9) | 60.6 (11.8) | 58.9 (13.8) | 70.3 (16.6) | 75 |
| Comorbidity, n (%) | ||||||
| Hypertension | 19 (59.3%) | 11 (52.4%) | 10 (62.5%) | 1 (20%) | 8 (72.7%) | 394 (78.3%) |
| Diabetes mellitus | 10 (31.3%) | 8 (38.1%) | 5 (31.3%) | 3 (60%) | 2 (18.2%) | 152 (30.2%) |
| Heart failure | 11 (34.4%) | 8 (38.1%) | 6 (37.5%) | 2 (40%) | 3 (27.3%) | 182 (36.2%) |
| Previous ischemic stroke | 14 (43.8%) | 8 (38.1%) | 5 (31.3%) | 3 (60%) | 6 (54.6%) | 47 (9.3%) |
| Acute coronary syndrome | 3 (9.4%) | 3 (14.3%) | 2 (12.5%) | 1 (20%) | 0 (0%) | 178 (35.4%) |
| Previous systemic embolism | 6 (18.8%) | 5 (23.8%) | 5 (31.3%) | 0 (0%) | 1 (9.1%) | 36 (7.2%) |
| Creatinine clearance, mL/min (%) | ||||||
| ≥80 | 9 (28.1%) | 4 (19.0%) | 3 (18.8%) | 1 (20%) | 5 (45.5%) | 108 (21.5%) |
| 30–80 | 20 (62.5%) | 14 (66.7%) | 10 (62.5%) | 4 (80%) | 6 (54.6%) | 290 (57.6%) |
| <30 | 3 (9.4%) | 3 (14.3%) | 3 (18.8%) | 0 (0%) | 0 (0%) | 91 (18.1%) |
| Dabigatran indications | ||||||
| Atrial fibrillation | 27 (84.4%) | 19 (90.5%) | 14 (87.5%) | 5 (100%) | 8 (72.7%) | 478 (95%) |
| Systemic embolism | 5 (15.6%) | 4 (19.0%) | 4 (25%) | 0 (0%) | 1 (9.1%) | 9 (1.8) |
| CHA2DS2-VASc score, median | 4.9 | 5.2 | 5.3 | 5.0 | 4.2 | N/A |
| HAS-BLED score, median | 2.7 | 2.9 | 3.2 | 1.8 | 2.5 | N/A |
| Initial NIHSS score, median | 13.5 | 15 | N/A | 15 | 13.2 | N/A |
| Daily dose of dabigatran, n (%) | ||||||
| 150 mg twice daily | 6 (18.8%) | 5 (23.8%) | 4 (25%) | 1 (20%) | 1/7 (14.3%) | 151 (30%) |
| 110 mg twice daily | 22 (68.8%) | 16 (76.2%) | 12 (75%) | 4 (80%) | 6/7 (85.7%) | 311 (61.8%) |
| Successful hemostasis, n (%) | 24/26 (92.3%) | 20/21 (95.2%) | 15 (93.8%) | 5 (100%) | 4/5 (80%) | 80.4% |
| Complete reversal of anticoagulant effects, n (%) a | 11/25 (44.0%) | 11/15 (73.3%) | 9/12 (75%) | 2/3 (66.7%) | 0/10 (0%) | N/A |
| Mortality, n (%) b | 5 (15.6%) | 2 (9.5%) | 2 (12.5%) | 0 (0%) | 3 (27.3%) | 13.1% |
| Thromboembolic events, n (%) c | 1 (3.1%) | 1 (4.8%) | 0 (0%) | 1 (20%) | 0 (0%) | 24 (4.8%) |
| Rebleeding rate, n (%) | 1/26 (3.9%) | 1/21 (4.8%) | 1 (6.3%) | 0 (0%) | 0/5 (0%) | 10 (2.0%) |
| Resumption of DOAC, n (%) | 20 (62.5%) | 14 (66.7%) | 10 (62.5%) | 4 (80%) | 6 (54.5%) | N/A |
| Choice of DOAC after resumption, n (%) | N/A | |||||
| Dabigatran | 9 (28.1%) | 5 (23.8%) | 3 (18.8%) | 2 (40%) | 4 (36.4%) | N/A |
| Apixaban | 4 (12.5%) | 3 (14.3%) | 2 (12.5%) | 1 (20%) | 1 (9.1%) | N/A |
| Edoxaban | 2 (6.3%) | 2 (9.5%) | 2 (12.5%) | 0 (0%) | 0 (0%) | N/A |
| Rivaroxaban | 2 (6.3%) | 1 (4.8%) | 1 (6.3%) | 0 (0%) | 1 (9.1%) | N/A |
| Other anticoagulants/antiplatelets | 3 (9.4%) | 3 (14.3%) | 2 (12.5%) | 1 (20%) | 0 (0%) | N/A |
| Adverse side effects d, n (%) | 3 (9.4%) | 2 (9.5%) | 0 (0%) | 2 (40%) | 1 (9.1%) | 117 (23.3%) |
| Laboratory data before idarucizumab | ||||||
| aPTT, median (s) | 43.6 | 42.3 | 52.9 | 33.7 | 35.7 | N/A |
| Prolonged aPTT, n (%) | 17 (53.1%) | 16 (76.2%) | 13 (81.3%) | 3 (60%) | 1 (9.1%) | 372 (74.2%) |
