Neuropsychiatric Symptoms as Indicators of Fall Risk in Geriatric Inpatients
Abstract
1. Introduction
2. Materials and Methods
2.1. Design of Study, Participants, and Measurements
2.2. The Neuropsychiatric Inventory–Questionnaire (NPIQ)
2.3. Statistical Analysis
2.4. Ethics
3. Results
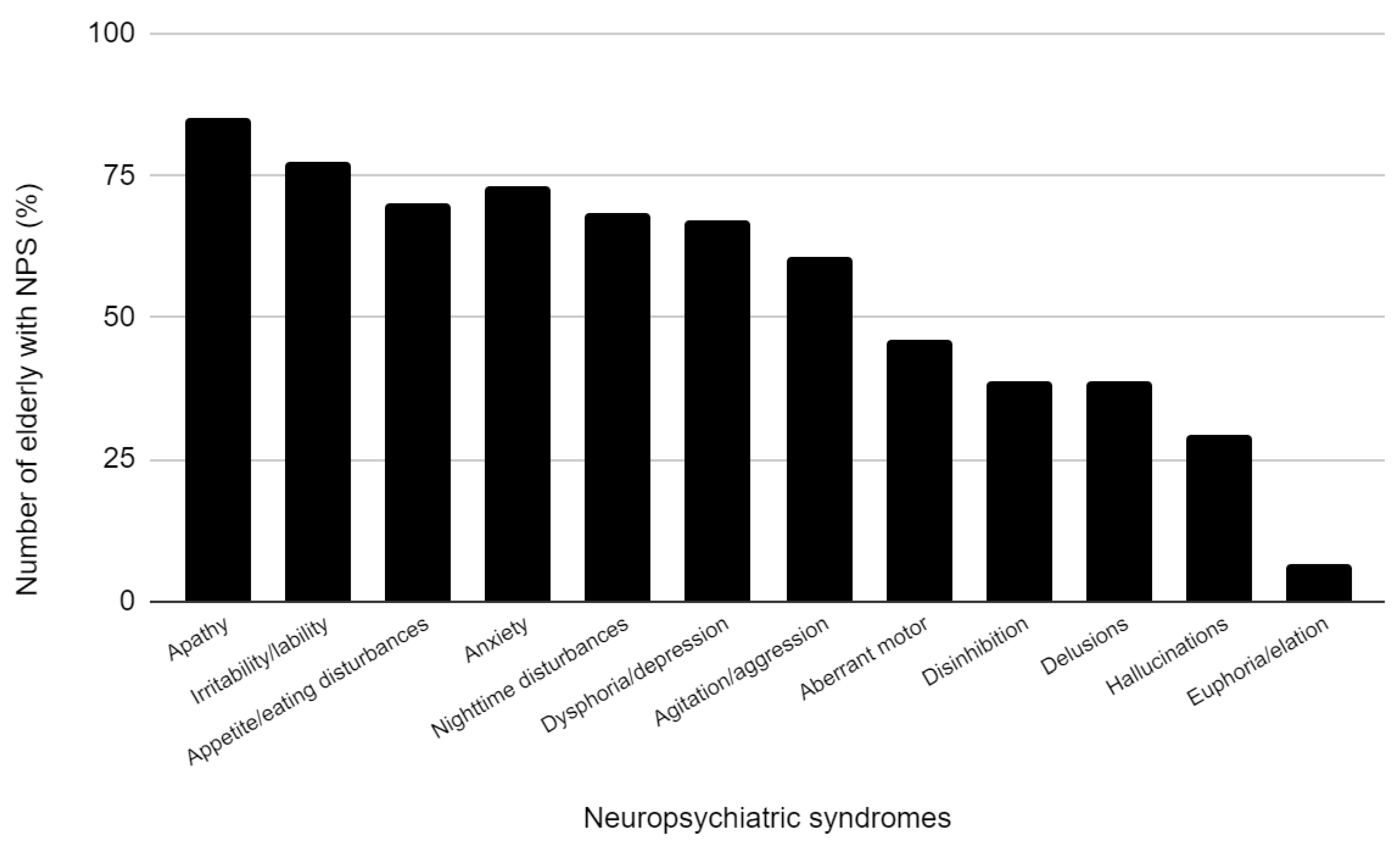
3.1. Population Characteristics and Prevalence of Neuropsychiatric Symptoms
3.2. Dementia and Risk of Falls, Functional Status
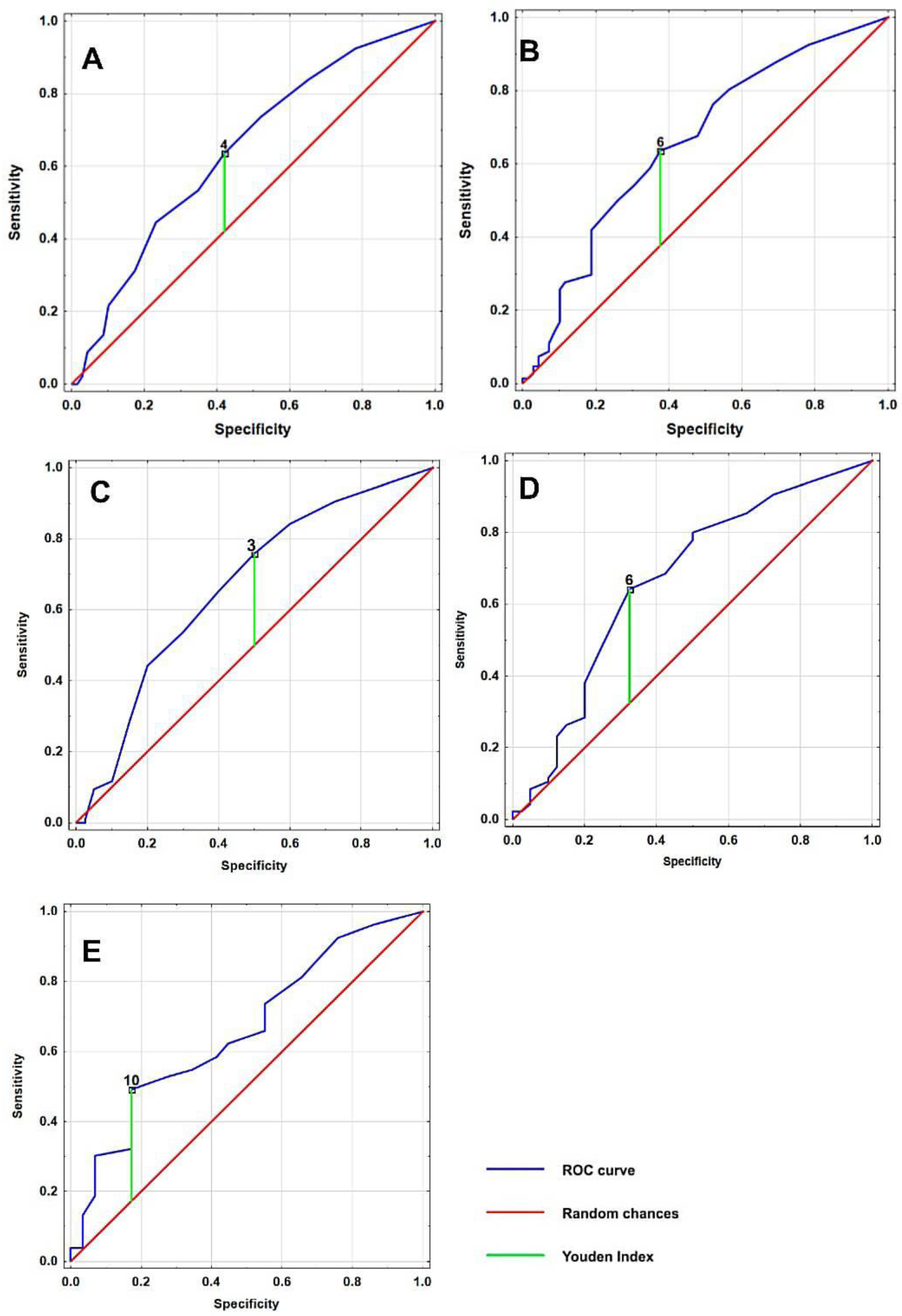
3.3. Receiver Operating Characteristic (ROC) Curve Analysis
3.4. Association between NPS and High Fall Risk
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aalten, P.; Verhey, F.R.; Boziki, M.; Bullock, R.; Byrne, E.J.; Camus, V.; Caputo, M.; Collins, D.; De Deyn, P.P.; Elina, K.; et al. Neuropsychiatric syndromes in dementia. Results from the European Alzheimer Disease Consortium: Part I. Dement. Geriatr. Cogn. Disord. 2007, 24, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Martínez, M.; Molano, A.; Castro, J.; Zarranz, J.J. Prevalence of neuropsychiatric symptoms in mild cognitive impairment and Alzheimer’s disease, and its relationship with cognitive impairment. Curr. Alzheimer Res. 2010, 7, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Liew, T.M. Neuropsychiatric symptoms in cognitively normal older persons, and the association with Alzheimer’s and non-Alzheimer’s dementia. Alzheimers Res. Ther. 2020, 12, 35. [Google Scholar] [CrossRef] [PubMed]
- Majer, R.; Adeyi, O.; Bagoly, Z.; Simon, V.; Csiba, L.; Kardos, L.; Hortobágyi, T.; Frecska, E. Neuropsychiatric symptoms, quality of life and caregivers’ burden in dementia. Open Med. 2020, 15, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Liampas, I.; Siokas, V.; Lyketsos, C.G.; Dardiotis, E. The Relationship between Neuropsychiatric Symptoms and Cognitive Performance in Older Adults with Normal Cognition. Medicina 2022, 58, 1586. [Google Scholar] [CrossRef]
- Moncada, L.V.V.; Mire, L.G. Preventing Falls in Older Persons. Am. Fam. Physician 2017, 96, 240–247. [Google Scholar]
