Cut-Off Value of Voluntary Peak Cough Flow in Patients with Parkinson’s Disease and Its Association with Severe Dysphagia: A Retrospective Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Methods
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pal, P.K.; Sathyaprabha, T.N.; Tuhina, P.; Thennarasu, K. Pattern of subclinical pulmonary dysfunctions in Parkinson’s disease and the effect of levodopa. Mov. Disord. 2007, 22, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Hegland, K.W.; Okun, M.S.; Troche, M.S. Sequential voluntary cough and aspiration or aspiration risk in Parkinson’s disease. Lung 2014, 192, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Ebihara, S.; Saito, H.; Kanda, A.; Nakajoh, M.; Takahashi, H.; Arai, H.; Sasaki, H. Impaired efficacy of cough in patients with Parkinson disease. Chest 2003, 124, 1009–1015. [Google Scholar] [CrossRef] [PubMed]
- Bolser, D.C.; Gestreau, C.; Morris, K.F.; Davenport, P.W.; Pitts, T.E. Central neural circuits for coordination of swallowing, breathing, and coughing: Predictions from computational modeling and simulation. Otolaryngol. Clin. N. Am. 2013, 46, 957–964. [Google Scholar] [CrossRef]
- Troche, M.S.; Brandimore, A.E.; Godoy, J.; Hegland, K.W. A framework for understanding shared substrates of airway protection. J. Appl. Oral Sci. 2014, 22, 251–260. [Google Scholar] [CrossRef]
- Costa, M.M.; Lemme, E.M. Coordination of respiration and swallowing: Functional pattern and relevance of vocal folds closure. Arq. Gastroenterol. 2010, 47, 42–48. [Google Scholar] [CrossRef]
- Eccles, R. Central mechanisms IV: Conscious control of cough and the placebo effect. In Pharmacology and Therapeutics of Cough. Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2009; pp. 241–262. [Google Scholar] [CrossRef]
- Hegland, K.W.; Bolser, D.C.; Davenport, P.W. Volitional control of reflex cough. J. Appl. Physiol. 2012, 113, 39–46. [Google Scholar] [CrossRef]
- Zhang, C.X.; Deng, J.; Li, Y.; Niu, G.Y.; Li, M.N.; Zhang, B.; Wang, J.J.; Liu, Y.L.; Fang, B.Y.; Xi, J.N.; et al. Abnormal Pulmonary Function in Early Parkinson’s Disease: A Preliminary Prospective Observational Study. Lung 2022, 200, 325–329. [Google Scholar] [CrossRef]
- McCool, F.D. Global physiology and pathophysiology of cough: ACCP evidence-based clinical practice guidelines. Chest 2006, 129, 48S–53S. [Google Scholar] [CrossRef]