| INR, median, median (s) | 2.1 | 2.3 | 2.6 | 1.2 | 1.8 | N/A |
| Platelet count, median (1000/μL) | 194.9 | 205.0 | 187.3 | 261.6 | 175.7 | N/A |
| Hemoglobin, median (g/dL) | 11.2 | 10.2 | 9.2 | 13.4 | 13.2 | N/A |
| Laboratory data after idarucizumab | ||||||
| aPTT, median (s) | 34.5 | 33.1 | 35 | 25.6 | 38.6 | N/A |
| INR, median, median (s) | 1.3 | 1.3 | 1.3 | 1.1 | 1.3 | N/A |
| Patients, n (%) | Hemostasis, n (%) | Post-Infusion Bleeding, n (%) | Thromboembolic or Adverse Events a, n (%) | Mortality b, n (%) | |
|---|---|---|---|---|---|
| Total patients | 32 (100%) | 24/26 (92.3%) | 1/26 (3.9%) | 1 (3.1%) | 5 (15.6%) |
| Ineligible patients | 11 (34.3%) | 4/5 (80%) | 0/5 (0%) | 0 (0%) | 3/11 (27.3%) |
| Before TPA c | 5 (15.6%) | N/A | N/A | 0/5 (0%) | 0/21 (0%) |
| Did not take dabigatran | 4 (12.5%) | 3/4 (75%) | 1/4 (25%) | 0/4 (0%) | 2/4 (50%) |
| Minor bleeding | 1 (3.1%) | 1/1 (100%) | 0/1 (%) | 0/1 (0%) | 0/1 (0%) |
| Sepsis without active bleeding | 1 (3.1%) | N/A | N/A | 0/1 (0%) | 1/1 (100%) |
| Eligible patients | 21 (65.6%) | 20/21 (95.2%) | 1/21 (4.8%) | 1/21 (4.8%) | 2/21 (9.5%) |
| Patient Number | Gender (Age) | CCR, mL/min | DOAC Dosage | HASBLEED Score | CHA2DS2-VASc Score | Eligibility for REVERSE AD | Hemostasis | Thromboembolic or Adverse Effects | Mortality |
|---|---|---|---|---|---|---|---|---|---|
| Group A: uncontrollable or life-threatening bleeding | |||||||||
| 1 | Male (89) | 21.2 | Dabigatran, 150 mg, BID | 4 | 4 | Yes (GI bleeding) | Yes | No | Yes |
| 2 | Male (65) | 40.1 | Dabigatran, 150 mg, BID | 5 | 4 | Yes (ICH) | No | No | No |
| 3 | Female (92) | 64.1 | Dabigatran, 150 mg, BID | 2 | 8 | Yes (GIB) | Yes | No | No |
| 4 | Female (89) | 31.3 | Dabigatran, 150 mg, BID | 3 | 5 | Yes (GIB) | Yes | No | No |
| 5 | Male (68) | 56.7 | Dabigatran, 110 mg, BID | 3 | 6 | Yes (Compartment syndrome) | Yes | No | No |
| 6 | Female (84) | 50.4 | Dabigatran, 150 mg, BID | 4 | 7 | Yes (Compartment syndrome) | Yes | No | No |
| 7 | Female (85) | 72.5 | Dabigatran, 150 mg, BID | 4 | 5 | Yes (ICH) | Yes | No | No |
| 8 | Male, (87) | 58.5 | Dabigatran, 150 mg, BID | 2 | 7 | Yes (ICH) | Yes | No | No |
| 9 | Female (91) | 34 | Dabigatran, 150 mg, BID | 4 | 6 | Yes (Compartment syndrome) | Yes | No | No |
| 10 | Male (75) | 82.4 | Dabigatran, 150 mg, BID | 2 | 3 | Yes (ICH) | Yes | No | No |
| 11 | Male (76) | 83.1 | Dabigatran, 150 mg, BID | 3 | 5 | Yes (GIB) | Yes | No | No |
| 12 | Female (77) | 14.25 | Dabigatran, 110 mg, BID | 6 | 6 | Yes (Hemoptysis) | Yes | No | No |
| 13 | Female (88) | 46.8 | Dabigatran, 150 mg, BID | 3 | 7 | Yes (GIB) | Yes | No | No |
| 14 | Female (64) | 6.2 | Dabigatran, 110 mg, BID | 2 | 2 | Yes (GIB) | Yes | No | No |
| 15 | Male (82) | 39.1 | Dabigatran, 110 mg, BID | 3 | 5 | Yes (GIB) | N/A | No | Yes |
| 16 | Male (87) | 91.4 | Dabigatran, 150 mg, BID | 1 | 5 | Yes (GIB) | N/A | No | No |
