Abstract
Background: Falls in older people have a significant impact on public health. The scientific literature has provided evidence about the necessity for older adults to be physically active, since it reduces the incidence of falls, several diseases, and deaths, and can even slow down some effects of aging. The primary aim of our study is to identify if physical performances and risk of falling are related to 1-, 2-, 3-, 4-, and 5-year mortality. Its secondary aim is to establish if people with both severely impaired physical performance and a high risk of falling also present impairment in other geriatric domains. Methods: In this prospective study, we enrolled subjects aged 65 years or more, subjected them to comprehensive assessment (including assessment of risk of falling, physical capacities, comorbidities, autonomies in daily living, cognitive abilities, mood, and nutritional status), and followed them for 5 years. Results: We included 384 subjects, 280 of whom were women (72.7%), with a median age of 81 years. Our results showed that physical performances and risk of falling are highly correlated to each other (rho = 0.828). After divided the sample into three groups (people without augmented risk of falling and able to perform adequate physical activity; people with moderate risk of falling and/or disability; people with severe risk of falling and/or disability), we found that the more severe the disability and risk of falling were, the more compromised the other geriatric domains were. Moreover, the survival probability progressively increased following the same trend, amounting to only 41% in severely compromised people, 51.1% in moderately compromised people, and 62.8% in people without physical compromise nor an augmented falling risk (p = 0.0124). Conclusions: Poor physical performance combined with a high risk of falling, correlated with each other, are associated with higher mortality and impairment in multiple domains in older adults.
1. Background
The aging of the population represents an increasing concern worldwide [1]. In Italy, life expectancy at birth is 82.6 years, and in 2022 more than 22,000 people have lived 100 or more years [2]. Indeed, it is not only significant from merely a demographic point of view, but also from a public health one. People, who will exponentially increase in number during the next decades [3], may experience changes in muscle mass, balance, and, consequently, risk of injury and disease as they age [4]. It goes without saying that, together with the physical concerns, there are mental and psychosocial issues to be considered, as well as internal medicine issues, since old-aged people are also more likely to experience cardiovascular, neurological, endocrinological, and gastroenterological (among others) diseases [5,6,7]. Additionally, aging also has a significant impact on society in terms of economic costs [8]. As people grow older, they are more likely to require financial assistance, with an increase in demand for services such as long-term care, transportation, and other forms of medical and pharmacological assistance. In this connection, many studies have shown that pharmacological therapy has to deal not only with differences in terms of metabolism in older subjects [9], but also with frequent inappropriate prescriptions [10,11,12], which in turn bring about the increase in the already mentioned risk of falling [13]. As such, comprehensive geriatric assessment has represented and still represents the most useful specialist tool to holistically frame the state of older adults [14,15,16], including the assessment of physical performance [17] and risk of falling [18]. It is known that one of the most significant problems in public health is represented by falls [19], and consequently, bone fractures, which in turn bring hospitalizations, disabilities, and deaths [20,21,22], but it is less known how to identify all the risk factors [23], thus defining what exactly “fall risk” means. It seems to include obvious physical aspects, such as balance and gait, but also cognitive impairment, the assumption of several classes of medications, and environmental matters [24], and can be summarized as defining it as a multifactorial condition which results in negative outcomes, and on the factors for which it is possible to work on in order to avoid them [25]. In such context, even the objective measurement of the sole physical abilities could not be enough to understand and prevent the risk. With the increasing of the global average age, several studies are focusing on the assessment of physical activities [26,27], as physical exercise has been progressively established as a full-fledged therapy [28,29,30]. Physical training proved to be effective even in frail older people in reducing the risk of developing musculoskeletal diseases, muscular weakness, and preventing falls [31]. Distancing from mere osteoarticular matters, physical exercise also reduces metabolic diseases [32], cardiovascular accidents [33], and cognitive impairment [34], not to mention quality of life [35] and depressive symptoms [36]. Unfortunately, it has been reported that, nowadays, some people still believe that training leads to poor benefits, and, on the contrary, can even be dangerous [37]. Current scientific evidence suggests that adequate exercise, in terms of quantity and quality, can even partially reverse some negative effects of aging [38]. Unfortunately, the global socioeconomic disparity is also reflected in global activity inequality, which represents a predictor of obesity prevalence [39].