- Rubenstein, L.Z.; Josephson, K.R. The epidemiology of falls and syncope. Clin. Geriatr. Med. 2002, 18, 141–158. [Google Scholar] [CrossRef]
- Roitto, H.M.; Öhman, H.; Salminen, K.; Kautiainen, H.; Laurila, J.; Pitkälä, K.H. Neuropsychiatric Symptoms as Predictors of Falls in Long-Term Care Residents with Cognitive Impairment. J. Am. Med. Dir. Assoc. 2020, 21, 1243–1248. [Google Scholar] [CrossRef]
- Roitto, H.M.; Kautiainen, H.; Öhman, H.; Savikko, N.; Strandberg, T.E.; Raivio, M.; Laakkonen, M.L.; Pitkälä, K.H. Relationship of Neuropsychiatric Symptoms with Falls in Alzheimer’s Disease—Does Exercise Modify the Risk? J. Am. Geriatr. Soc. 2018, 66, 2377–2381. [Google Scholar] [CrossRef]
- Saari, T.; Hallikainen, I.; Hintsa, T.; Koivisto, A.M. Neuropsychiatric symptoms and activities of daily living in Alzheimer’s disease: ALSOVA 5-year follow-up study. Int. Psychogeriatr. 2020, 32, 741–751. [Google Scholar] [CrossRef]
- Mazur, K.; Wilczyński, K.; Szewieczek, J. Geriatric falls in the context of a hospital fall prevention program: Delirium, low body mass index, and other risk factors. Clin. Interv. Aging 2016, 11, 1253–1261. [Google Scholar]
- White, N.; Leurent, B.; Lord, K.; Scott, S.; Jones, L.; Sampson, E.L. The management of behavioural and psychological symptoms of dementia in the acute general medical hospital: A longitudinal cohort study. Int. J. Geriatr. Psychiatry 2017, 32, 297–305. [Google Scholar] [CrossRef]
- Buhr, G.T.; White, H.K. Difficult Behaviors in Long-term Care Patients with Dementia. J. Am. Med. Dir. Assoc. 2007, 8, e101–e113. [Google Scholar] [CrossRef]
- Kaufer, D.I.; Cummings, J.L.; Ketchel, P.; Smith, V.; MacMillan, A.; Shelley, T.; Lopez, O.L.; DeKosky, S.T. Validation of the NPIQ, a Brief Clinical Form of the Neuropsychiatric Inventory. J. Neuropsychiatry Clin. Neurosci. 2000, 12, 233–239. [Google Scholar] [CrossRef]
- Tapiainen, V.; Lavikainen, P.; Koponen, M.; Taipale, H.; Tanskanen, A.; Tiihonen, J.; Hartikainen, S.; Tolppanen, A.M. The Risk of Head Injuries Associated with Antipsychotic Use Among Persons with Alzheimer’s disease. J. Am. Geriatr. Soc. 2020, 68, 595–602. [Google Scholar] [CrossRef]
