Prognostic Impact of Global Longitudinal Strain and NT-proBNP on Early Development of Cardiotoxicity in Breast Cancer Patients Treated with Anthracycline-Based Chemotherapy
Abstract
1. Introduction
2. Methods and Materials
2.1. Study Population
- Age > 18 years
- Ist–IIIrd stage of breast cancer (non-metastatic disease)
- Qualification for chemotherapy regimens with conventional doxorubicin
- Normal systolic LV function (baseline LV EF ≥ 55%), no typical signs of heart failure before the onset of anticancer treatment
- Previous radiation therapy, involving the heart and previous chemotherapy
- Known significant LV and right ventricular (RV) dysfunction, severe valvular heart disease, arrhythmias, mental illness
- Contraindication for doxorubicin-based chemotherapy
- Poor-quality echocardiography windows
- AC (doxorubicin plus cyclophosphamide)
- AC-paclitaxel (doxorubicin plus cyclophosphamide followed by paclitaxel)
- AC-docetaxel (doxorubicin plus cyclophosphamide followed by docetaxel)
- FAC (5-FU plus doxorubicin plus cyclophosphamide)
- TAC (docetaxel plus doxorubicin plus cyclophosphamide)
- FAC-docetaxel (5-FU plus doxorubicin plus cyclophosphamide followed by docetaxel)
2.2. Echocardiography
2.3. Biochemical Markers
2.4. The Six-Minute Walking Test
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, J.; Lerman, A. Evaluation and management of patients with heart disease and cancer: Cardio-oncology. Mayo Clin. Proc. 2014, 89, 1287–1306. [Google Scholar] [CrossRef] [PubMed]
- Octavia, Y.; Tocchetti, C.G.; Gabrielson, K.L.; Janssens, S.; Crijns, H.J.; Moens, A.L. Doxorubicin-induced cardiomyopathy: From molecular mechanisms to therapeutic strategies. J. Mol. Cell. Cardiol. 2012, 52, 1213–1225. [Google Scholar] [CrossRef] [PubMed]
- Lbini, A.; Pennesi, G. Cardiotoxicity of anticancer drugs: The need for cardio-oncology and cardio-oncological prevention. J. Natl. Cancer Inst. 2010, 102, 14–25. [Google Scholar] [CrossRef]