Since poor physical capacities and the co-presence of several risk factors for falling are indeed multifactorial [25,40], apart from therapeutic interventions, it is necessary to early identify people at greater risk and understand the long-time outcomes the experience.
Taking the above into consideration, we decided to design the present prospective study, the primary aim of which is to identify if physical performance and risk of falling is related to 1-, 2-, 3-, 4-, and 5-year mortality, and the secondary aim of which is to establish if people with both severely impaired physical performance and a high risk of falling also present impairment in other geriatric domains.
2. Methods
2.1. Design of the Study
This prospective observational cohort study included subjects who were evaluated at the Geriatric Outpatient Service of the University Hospital of Monserrato, Cagliari, Italy, from January 2010 to December 2017, and followed for sixty months.
2.2. Study Size
The given confidence level: 95%, confidence interval: 5%, standard deviation (SD): 0.5, Z-score (z): 1.96, and error margin (e): 5%, according to the following formula:
The final sample (N) consisted of 384 subjects.
2.3. Inclusion Criteria
Subject age ≥ 65 years; having been subjected to two tests assessing physical performance: Physical Performance Test (PPT) and Performance Oriented Mobility Assessment (POMA)
2.4. Exclusion Criteria
Comorbidity Index Rating Scale (CIRS) > 30.
2.5. Assessment
The enrolled subjects were evaluated with the following assessments:
- PPT [17], for the assessment of physical performance status. Scores ≥ 20 are indicative of adequate physical capacities, 11–19 indicate moderate disability, and <11 indicate severe disability. It is divided into items, each of which explores the ability to perform a standardized activity, namely, writing a sentence, simulating eating, putting a book on a shelf, putting on and removing a jacket, picking up a coin from the floor, turning 360°, and walking for 15 m: 0 to 4 points are given to each item according to the time spent or the confidence in performing the task.
- POMA [18], for the assessment of the risk of falling. Scores > 24 are indicative of non-increased risk of fall, 20–24 indicate moderate risk, and <20 indicate high risk (or indicate non-ambulatory subjects if <2). It is divided into two sections: “balance” and “gait”. In “balance” section, scoring from 0 to 16, it is tested the balance in different activities, namely sitting, arising, immediate standing, sustained standing, nudging, turning 360°, sitting down; in “gait” section”, scoring from 0 to 12 it is tested the ability and the confidence in moving, examining the characteristics of the steps, the path, and the trunk. The sum of such sections gives the total score.
- CIRS [41], for the assessment of the comorbidity burden. It evaluates 14 categories of pathologies concerning some organs and systems: hypertension, cardiological, vascular, hematopoietic, respiratory, eye–ear–nose–throat–larynx, upper and lower gastroenterological, liver–pancreatic, renal, genitourinary, musculoskeletal, neurological, endocrinological, psychiatric, and behavioral diseases. Each category is given a score from 1 (minimum impairment) to 5 (severe disease or risk of life), the sum of which defines the total score. Such score can be divided for the number of categories to obtain the complex comorbidity index. The number of categories scoring 3 or more defines the severity index.
- Mini Mental State Examination (MMSE) [42], for cognitive assessment. It examines different domains (temporal orientation—from 0 to 5 points, spatial orientation—from 0 to 5 points, immediate memory—from 0 to 3 points, attention—from 0 to 5 points, delayed memory—from 0 to 3 points, language—from 0 to 7 points, and praxis—from 0 to 2 points). Conversion tables are available to avoid age or school influences. Scores < 26 are suggestive of mild-to-severe cognitive impairment [43]
- Geriatric Depression Scale (GDS) [44], for mood assessment. It is made up of 15 yes/no questions regarding satisfaction, dropped interests, happiness, boredom, good spirit, fears, subjective utility, energy, and hope. Each question is given 1 (depressed) or 0 (non-depressed) points. Scores > 5 are suggestive of deflected mood.