- Isaacson, S.H.; Citrome, L. Hallucinations and delusions associated with Parkinson’s disease psychosis: Safety of current treatments and future directions. Expert Opin. Drug Saf. 2022, 21, 873–879. [Google Scholar] [CrossRef]
- Byun, M.; Kim, J.; Kim, M. Physical and Psychological Factors Affecting Falls in Older Patients with Arthritis. Int. J. Environ. Res. Public Health 2020, 17, 1098. [Google Scholar] [CrossRef]
- Hallford, D.J.; Nicholson, G.; Sanders, K.; McCabe, M.P. The Association Between Anxiety and Falls: A Meta-Analysis. J. Gerontol. B Psychol. Sci. 2017, 72, 729–741. [Google Scholar] [CrossRef]
- Holloway, K.L.; Williams, L.J.; Brennan-Olsen, S.L.; Morse, A.G.; Kotowicz, M.A.; Nicholson, G.C.; Pasco, J.A. Anxiety disorders and falls among older adults. J. Affect. Disord. 2016, 205, 20–27. [Google Scholar]
- Wilczyński, K.; Gorczyca, M.; Gołębiowska, J.; Szewieczek, J. Anticholinergic Burden of Geriatric Ward Inpatients. Medicina 2021, 57, 1115. [Google Scholar] [CrossRef]
- Sylliaas, H.; Selbaek, G.; Bergland, A. Do behavioral disturbances predict falls among nursing home residents? Aging Clin. Exp. Res. 2012, 24, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Balzer, K.; Bremer, M.; Schramm, S.; Lühmann, D.; Raspe, H. Falls prevention for the elderly. GMS Health Technol. Assess. 2012, 8. [Google Scholar] [CrossRef]
- Gamble, L.D.; Matthews, F.E.; Jones, I.R.; Hillman, A.E.; Woods, B.; Macleod, C.A.; Martyr, A.; Collins, R.; Pentecost, C.; Rusted, J.M.; et al. Characteristics of people living with undiagnosed dementia: Findings from the CFAS Wales study. BMC Geriatr. 2022, 22, 409. [Google Scholar] [CrossRef]