- Tan, C.; Tasaka, H. Daunomycin, an antitumor antibiotic, in the treatment of neoplastic disease. Clinical evaluation with special reference to childhood leukemia. Cancer 1967, 20, 333–353. [Google Scholar] [CrossRef]
- Plana, J.C.; Galderisi, M.; Barac, A.; Ewer, M.S.; Ky, B.; Scherrer-Crosbie, M.; Ganame, J.; Sebag, I.A.; Agler, D.A.; Badano, L.P.; et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: A report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2014, 27, 911–939. [Google Scholar] [CrossRef]
- Zamorano, J.L.; Lancellotti, P.; Rodriguez Muñoz, D.; Aboyans, V.; Asteggiano, R.; Galderisi, M.; Habib, G.; Lenihan, D.J.; Lip, G.Y.H.; Lyon, A.R.; et al. ESC Scientific Document Group. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 2768–2801. [Google Scholar] [CrossRef]
- Barrett-Lee, P.J.; Dixon, J.M.; Farrell, C.; Jones, A.; Leonard, R.; Murray, N.; Palmieri, C.; Plummer, C.J.; Stanley, A.; Verrill, M.W. Expert opinion on the use of anthracyclines in patients with advanced breast cancer at cardiac risk. Ann. Oncol. 2009, 20, 816–827. [Google Scholar] [CrossRef]
- Feola, M.; Garrone, O.; Occelli, M.; Francini, A.; Biggi, A.; Visconti, G.; Albrile, F.; Bobbio, M.; Merlano, M. Cardiotoxicity after anthracycline chemotherapy in breast carcinoma: Effects on left ventricular ejection fraction, troponin I and brain natriuretic peptide. Int. J. Cardiol. 2011, 148, 194–198. [Google Scholar] [CrossRef]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, e596–e646. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the american society of echocardiography and the european association of cardiovascular imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef]
- Marwick, T.H.; Leano, R.L.; Brown, J.; Sun, J.-P.; Hoffmann, R.; Lysyansky, P.; Becker, M.; Thomas, J.D. Myocardial strain measurement with 2-dimensional speckle-tracking echocardiography: Definition of normal range. JACC Cardiovasc. Imaging 2009, 2, 80–84. [Google Scholar] [CrossRef]