- Activities of Daily Living (ADL), expressed as Barthel Index, and Instrumental Activities of Daily Living (IADL) [45], for the assessment of residual autonomies. ADL evaluate feeding (from 0 to 10 points), bathing (from 0 to 5 points), grooming (from 0 to 5 points), dressing (from 0 to 10 points), urinary continence (from 0 to 10 points), bowel continence (from 0 to 10 points), toilet use (from 0 to 10 points), transfers (from 0 to 15 points), mobility (from 0 to 15), walking stairs (from 0 to 10): the sum of each defines the Barthel Index, which is higher when the level of independence in such activities is high. IADL evaluate the ability to use the telephone, shopping, food preparation, housekeeping, laundry, transportation, responsibility for medications, handling finances: each item is given 0 (dependent) or 1 point (independent).
- Mini Nutritional Assessment (MNA) [46,47], for the assessment of nutritional status. It evaluates anthropometric measures (body mass index, mid-arm, and calf circumferences), food intake, weight loss, mobility, recent stress, neuropsychological status, independence, drugs taken, pressure ulcers, as well as how may full meals are eaten daily, markers for protein, fruit, vegetables, and fluids intake, mode of feeding, and subjective view about nutritional and general status. Scores < 17 indicate malnutrition and 17–23.5 indicate risk of malnutrition.
- Number of different drugs taken.
2.6. Statistical Analysis
Variables were expressed as medians and interquartile ranges (IQR) or in percentages (%), where appropriate. The Kolmogorov–Smirnov test was used to test normal distribution. Spearman’s coefficient of rank correlation (rho) was used to correlate the PPT and POMA scores. The Kruskal–Wallis test for independent sample was used to compare the groups on dependent variables. The Conover test was used for post-hoc analysis. Kaplan–Meier curves were designed in order to estimate the survival probability: their results were expressed as hazard