- Hodgson, N.A.; Gitlin, L.N.; Winter, L.; Czekanski, K. Undiagnosed illness and neuropsychiatric behaviors in community residing older adults with dementia. Alzheimer Dis. Assoc. Disord. 2011, 25, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Eikelboom, W.S.; Singleton, E.; van den Berg, E.; Coesmans, M.; Mattace Raso, F.; van Bruchem, R.L.; Goudzwaard, J.A.; de Jong, F.J.; Koopmanschap, M.; den Heijer, T.; et al. Early recognition and treatment of neuropsychiatric symptoms to improve quality of life in early Alzheimer’s disease: Protocol of the BEAT-IT study. Alzheimers Res. Ther. 2019, 11, 48. [Google Scholar] [CrossRef]
- Liang, Y.J.; Su, Q.W.; Sheng, Z.R.; Weng, Q.Y.; Niu, Y.F.; Zhou, H.D.; Liu, C.B. Effectiveness of Physical Activity Interventions on Cognition, Neuropsychiatric Symptoms, and Quality of Life of Alzheimer’s Disease: An Update of a Systematic Review and Meta-Analysis. Front. Aging Neurosci. 2022, 14, 155. [Google Scholar] [CrossRef]
- Tao, Y.; Peters, M.E.; Drye, L.T.; Devanand, D.P.; Mintzer, J.E.; Pollock, B.G.; Porsteinsson, A.P.; Rosenberg, P.B.; Schneider, L.S.; Shade, D.M.; et al. Sex Differences in the Neuropsychiatric Symptoms of Patients with Alzheimer’s Disease. Am. J. Alzheimer’s Dis. Dement. 2018, 33, 450–457. [Google Scholar] [CrossRef]
- Eikelboom, W.S.; Pan, M.; Ossenkoppele, R.; Coesmans, M.; Gatchel, J.R.; Ismail, Z.; Lanctôt, K.L.; Fischer, C.E.; Mortby, M.E.; van den Berg, E.; et al. Sex differences in neuropsychiatric symptoms in Alzheimer’s disease dementia: A meta-analysis. Alzheimer’s Res. Ther. 2022, 14, 48. [Google Scholar] [CrossRef]
- Thompson, A.E.; Anisimowicz, Y.; Miedema, B.; Hogg, W.; Wodchis, W.P.; Aubrey-Bassler, K. The influence of gender and other patient characteristics on health care-seeking behaviour: A QUALICOPC study. BMC Fam. Pract. 2016, 17, 38. [Google Scholar] [CrossRef]
| Variable | Total Cohort | High Fall Risk | No High Fall Risk | p-Value | Women | Men | p-Value |
|---|---|---|---|---|---|---|---|
| n = 234 | |||||||
| Age (years) | 80.70 ± 6.60 | 81.99 ± 6.21 | 77.69 ± 6.03 | <0.001 | 81.20 ± 6.21 | 79.95 ± 7.09 | 0.164 |
| Sex (percentage of females) | 63 | 64 | 58 | 0.379 | - | - | - |
| Dementia (%) | 62.40 | 72.97 | 37.68 | <0.001 | 64.67 | 58.14 | 0.319 |