- Holland, A.E.; Spruit, M.A. An official European Respiratory Society/American Thoracic Society technical standard: Field walking tests in chronic respiratory disease. Eur. Respir. J. 2014, 44, 1428–1446. [Google Scholar] [CrossRef]
- Von Hoff, D.D.; Layard, M.W.; Basa, P.; Davis, H.L., Jr.; Von Hoff, A.L.; Rozencweig, M.; Muggia, F.M. Risk factors for doxorubicin-induced congestive heart failure. Ann. Intern. Med. 1979, 91, 710–717. [Google Scholar] [CrossRef]
- Hamo, C.E.; Bloom, M.W. Cancer and Heart Failure: Understanding the Intersection. Card. Fail. Rev. 2017, 3, 66–70. [Google Scholar] [CrossRef]
- Mitry, M.A.; Edwards, J.G. Doxorubicin induced heart failure: Phenotype and molecular mechanisms. IJC Hear. Vasc. 2015, 10, 17–24. [Google Scholar] [CrossRef]
- Kalivendi, S.V.; Kotamraju, S. Doxorubicin-induced apoptosis is associated with increased transcription of endothelial nitric-oxide synthase: Effect of antiapoptotic antioxidants and calcium. J. Biol. Chem. 2001, 276, 47266–47276. [Google Scholar] [CrossRef]
- Simunek, T.; Sterba, M. Anthracycline-induced cardiotoxicity: Overview of studies examining the roles of oxidative stress and free cellular iron. Pharmacol. Rep. 2009, 61, 154–171. [Google Scholar] [CrossRef]
- Floyd, J.D.; Nguyen, D.T. Cardiotoxicity of cancer therapy. J. Clin. Oncol. 2005, 23, 7685–7696. [Google Scholar] [CrossRef]
- Thorn, C.F.; Oshiro, C. Doxorubicin pathways: Pharmacodynamics and adverse effects. Pharmacogenet. Genom. 2011, 21, 440–446. [Google Scholar] [CrossRef]
- Kremer, L.C.M.; van der Pal, H.J.H.; Offringa, M.; van Dalen, E.C.; Voûte, P.A. Frequency and risk factors of subclinical cardiotoxicity after anthracycline therapy in children: A systematic review. Ann. Oncol. 2002, 13, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Lipshultz, S.E.; Lipsitz, S.R.; Mone, S.M.; Goorin, A.M.; Sallan, S.E.; Sanders, S.P.; Orav, E.J.; Gelber, R.D.; Colan, S.D. Female sex and drug dose as risk factors for late cardiotoxic effects of doxorubicin therapy for childhood cancer. New Engl. J. Med. 1995, 332, 1738–1744. [Google Scholar] [CrossRef] [PubMed]