- Fontana, G.A.; Lavorini, F. Cough motor mechanisms. Respir. Physiol. Neurobiol. 2006, 152, 266–281. [Google Scholar] [CrossRef]
- Curtis, J.A.; Troche, M.S. Handheld cough testing: A novel tool for cough assessment and dysphagia screening. Dysphagia 2020, 35, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Pitts, T.; Bolser, D.; Rosenbek, J.; Troche, M.; Okun, M.S.; Sapienza, C. Impact of expiratory muscle strength training on voluntary cough and swallow function in Parkinson disease. Chest 2009, 135, 1301–1308. [Google Scholar] [CrossRef]
- Silverman, E.P.; Carnaby, G.; Singletary, F.; Hoffman-Ruddy, B.; Yeager, J.; Sapienza, C. Measurement of voluntary cough production and airway protection in Parkinson disease. Arch. Phys. Med. Rehabil. 2016, 97, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Troche, M.S.; Schumann, B.; Brandimore, A.E.; Okun, M.S.; Hegland, K.W. Reflex cough and disease duration as predictors of swallowing dysfunction in Parkinson’s disease. Dysphagia 2016, 31, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.W.; Dunn, J.R.; Gray, W.K. Self-reported dysphagia and its correlates within a prevalent population of people with Parkinson’s disease. Dysphagia 2011, 26, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Ali, G.; Wallace, K.; Schwartz, R.; DeCarle, D.; Zagami, A.; Cook, I. Mechanisms of oral-pharyngeal dysphagia in patients with Parkinson’s disease. Gastroenterology 1996, 110, 383–392. [Google Scholar] [CrossRef]
- Nagaya, M.; Kachi, T.; Yamada, T. Effect of swallowing training on swallowing disorders in Parkinson’s disease. Scand. J. Rehabil. Med. 2000, 32, 11–15. [Google Scholar] [CrossRef]
- Jankovic, J. Parkinson’s disease: Clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatry 2008, 79, 368–376. [Google Scholar] [CrossRef]
- Leopold, N.A.; Kagel, M.C. Pharyngo-esophageal dysphagia in Parkinson’s disease. Dysphagia 1997, 12, 11–18. [Google Scholar] [CrossRef]
- Wang, C.M.; Shieh, W.Y.; Weng, Y.H.; Hsu, Y.H.; Wu, Y.R. Non-invasive assessment determine the swallowing and respiration dysfunction in early Parkinson’s disease. Park. Relat. Disord. 2017, 42, 22–27. [Google Scholar] [CrossRef]
- Pitts, T. Airway protective mechanisms. Lung 2014, 192, 27–31. [Google Scholar] [CrossRef]