| History of stroke (%) | 11.50 | 17.93 | 4.35 | 0.007 | 10.96 | 15.12 | 0.355 |
| Diabetes (%) | 34.60 | 34.69 | 33.33 | 0.844 | 31.76 | 40.70 | 0.167 |
| Systolic blood pressure (mmHg) | 141. ± 24 | 138.2 ± 25 | 147 ± 22 | 0.014 | 145.01 ± 25 | 136 ± 22 | 0.005 |
| Diastolic blood pressure (mmHg) | 79.30 ± 12.8 | 77.92 ± 12.88 | 82.16 ± 12.81 | 0.023 | 81.21 ± 12.99 | 76.67 ± 11.69 | 0.005 |
| Body mass index | 26.70 ± 5.91 | 26.51 ± 5.25 | 27.20 ± 7.12 | 0.902 | 26.46 ± 5.29 | 27.21 ± 6.9 | 0.582 |
| Barthel Index (score) | 71.80 ± 27.30 | 61.59 ± 27.19 | 93.33 ± 7.16 | <0.001 | 71.07 ± 25.90 | 71.45 ± 29.84 | 0.317 |
| IADL Index (score) | 17.55 ± 6.24 | 15.15 ± 5.26 | 22.90 ± 4.71 | <0.001 | 17.58 ± 6.00 | 17.45 ± 6.63 | 0.913 |
| Mini-Mental State Examination (score) | 19.80 ± 8.31 | 17.97 ± 8.45 | 24.32 ± 5.3 | <0.001 | 19.89 ± 8.13 | 19.74 ± 8.62 | 0.968 |
| Total number of neuropsychiatric symptoms | 4.41 ± 3.09 | 4.89 ± 3.02 | 3.41 ± 3.09 | <0.001 | 4.41 ± 3.11 | 4.43 ± 3.13 | 0.947 |
| Apathy/indifference, prevalence (%) | 58.10 | 63.51 | 49.28 | 0.047 | 59.33 | 59.30 | 0.1 |
| Apathy/indifference, intensity (score) | 1.17 ± 1.17 | 1.31 ± 1.17 | 0.86 ± 1.07 | 0.008 | 1.20 ± 1.18 | 1.14 ± 1.14 | 0.722 |
| Irritability/lability, prevalence (%) | 53.80 | 57.43 | 44.93 | 0.085 | 52.00 | 55.81 | 0.572 |
| Irritability/lability, intensity (score) | 1.00 ± 1.07 | 1.07 ± 1.08 | 0.83 ± 1.04 | 0.10 | 0.97 ± 1.08 | 1.00 ± 1.08 | 0.618 |
| Appetite/eating disturbances, prevalence (%) | 53.80 | 55.40 | 47.83 | 0.298 | 54.00 | 51.16 | 0.67 |
| Appetite/eating disturbances, intensity (score) | 1.07 ± 1.14 | 1.14 ± 1.18 | 0.86 ± 1.03 | 0.105 | 1.09 ± 1.15 | 0.99 ± 1.13 | 0.512 |
| Anxiety prevalence (%) | 50.00 | 55.05 | 37.68 | 0.025 | 52.67 | 43.02 | 0.154 |
| Anxiety intensity (score) | 0.91 ± 1.05 | 1.00 ± 1.07 | 0.67 ± 0.98 | 0.002 | 0.98 ± 1.08 | 0.76 ± 0.96 | 0.133 |
| Nighttime disturbances, prevalence (%) | 47.90 | 54.05 | 37.68 | 0.025 | 45.33 | 52.33 | 0.301 |
| Nighttime disturbances, intensity (score) | 0.86 ± 1.04 | 0.99 ± 1.06 | 0.62 ± 0.91 | 0.013 | 0.81 ± 1.01 | 0.97 ± 1.07 | 0.257 |