- Aminkeng, F.; Ross, C.J.D.; Rassekh, S.R.; Hwang, S.; Rieder, M.J.; Bhavsar, A.P.; Smith, A.; Sanatani, S.; Gelmon, K.A.; Bernstein, D.; et al. Recommendations for genetic testing to reduce the incidence of anthracycline-induced cardiotoxicity. Br. J. Clin. Pharmacol. 2016, 82, 683–695. [Google Scholar] [CrossRef] [PubMed]
- Hershman, D.L.; Shao, T. Anthracycline cardiotoxicity after breast cancer treatment. Oncology 2009, 23, 227–234. [Google Scholar]
- Billingham, M.E.; Mason, J.W.; Bristow, M.R.; Daniels, J.R. Anthracycline cardiomyopathy monitored by morphologic changes. Cancer Treat. Rep. 1978, 62, 865–872. [Google Scholar]
- Kalam, K.; Otahal, P. Prognostic implications of global LV dysfunction: A systematic review and meta-analysis of global longitudinal strain and ejection fraction. Heart 2014, 100, 1673–1680. [Google Scholar] [CrossRef]
- Curigliano, G.; Cardinale, D.; Suter, T.; Plataniotis, G.; de Azambuja, E.; Sandri, M.T.; Criscitiello, C.; Goldhirsch, A.; Cipolla, C.; Roila, F. Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO Clinical Practice Guidelines. Ann. Oncol. 2012, 23, vii155–vii166. [Google Scholar] [CrossRef]
- Negishi, K.; Negishi, T. Independent and incremental value of deformation indices for prediction of trastuzumab-induced cardiotoxicity. J. Am. Soc. Echocardiogr. 2013, 26, 493–498. [Google Scholar] [CrossRef]
- Sawaya, H.; Sebag, I.A.; Plana, J.C.; Januzzi, J.L.; Ky, B.; Cohen, V.; Gosavi, S.; Carver, J.R.; Wiegers, S.E.; Martin, R.P.; et al. Early detection and prediction of cardiotoxicity in chemotherapy-treated patients. Am. J. Cardiol. 2011, 107, 1375–1380. [Google Scholar] [CrossRef]
- Tang, Q.; Jiang, Y.; Xu, Y.; Xia, H. Speckle tracking echocardiography predicts early subclinical anthracycline cardiotoxicity in patients with breast cancer. J. Clin. Ultrasound 2016, 45, 222–230. [Google Scholar] [CrossRef]