- Brennan, M.; McDonnell, M.J.; Duignan, N.; Gargoum, F.; Rutherford, R.M. The use of cough peak flow in the assessment of respiratory function in clinical practice—A narrative literature review. Respir. Med. 2022, 193, 106740. [Google Scholar] [CrossRef] [PubMed]
- Pitts, T.; Troche, M.; Mann, G.; Rosenbek, J.; Okun, M.S.; Sapienza, C. Using voluntary cough to detect penetration and aspiration during oropharyngeal swallowing in patients with Parkinson disease. Chest 2010, 138, 1426–1431. [Google Scholar] [CrossRef]
- Logemann, J.A. The evaluation and treatment of swallowing disorders. Curr. Opin. Otolaryngol. Head Neck Surg. 1998, 6, 395–400. [Google Scholar] [CrossRef]
- Rosenbek, J.C.; Robbins, J.A.; Roecker, E.B.; Coyle, J.L.; Wood, J.L. A penetration-aspiration scale. Dysphagia 1996, 11, 93–98. [Google Scholar] [CrossRef]
- Silverman, E.P.; Carnaby-Mann, G.; Pitts, T.; Davenport, P.; Okun, M.S.; Sapienza, C. Concordance and discriminatory power of cough measurement devices for individuals with Parkinson disease. Chest 2014, 145, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Leow, L.P.; Huckabee, M.L.; Anderson, T.; Beckert, L. The Impact of Dysphagia on Quality of Life in Ageing and Parkinson’s Disease as Measured by the Swallowing Quality of Life (SWAL-QOL) Questionnaire. Dysphagia 2010, 25, 216–220. [Google Scholar] [CrossRef]
- Rodrigues, B.; Nobrega, A.C.; Sampaio, M.; Argolo, N.; Melo, A. Silent Saliva Aspiration in Parkinson’s Disease. Mov. Disord. 2011, 26, 138–141. [Google Scholar] [CrossRef]
- Suttrup, I.; Warnecke, T. Dysphagia in Parkinson’s disease. Dysphagia 2016, 31, 24–32. [Google Scholar] [CrossRef]
- Bach, J.R.; Saporito, L.R. Criteria for extubation and tracheostomy tube removal for patients with ventilatory failure: A different approach to weaning. Chest 1996, 110, 1566–1571. [Google Scholar] [CrossRef]
- Han, Y.J.; Lee, J.; Sohn, D.G.; Park, G.-Y.; Kim, Y.; Park, H.-Y.; Jung, S.-A.; Im, S. Cut-off Values of the Respiratory Muscle Power and Peak Cough Flow in Post-Stroke Dysphagia. Medicina 2020, 56, 635. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Takahashi, M.; Wada, F.; Hachisuka, K. Differences in the peak cough flow among stroke patients with and without dysphagia. J. UOEH 2013, 35, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Mann, G. MASA: The Mann Assessment of Swallowing Ability; Singular/Thomson Learning: Clifton Park, NY, USA, 2002. [Google Scholar]