| Dysphoria/depression, prevalence (%) | 44.40 | 44.59 | 40.58 | 0.58 | 45.33 | 41.86 | 0.605 |
| Dysphoria/depression, intensity (score) | 0.75 ± 0.99 | 0.78 ± 1.01 | 0.62 ± 0.91 | 0.345 | 0.83 ± 1.10 | 0.62 ± 0.84 | 0.255 |
| Agitation/aggression, prevalence (%) | 37.60 | 42.57 | 23.19 | 0.006 | 39.33 | 32.56 | 0.3 |
| Agitation/aggression, intensity (score) | 0.66 ± 0.96 | 0.78 ± 1.03 | 0.41 ± 0.81 | 0.006 | 0.68 ± 0.98 | 0.63 ± 0.98 | 0.491 |
| Aberrant motor, prevalence (%) | 29.10 | 35.13 | 18.84 | 0.015 | 30 | 30.40 | 0.822 |
| Aberrant motor, intensity (score) | 0.57 ± 0.99 | 0.68 ± 1.02 | 0.38 ± 0.84 | 0.02 | 0.59 ± 1 | 0.60 ± 1 | 0.851 |
| Disinhibition, prevalence (%) | 23.10 | 26.35 | 18.84 | 0.227 | 22.67 | 24.41 | 0.759 |
| Disinhibition, intensity (score) | 0.42 ± 0.86 | 0.51 ± 0.96 | 0.29 ± 0.67 | 0.16 | 0.42 ± 0.86 | 0.43 ± 0.86 | 0.814 |
| Delusions, prevalence (%) | 22.20 | 29.00 | 10.14 | 0.02 | 21.33 | 24.42 | 0.5 |
| Delusions, intensity (score) | 0.41 ± 0.83 | 0.55 ± 0.95 | 0.18 ± 0.6 | 0.002 | 0.40 ± 0.84 | 0.44 ± 0.86 | 0.62 |
| Hallucinations, prevalence (%) | 16.2 | 23.65 | 4.35 | <0.001 | 14.67 | 22.09 | 0.147 |
| Hallucinations, intensity (score) | 0.29 ± 0.72 | 0.41 ± 0.8 | 0.09 ± 0.45 | 0.002 | 0.27 ± 0.71 | 0.35 ± 0.86 | 0.194 |
| Euphoria/elation, prevalence (%) | 4.70 | 2.70 | 7.24 | 0.231 | 4.67 | 4.65 | 0.1 |
| Euphoria/elation, intensity (score) | 0.09 ± 0.38 | 0.06 ± 0.31 | 0.11 ± 0.47 | 0.841 | 0.09 ± 0.40 | 0.08 ± 0.35 | 0.89 |
| Number of medications | 8.35 ± 4.05 | 8.36 ± 3.75 | 8.39 ± 4.77 | 0.787 | 8.45 ± 4.31 | 8.28 ± 3.57 | 0.868 |
| Number of hospital days | 5.96 ± 2.97 | 6.22 ± 3.13 | 5.53 ± 2.78 | 0.067 | 5.87 ± 2.90 | 6.12 ± 3.09 | 0.609 |
| Charlson comorbidity index | 10.80 ± 2.62 | 7.63 ± 2.03 | 6.39 ± 2.41 | <0.001 | 7.03 ± 2.12 | 7.62 ± 2.41 | 0.04 |
| Outcomes | Cut-Off Value | Sensitivity | Specificity | Youden Index | AUC | p Value |
|---|---|---|---|---|---|---|
| Complete cohort | ||||||