- Charbonnel, C.; Convers-Domart, R.; Rigaudeau, S.; Taksin, A.L.; Baron, N.; Lambert, J.; Ghez, S.; Georges, J.-L.; Farhat, H.; Lambert, J.; et al. Assessment of global longitudinal strain at low-dose anthracycline-based chemotherapy, for the prediction of subsequent cardiotoxicity. Eur. Hear. J. Cardiovasc. Imaging 2017, 18, 392–401. [Google Scholar] [CrossRef]
- Porcel, J.M. Utilization of B-type natriuretic peptide and NT-proBNP in the diagnosis of pleural effusions due to heart failure. Curr. Opin. Pulm. Med. 2011, 17, 215–219. [Google Scholar] [CrossRef]
- Tsai, S.-H.; Lin, Y.-Y.; Chu, S.-J.; Hsu, C.-W.; Cheng, S.-M. Interpretation and use of natriuretic peptides in non-congestive heart failure settings. Yonsei Med J. 2010, 51, 151–163. [Google Scholar] [CrossRef]
- Gimeno, E.; Gomez, M. NT-proBNP: A cardiac biomarker to assess prognosis in non-Hodgkin lymphoma. Leuk. Res. 2011, 35, 715–720. [Google Scholar] [CrossRef]
- Dodos, F.; Halbsguth, T. Usefulness of myocardial performance index and biochemical markers for early detection of anthracycline-induced cardiotoxicity in adults. Clin. Res. Cardiol. 2008, 97, 318–326. [Google Scholar] [CrossRef]
- Uszko-Lencer, N.H.; Mesquita, R.; Janssen, E.; Werter, C.; Rocca, H.-P.B.-L.; Pitta, F.; Wouters, E.F.; Spruit, M.A. Reliability, construct validity and determinants of 6-minute walk test performance in patients with chronic heart failure. Int. J. Cardiol. 2017, 240, 285–290. [Google Scholar] [CrossRef]
- Travensolo, C.D.F.; Arcuri, J.F.; Polito, M.D. Validity and reliability of the 6-min step test in individuals with coronary artery disease. Physiother. Res. Int. 2019, 25, e1810. [Google Scholar] [CrossRef]
- Schmidt, K.; Vogt, L.; Thiel, C.; Jäger, E.; Banzer, W. Validity of the six-minute walk test in cancer patients. Int. J. Sport Med. 2013, 34, 631–636. [Google Scholar] [CrossRef]
- Larsen, C.M.; Mulvagh, S.L. Cardio-oncology: What you need to know now for clinical practice and echocardiography. Echo Res Pract. 2017, 4, R33–R41. [Google Scholar] [CrossRef]