- Cereda, E.; Cilia, R.; Klersy, C.; Canesi, M.; Zecchinelli, A.L.; Mariani, C.B.; Tesei, S.; Sacilotto, G.; Meucci, N.; Zini, M. Swallowing disturbances in Parkinson’s disease: A multivariate analysis of contributing factors. Park. Relat. Disord. 2014, 20, 1382–1387. [Google Scholar] [CrossRef]
- Claus, I.; Muhle, P.; Suttrup, J.; Labeit, B.; Suntrup-Krueger, S.; Dziewas, R.; Warnecke, T. Predictors of Pharyngeal Dysphagia in Patients with Parkinson’s Disease. J. Park. Dis. 2020, 10, 1727–1735. [Google Scholar] [CrossRef]
- Lee, J.J.; Ham, J.H.; Lee, P.H.; Sohn, Y.H. Gender differences in age-related striatal dopamine depletion in Parkinson’s disease. J. Mov. Disord. 2015, 8, 130. [Google Scholar] [CrossRef] [PubMed]
- Haaxma, C.A.; Bloem, B.R.; Borm, G.F.; Oyen, W.J.G.; Leenders, K.L.; Eshuis, S.; Booij, J.; Dluzen, D.E.; Horstink, M.W.I.M. Gender differences in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2007, 78, 819–824. [Google Scholar] [CrossRef]
| Score | Description of Events |
|---|---|
| 1 | Material does not enter the airway. |
| 2 | Material enters the airway, remains above the vocal folds, and is ejected from the airway. |
| 3 | Material enters the airway, remains above the vocal folds, and is not ejected from the airway. |
| 4 | Material enters the airway, contacts the vocal folds, and is ejected from the airway. |
| 5 | Material enters the airway, contacts the vocal folds, and is not ejected from the airway. |
| 6 | Material enters the airway, passes below the vocal folds, and is ejected into the larynx or out of the airway. |
| 7 | Material enters the airway, passes below the vocal folds, and is not ejected from the trachea despite effort. |
| 8 | Material enters the airway, passes below the vocal folds, and no effort is made to eject. |
| Aspiration Group (n = 125) | Non-Aspiration Group (n = 94) | p-Value | |
|---|---|---|---|
| Age (years) | 73.99 ± 8.87 | 71.88 ± 9.54 | 0.093 |
| Sex (Male/Female) | (81/44) | (46/48) | 0.019 * |
| BMI (kg/m2) | 21.48 ± 3.79 | 22.78 ± 3.72 | 0.012 * |
| Hoehn and Yahr scale | 3.45 ± 0.85 | 2.82 ± 0.92 | <0.001 * |
| MMSE | 21.18 ± 6.17 | 22.76 ± 6.83 | 0.075 |