| Group B: Emergency surgery or invasive procedures | |||||||||
| 17 | Female (92) | 70.4 | Dabigatran, 150 mg, BID | 1 | 3 | Yes (PPU) | Yes | Yes | No |
| 18 | Male, (70) | 56.7 | Dabigatran, 150 mg, BID | 2 | 6 | Yes (ICH) | Yes | No | No |
| 19 | Male (71) | 56.9 | Dabigatran, 150 mg, BID | 3 | 4 | Yes (ICH) | Yes | Yes | No |
| 20 | Female (71) | 105.8 | Dabigatran, 110 mg, BID | 1 | 6 | Yes (ICH) | Yes | No | No |
| 21 | Female (69) | 79 | Dabigatran, 150 mg, BID | 2 | 6 | Yes (ICH) | N/A | Yes | No |
| 17 | Female (92) | 70.4 | Dabigatran, 150 mg, BID | 1 | 3 | Yes (PPU) | Yes | Yes | No |
| Group C: Ineligible for clinical trials (RE-VERSE AD) | |||||||||
| 22 | Male (62) | 51.5 | Dabigatran, 150 mg, BID | 2 | 6 | NO (Pre-TPA/IA) | Yes | No | No |
| 23 | Female (63) | 59.6 | Dabigatran, 150 mg, BID | 3 | 7 | NO (Pre-TPA) | Yes | No | No |
| 24 | Female (70) | 87.3 | Dabigatran, 150 mg, BID | 3 | 3 | NO (Pre-TPA) | Yes | No | No |
| 25 | Male (53) | 70 | Dabigatran, 150 mg, BID | 2 | 1 | NO (Pre-TPA) | Yes | No | No |
| 26 | Male (91) | 105.9 | Dabigatran, 150 mg, BID | 3 | 4 | NO (Pre-TPA) | Yes | Yes | No |
| 27 | Male (79) | 47.9 | Rivaroxaban, 10 mg QD | 2 | 2 | NO (did not take dabigatran) | 1 | No | NO |
| 28 | Female (55) | 89.1 | Rivaroxaban, 1/4 10 mg BID | 4 | 6 | NO (did not take dabigatran) | 1 | No | Yes |
| 29 | Female (77) | 90.22 | Apixaban, 5 mg QD | 2 | 5 | NO (did not take dabigatran) | 0 | No | Yes |
| 30 | Male (64) | 34.4 | Rivaroxaban, 15 mg QD | 0 | 1 | NO (did not take dabigatran) | 1 | No | No |
| 31 | Female (87) | 71.9 | Dabigatran, 110 mg, BID | 4 | 6 | NO (Sepsis without active bleeding) | N/A | No | Yes |
| 32 | Female (64) | 121 | Dabigatran, 150 mg, BID | 2 | 5 | NO (minor bleeding) | 1 | No | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, J.-W.; Wang, C.-H.; Chu, C.-L.; Liao, S.-C. Effectiveness and Safety of Dabigatran Reversal with Idarucizumab in the Taiwanese Population: A Comparison Based on Eligibility for Inclusion in Clinical Trials. Medicina 2023, 59, 881. https://doi.org/10.3390/medicina59050881
Dai J-W, Wang C-H, Chu C-L, Liao S-C. Effectiveness and Safety of Dabigatran Reversal with Idarucizumab in the Taiwanese Population: A Comparison Based on Eligibility for Inclusion in Clinical Trials. Medicina. 2023; 59(5):881. https://doi.org/10.3390/medicina59050881
Chicago/Turabian StyleDai, Jhih-Wei, Chien-Ho Wang, Chan-Lin Chu, and Shu-Chen Liao. 2023. "Effectiveness and Safety of Dabigatran Reversal with Idarucizumab in the Taiwanese Population: A Comparison Based on Eligibility for Inclusion in Clinical Trials" Medicina 59, no. 5: 881. https://doi.org/10.3390/medicina59050881
APA StyleDai, J.-W., Wang, C.-H., Chu, C.-L., & Liao, S.-C. (2023). Effectiveness and Safety of Dabigatran Reversal with Idarucizumab in the Taiwanese Population: A Comparison Based on Eligibility for Inclusion in Clinical Trials. Medicina, 59(5), 881. https://doi.org/10.3390/medicina59050881