- Jones, R.; Swanton, C.; Ewer, M.S. Anthracycline cardiotoxicity. Expert Opin. Drug Saf. 2006, 5, 791–809. [Google Scholar] [CrossRef]
- Raj, S.; Franco, V.I.; Lipshultz, S.E. Anthracycline-induced cardiotoxicity: A review of pathophysiology, diagnosis, and treatment. Curr. Treat. Options Cardiovasc. Med. 2014, 16, 315. [Google Scholar] [CrossRef] [PubMed]
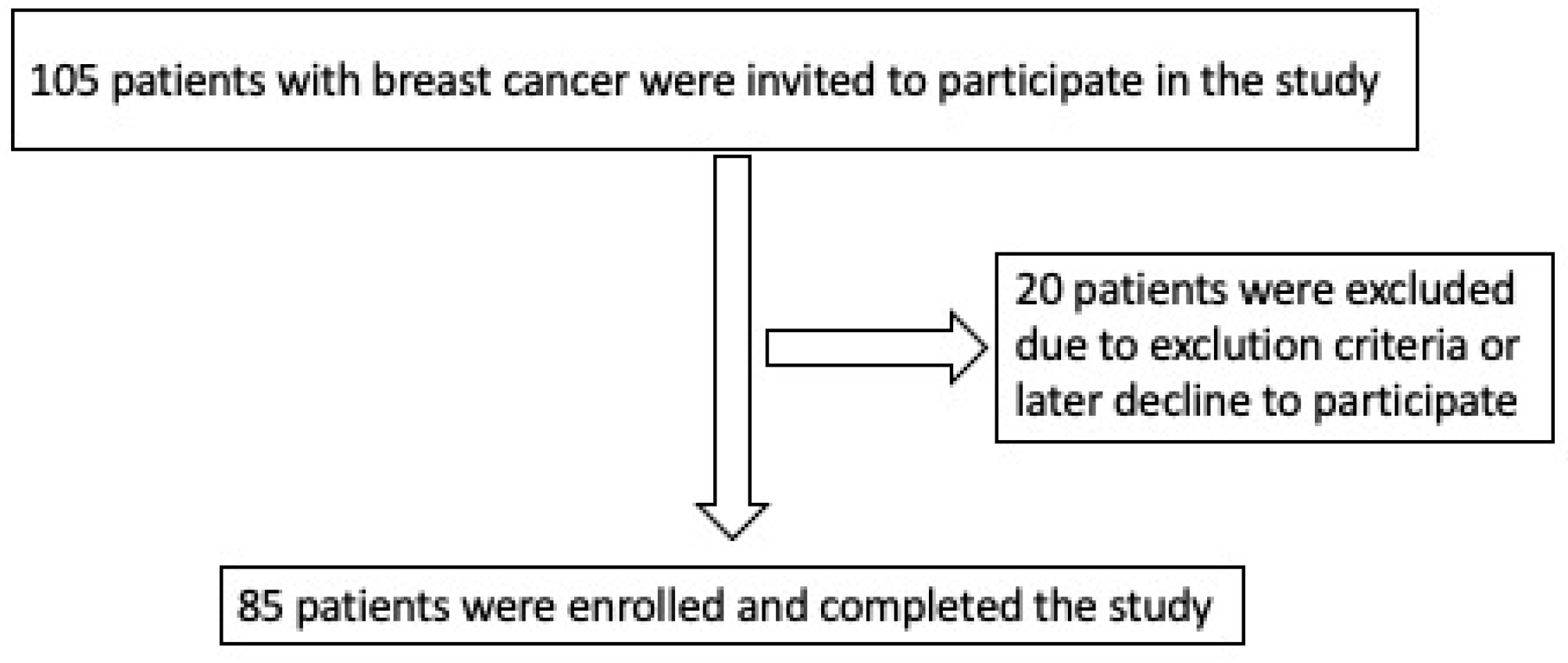
| All Patients (n = 85) | Noncardiotoxicity (n = 63; 74.1%) | Cardiotoxicity (n = 22; 25.9%) | p-Value | |
|---|---|---|---|---|
| Age, yrs | 54.5 ± 9.3 | 54.4 ± 8.5 | 54.6 ± 11.4 | 0.77 |
| BMI, kg/m2 | 27.7 ± 5.5 | 27.8 ± 5.4 | 27.5 ± 5.8 | 0.79 |
| CVD risk factors | ||||
| AH, n (%) | 36 (42.4) | 21 (33.3) | 15 (68.2) | 0.004 |
| Diabetes mellitus, n (%) | 16 (18.8) | 10 (15.9) | 6 (27.3) | 0.24 |
| Smoking, n (%) | 17 (20.0) | 12 (19.1) | 5 (22.7) | 0.26 |
| Family history of CVD, n (%) | 23 (27.1) | 12 (19.1) | 11 (50.0) | 0.005 |
| Dyslipidemia, n (%) | 23 (27.1) | 14 (22.2) | 9 (40.9) | 0.89 |
| Medications | ||||
| ACE inhibitors/ARBs | 24 (28.2) | 16 (25.4) | 8 (36.4) | 0.33 |
| β-blockers | 26 (30.6) | 14 (22.2) | 12 (54.5) | 0.005 |
| Diuretics | 6 (7.1) | 3 (4.8) | 3 (13.6) | 0.18 |
| Calcium channel blockers | 8 (9.4) | 4 (6.4) | 4 (18.2) | 0.10 |
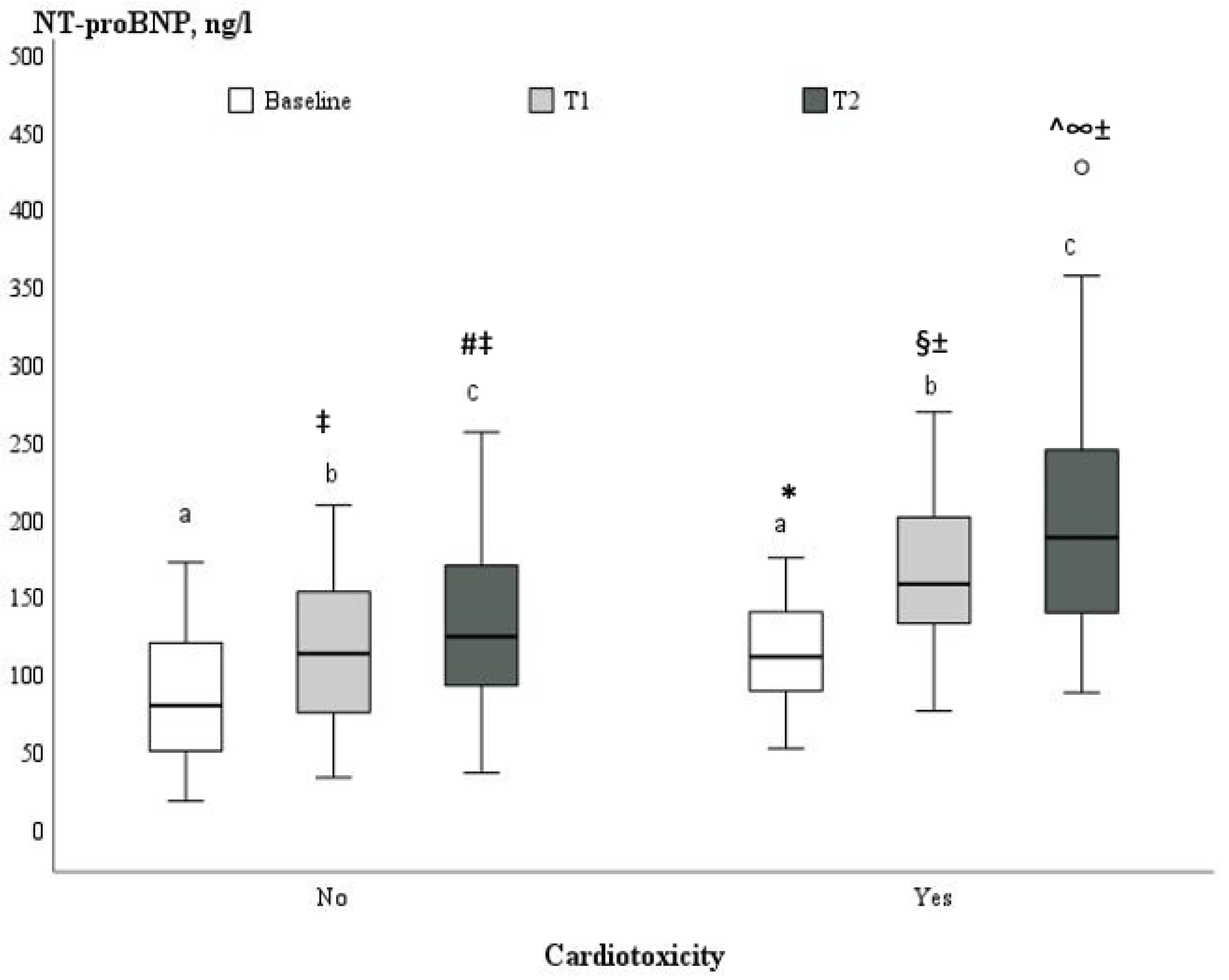
| NT pro-BNP (ng/L) | 94.8 ± 43.8 | 87.3 ± 44.3 | 113.7 ± 37.3 | 0.021 |
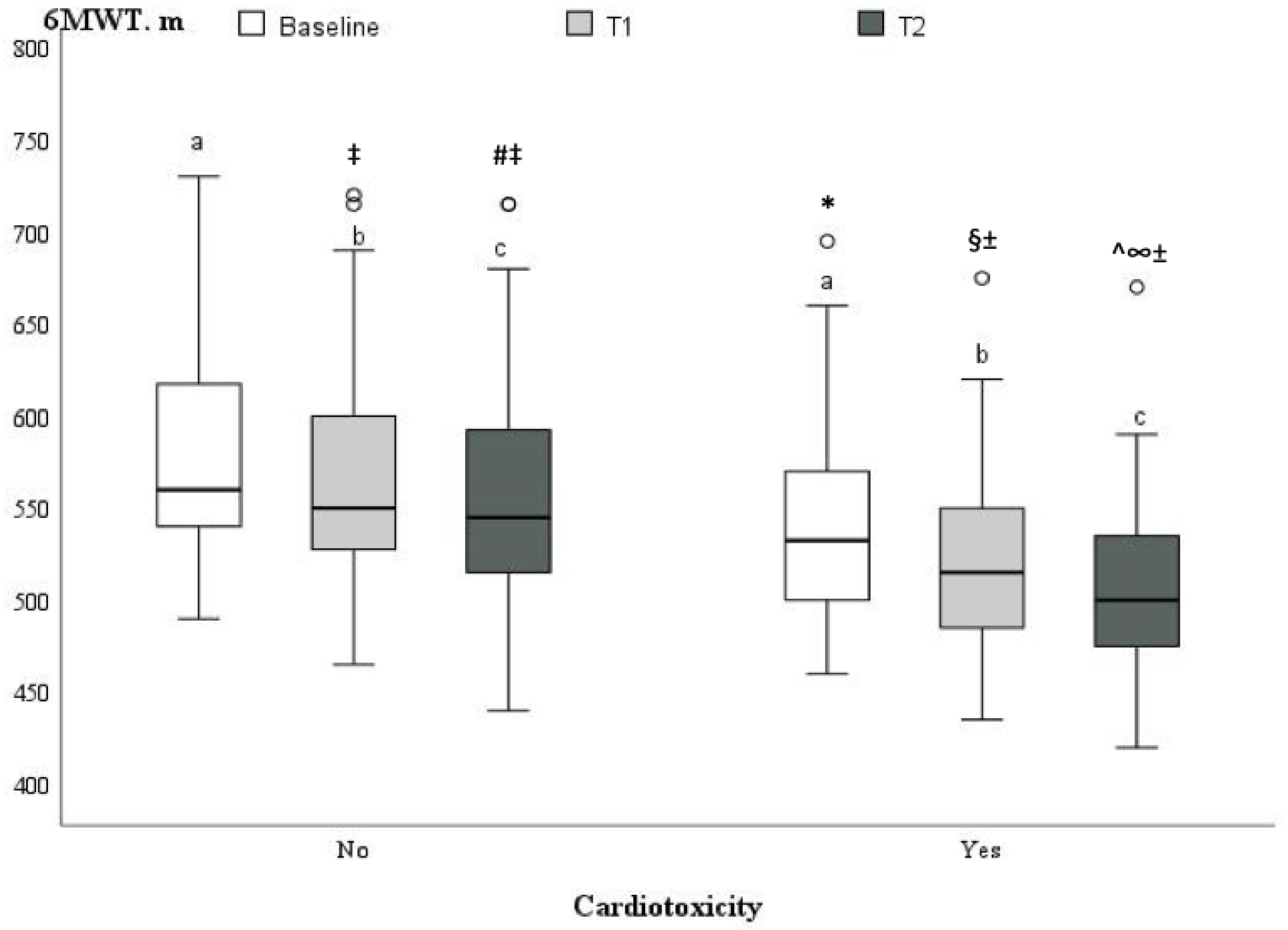
| 6MWT, m | 569.6 ± 59.4 | 579.3 ± 56.9 | 541.8 ± 59.0 | 0.005 |
| Regimen | All Patients (n = 85) | Noncardiotoxicity (n = 63; 74.1%) | Cardiotoxicity (n = 22; 25.9%) | p-Value |
|---|---|---|---|---|
| AC | 8 (9.4) | 6 (9.5) | 2 (9.1) | 1.00 |
| AC-paclitaxel | 55 (64.7) | 40 (63.5) | 15 (68.3) | 0.692 |
| AC-docetaxel | 8 (9.4) | 7 (11.1) | 1 (4.5) | 0.674 |
| FAC-docetaxel | 6 (7.1) | 5 (7.9) | 1 (4.5) | 1.00 |
| TAC | 5 (5.9) | 2 (3.2) | 3 (13.6) | 0.107 |
| FAC | 3 (3.5) | 3 (4.8) | 0 (-) | 0.565 |
| Doxorubicin cumulative dose (mg/m2) | 233.9 ± 29.6 | 234.6 ± 30.1 | 231.3 ± 28.5 | 0.80 |