| Smoking | 32 (25.6%) | 17 (18.1%) | 0.181 |
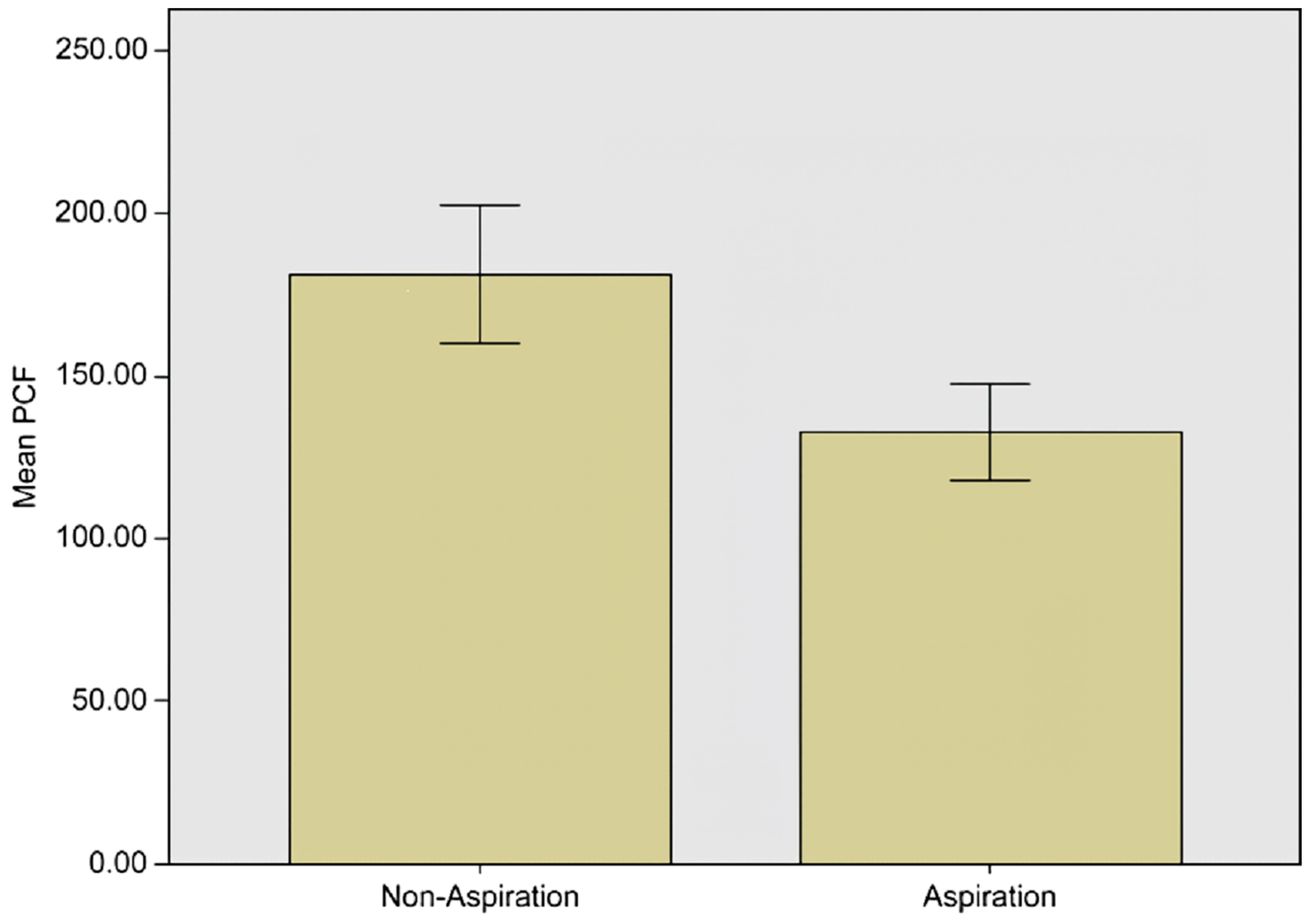
| PCF | 132.63 ± 83.62 | 181.38 ± 103.92 | <0.001 * |
| Hypertension (HTN) | 41 (32.8%) | 36 (38.3%) | 0.401 |
| Diabetes mellitus (DM) | 24 (19.2%) | 22 (23.4%) | 0.452 |
| PCF | Asp (+) | Asp (−) | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|---|
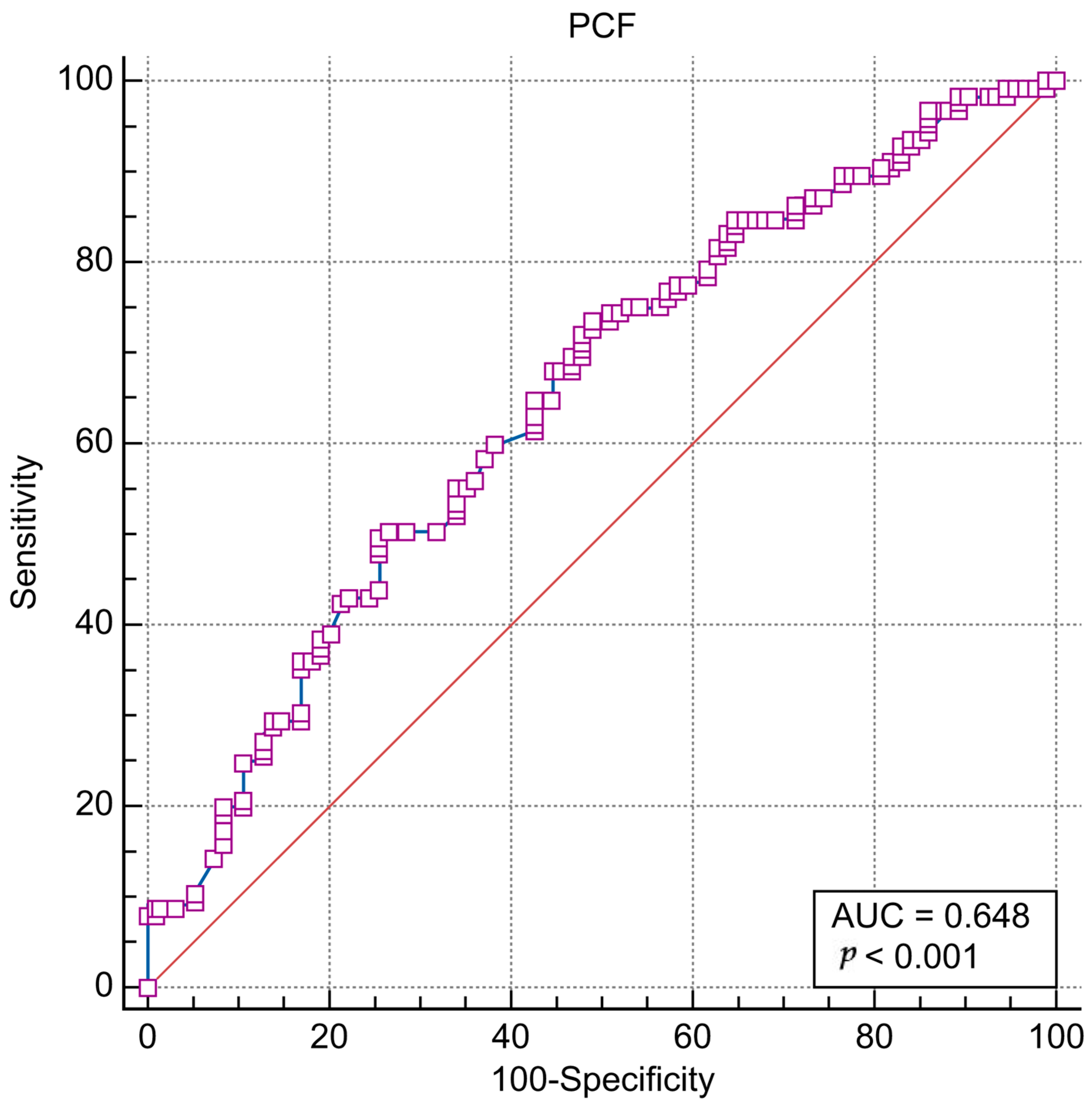
| PCF ≤ 153 | 92 | 46 | 73.60 (65.0–81.1) | 51.06 (40.5–61.5) | 66.7 (61.3–71.6) | 59.3 (50.5–67.4) |
| PCF > 153 | 33 | 48 |
| Variable | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Age (per 1 year) | 1.025 (0.996–1.056) | 0.095 | N/A | |
| Male sex | 1.921 (1.113–3.317) | 0.019 * | 3.264 (1.630–6.535) | 0.001 * |
| BMI (per 1 kg/m2) | 0.912 (0.848–0.982) | 0.014 * | 0.931 (0.857–1.012) | 0.095 |
| Hoehn and Yahr scale | 2.232 (1.598–3.117) | <0.001 * | 1.959 (1.379–2.783) | <0.001 * |
| MMSE | 0.961 (0.920–1.004) | 0.077 | N/A | |
| Smoking | 1.559 (0.805–3.019) | 0.188 | N/A | |
| PCF ≤ 153 | 2.909 (1.650–5.130) | <0.001 * | 3.648 (1.797–7.407) | <0.001 * |
| Hypertension | 0.786 (0.450–1.375) | 0.399 | N/A | |
| Diabetes mellitus | 0.778 (0.405–1.494) | 0.450 | N/A | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.-W.; Kim, S.-B.; Lee, J.-H.; Kim, S.-W. Cut-Off Value of Voluntary Peak Cough Flow in Patients with Parkinson’s Disease and Its Association with Severe Dysphagia: A Retrospective Pilot Study. Medicina 2023, 59, 921. https://doi.org/10.3390/medicina59050921
Lee K-W, Kim S-B, Lee J-H, Kim S-W. Cut-Off Value of Voluntary Peak Cough Flow in Patients with Parkinson’s Disease and Its Association with Severe Dysphagia: A Retrospective Pilot Study. Medicina. 2023; 59(5):921. https://doi.org/10.3390/medicina59050921
Chicago/Turabian StyleLee, Kyeong-Woo, Sang-Beom Kim, Jong-Hwa Lee, and Seong-Woo Kim. 2023. "Cut-Off Value of Voluntary Peak Cough Flow in Patients with Parkinson’s Disease and Its Association with Severe Dysphagia: A Retrospective Pilot Study" Medicina 59, no. 5: 921. https://doi.org/10.3390/medicina59050921
APA StyleLee, K.-W., Kim, S.-B., Lee, J.-H., & Kim, S.-W. (2023). Cut-Off Value of Voluntary Peak Cough Flow in Patients with Parkinson’s Disease and Its Association with Severe Dysphagia: A Retrospective Pilot Study. Medicina, 59(5), 921. https://doi.org/10.3390/medicina59050921