| Total number of neuropsychiatric symptoms | 4 | 0.64 | 0.42 | 0.21 | 0.65 | <0.001 |
| Intensity of total number of neuropsychiatric symptoms | 6 | 0.64 | 0.38 | 0.26 | 0.66 | <0.001 |
| Women | ||||||
| Total number of neuropsychiatric symptoms | 3 | 0.79 | 0.5 | 0.26 | 0.67 | 0.002 |
| Intensity of total number of neuropsychiatric symptoms | 6 | 0.67 | 0.33 | 0.32 | 0.67 | 0.002 |
| Men | ||||||
| Total number of neuropsychiatric symptoms | 6 | 0.51 | 0.26 | 0.18 | 0.65 | 0.062 |
| Intensity of total number of neuropsychiatric symptoms | 10 | 0.56 | 0.66 | 0.011 |
| Outcomes | OR | 95%CI | p Value |
|---|---|---|---|
| Complete Cohort | |||
| Total number of neuropsychiatric symptoms ≥ 4 | 2.50 | 1.35–4.65 | 0.004 |
| Total neuropsychiatric symptom intensity ≥ 6 | 2.77 | 1.49–5.16 | 0.001 |
| Women | |||
| Total number of neuropsychiatric symptoms ≥ 3 | 3.90 | 1.69–8.98 | 0.001 |
| Total neuropsychiatric symptom intensity ≥ 6 | 4.11 | 1.73–9.63 | 0.001 |
| Men | |||
| Total number of neuropsychiatric symptoms | (cutoffs not significant) | ||
| Total neuropsychiatric symptom intensity ≥ 10 | 3.86 | 1.25–11.96 | 0.019 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilczyński, K.; Gorczyca, M.; Grabarczyk, M.; Szewieczek, J. Neuropsychiatric Symptoms as Indicators of Fall Risk in Geriatric Inpatients. Medicina 2023, 59, 887. https://doi.org/10.3390/medicina59050887
Wilczyński K, Gorczyca M, Grabarczyk M, Szewieczek J. Neuropsychiatric Symptoms as Indicators of Fall Risk in Geriatric Inpatients. Medicina. 2023; 59(5):887. https://doi.org/10.3390/medicina59050887
Chicago/Turabian StyleWilczyński, Krzysztof, Marta Gorczyca, Małgorzata Grabarczyk, and Jan Szewieczek. 2023. "Neuropsychiatric Symptoms as Indicators of Fall Risk in Geriatric Inpatients" Medicina 59, no. 5: 887. https://doi.org/10.3390/medicina59050887
APA StyleWilczyński, K., Gorczyca, M., Grabarczyk, M., & Szewieczek, J. (2023). Neuropsychiatric Symptoms as Indicators of Fall Risk in Geriatric Inpatients. Medicina, 59(5), 887. https://doi.org/10.3390/medicina59050887