| Variables | T0 | T1 | T2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All Patients (n = 85) | Cardiotoxicity | Cardiotoxicity | Cardiotoxicity | |||||||
| No (n = 63; 74.1%) | Yes (n = 22; 25.9%) | p-Value | No (n = 63; 74.1%) | Yes (n = 22; 25.9%) | p-Value | No (n = 63; 74.1%) | Yes (n = 22; 25.9%) | p-Value | ||
| LVEF (%) | 60.6 ± 1.8 | 60.1 ± 1.6 | 61.8 ±1.8 | <0.001 | 57.1 ± 1.4 ± | 54.0 ± 1.6 * | <0.001 | 55.8 ± 1.6 ±^ | 49.9 ± 2.1 *§ | <0.001 |
| GLS (%) | −21.1± 0.5 | −21.1 ± 0.5 | −21.0 ± 0.5 | 0.52 | −19.3 ± 0.9 ± | −17.8 ± 0.4 * | <0.001 | −18.5 ± 0.9 ±^ | −16.5 ± 11.1 *§ | <0.001 |
| LVEDD (mm) | 46.1 ± 3.9 | 45.9 ± 4.2 | 46.8 ± 2.6 | 0.018 | 46.2 ± 3.8 ± | 47.1 ± 3.0 | 0.04 | 46.6 ± 3.8 ±^ | 47.1 ± 3.9 | 0.019 |
| LVEDD index (mm/m2) | 25.1 ± 2.8 | 24.8 ± 2.7 | 25.6 ± 2.8 | 0.17 | 25.0 ± 2.6 ± | 25.7 ± 2.9 | 0.21 | 25.5 ± 3.7 ±^ | 25.8 ± 3.0 | 0.20 |
| MAPSE (mm) | 14.8 ± 1.9 | 14.7 ± 2.0 | 14.9 ± 1.4 | 0.68 | 13.9 ± 1.6 ± | 13.1 ± 1.0 * | 0.043 | 13.3 ± 1.7 ±^ | 13.0 ± 1.8 *§ | 0.30 |
| S’ mean (cm/s) | 9.0 ± 1.3 | 9.1 ± 1.3 | 8.9 ± 1.2 | 0.98 | 8.4 ± 1.1 ± | 7.9 ± 1.1 * | 0.08 | 8.0 ± 1.2 ±^ | 7.6 ± 1.1 *§ | 0.18 |
| E (cm/s) | 72.7 ± 16.7 | 72.5 ± 15.6 | 73.2 ± 19.8 | 0.50 | 70.0 ±13.8 ± | 69.4 ±12.4 * | 0.85 | 67.3 ± 14.0 ±^ | 66.3 ± 11.3 * | 0.85 |
| A (cm/s) | 73.7 ± 17.7 | 75.0 ± 17.9 | 70.1 ± 17.2 | 0.29 | 76.2 ± 14.7 | 73.6 ± 14.8 | 0.46 | 79.0 ± 17.6 ± | 74.0 ± 17.7 | 0.30 |
| E/A ratio | 1.07 ± 0.4 | 1.04 ± 0.4 | 1.15 ± 0.3 | 0.08 | 0.96 ± 0.3 ± | 0.98 ± 0.3 * | 0.40 | 0.89 ± 0.3 ±^ | 0.95 ± 0.3 *§ | 0.29 |
| E’ mean (cm/s) | 11.4 ± 2.3 | 11.4 ± 2.4 | 11.6 ± 2.1 | 0.88 | 10.3 ± 2.1 ± | 10.3 ± 1.9 * | 0.86 | 9.7 ± 2.1 ±^ | 10.0 ± 2.4 *§ | 0.98 |
| E/E’ ratio | 6.7 ± 1.4 | 6.7 ± 1.4 | 6.9 ± 1.3 | 0.79 | 6.9 ± 1.3 ± | 6.8 ± 1.0 | 0.22 | 7.3 ± 1.6 ±^ | 7.2 ± 1.4 | 0.89 |
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR [95% CI] | p-Value | OR [95% CI] | p-Value | |
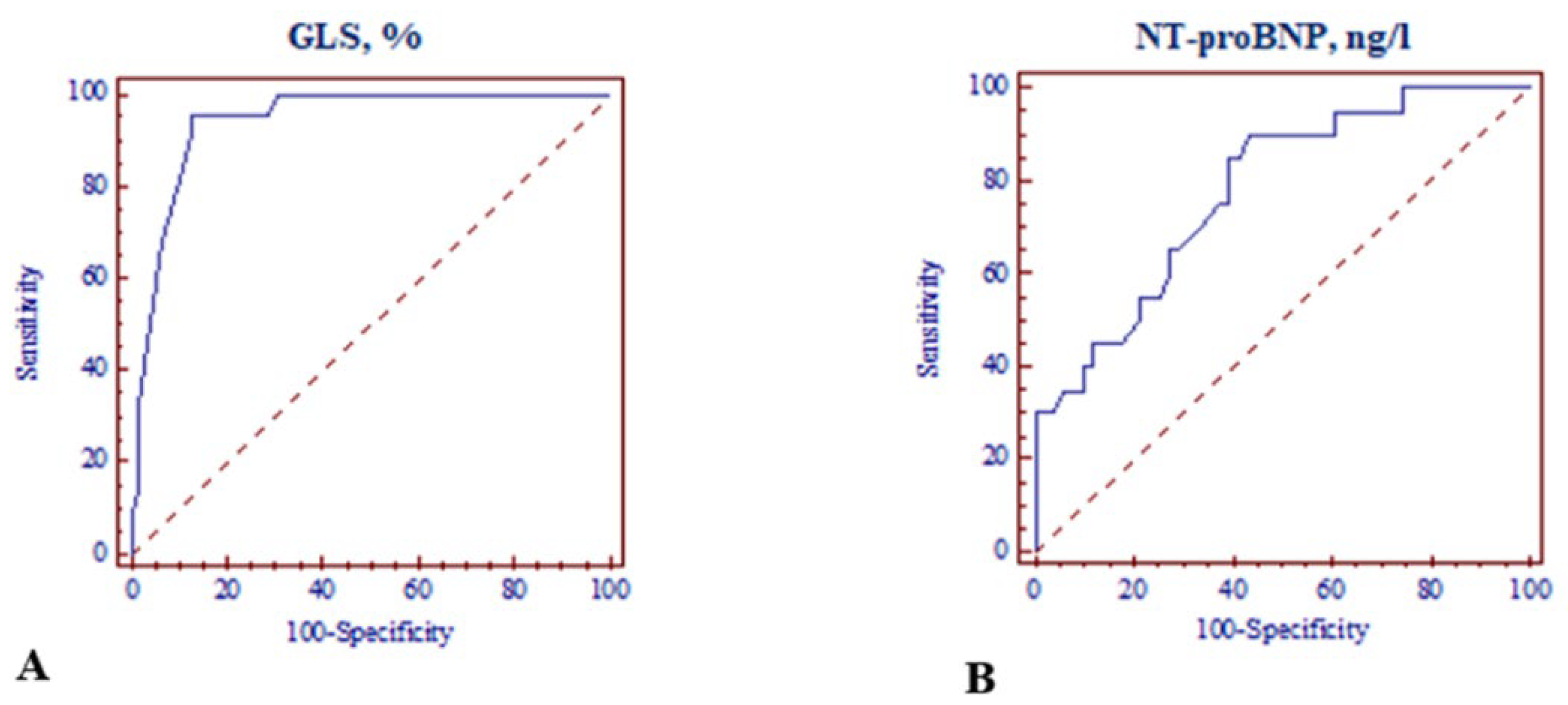
| GLS ≤ −18.0% | 30.933 [8.350–114.4590] | <0.001 | 12.849 [2.783–59.326] | 0.001 |
| NT-proBNP > 125 ng/L | 11.864 [2.487–56.595] | <0.001 | 7.091 [1.034–48.642] | 0.046 |
| Family history of CVD | 4.250 [1.493–12.095] | 0.005 | 2.874 [0.615–13.417] | 0.18 |
| AH | 4.286 [1.517–12.112] | 0.004 | 1.299 [0.300–18.105] | 0.73 |
| 6MWT ≤ 515 m | 7.200 [2.405–21.554] | <0.001 | 3.704 [0.758–9.777] | 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muckiene, G.; Vaitiekus, D.; Zaliaduonyte, D.; Zabiela, V.; Verseckaite-Costa, R.; Vaiciuliene, D.; Juozaityte, E.; Jurkevicius, R. Prognostic Impact of Global Longitudinal Strain and NT-proBNP on Early Development of Cardiotoxicity in Breast Cancer Patients Treated with Anthracycline-Based Chemotherapy. Medicina 2023, 59, 953. https://doi.org/10.3390/medicina59050953
Muckiene G, Vaitiekus D, Zaliaduonyte D, Zabiela V, Verseckaite-Costa R, Vaiciuliene D, Juozaityte E, Jurkevicius R. Prognostic Impact of Global Longitudinal Strain and NT-proBNP on Early Development of Cardiotoxicity in Breast Cancer Patients Treated with Anthracycline-Based Chemotherapy. Medicina. 2023; 59(5):953. https://doi.org/10.3390/medicina59050953
Chicago/Turabian StyleMuckiene, Gintare, Domas Vaitiekus, Diana Zaliaduonyte, Vytautas Zabiela, Raimonda Verseckaite-Costa, Dovile Vaiciuliene, Elona Juozaityte, and Renaldas Jurkevicius. 2023. "Prognostic Impact of Global Longitudinal Strain and NT-proBNP on Early Development of Cardiotoxicity in Breast Cancer Patients Treated with Anthracycline-Based Chemotherapy" Medicina 59, no. 5: 953. https://doi.org/10.3390/medicina59050953
APA StyleMuckiene, G., Vaitiekus, D., Zaliaduonyte, D., Zabiela, V., Verseckaite-Costa, R., Vaiciuliene, D., Juozaityte, E., & Jurkevicius, R. (2023). Prognostic Impact of Global Longitudinal Strain and NT-proBNP on Early Development of Cardiotoxicity in Breast Cancer Patients Treated with Anthracycline-Based Chemotherapy. Medicina, 59(5), 953. https://doi.org/10.3390/medicina59050953