ratios (HRs), for the comparison of which the Log-rank test was used. The multivariate analysis was conducted with a logistic regression—stepwise (p-values > 0.1 excluded by the model), the results of which were expressed as odds ratios (ORs), and Area Under the Receiver Operating Characteristic (ROC) curve (AUC).
The results are reported indicating p-values in reference to 95% confidence interval (CI).
MedCalc software (Version 20.218, Ostend, Belgium) was used for the statistical analysis.
3. Results
The study included 384 community-dwelling people aged 65 years or more, of whom 280 were women (72.7%), with a median age of 81 years. The characteristics of the sample are shown in Table 1 and Table 2.

Table 1.
Characteristics of the sample (multidimensional assessment).

Table 2.
Prevalence of comorbidities.
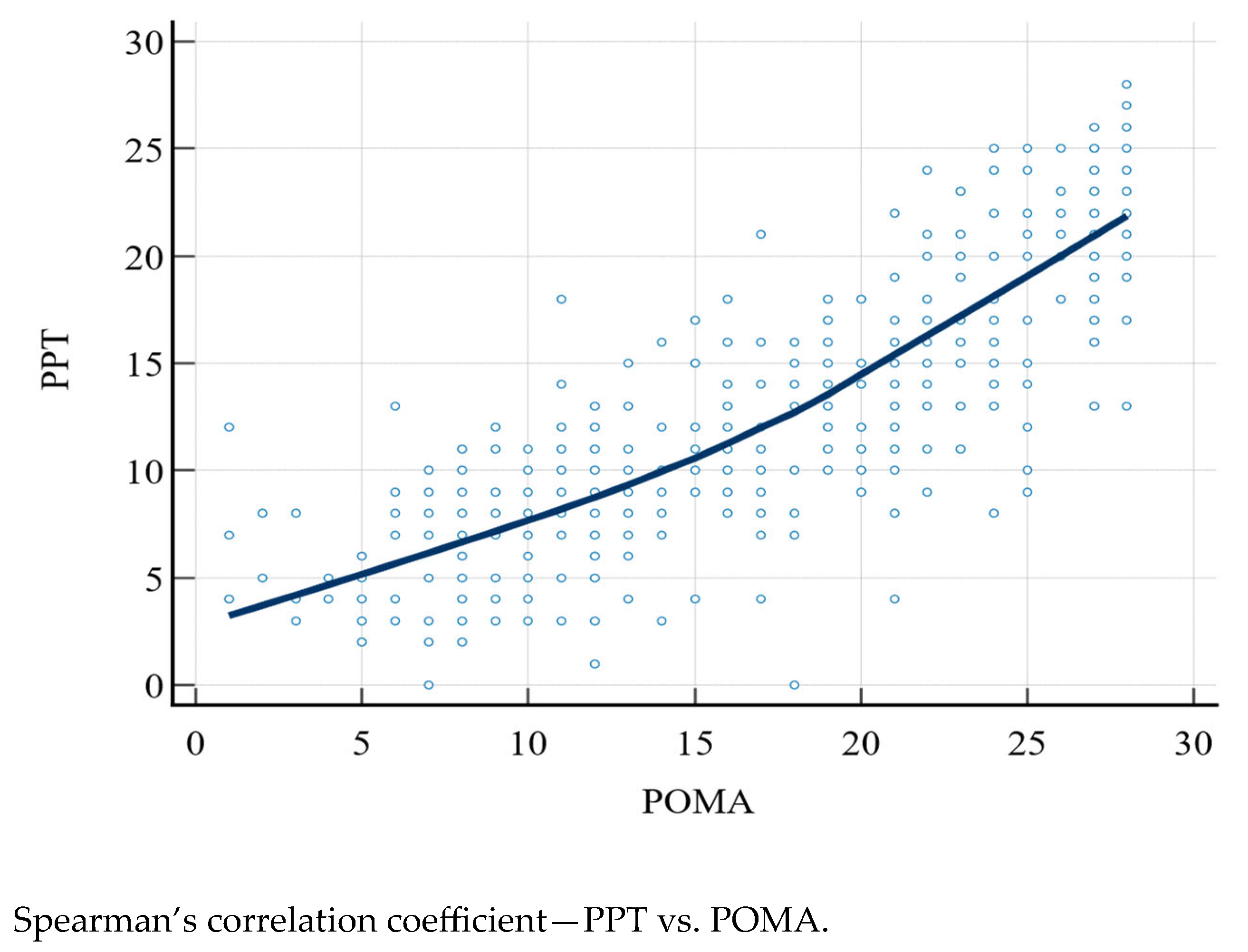
The PPT and POMA scores were compared with Spearman’s correlation coefficient (rho), which was 0.828 (95% CI: 0.793–0.857, p < 0.0001) (Figure 1).
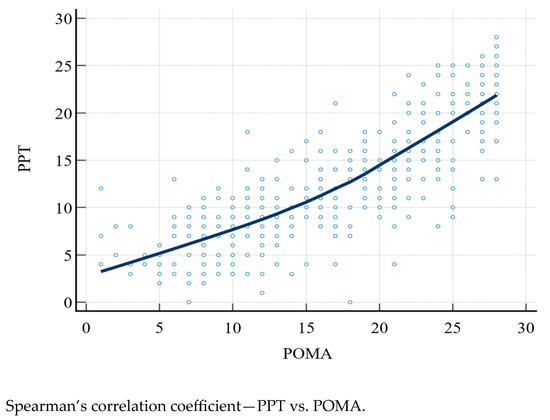
Figure 1.
PPT, Physical Performance Test; POMA, Performance Oriented Mobility Assessment.
We divided the sample into three groups, according to the abovementioned scores, thus obtaining group 1 (PPT ≥ 20 and POMA > 24, 43 subjects), group 2 (PPT of 11–19 and/or POMA of 20–24, 92 subjects), and group 3 (PPT < 11 and/or POMA < 20, 249 subjects). As in Table 3, the age was the only variable with no significant difference in the groups (p = 0.913). ADL, IADL, and MNA scores were significantly lower in group 3 than in group 2, and also in group 2 than in group 1 (p < 0.0001); similarly, the GDS scores were significantly higher in group 3 than in group 2, and in group 2 than in group 1 (p < 0.0001). The CIRS scores (p < 0.0001) and number of drugs taken (p = 0.0009) were significantly higher in group 3 than in the other two groups. Finally, the MMSE scores were higher in group 1 than in the other two groups (p = 0.001).

Table 3.
Comparison between groups.
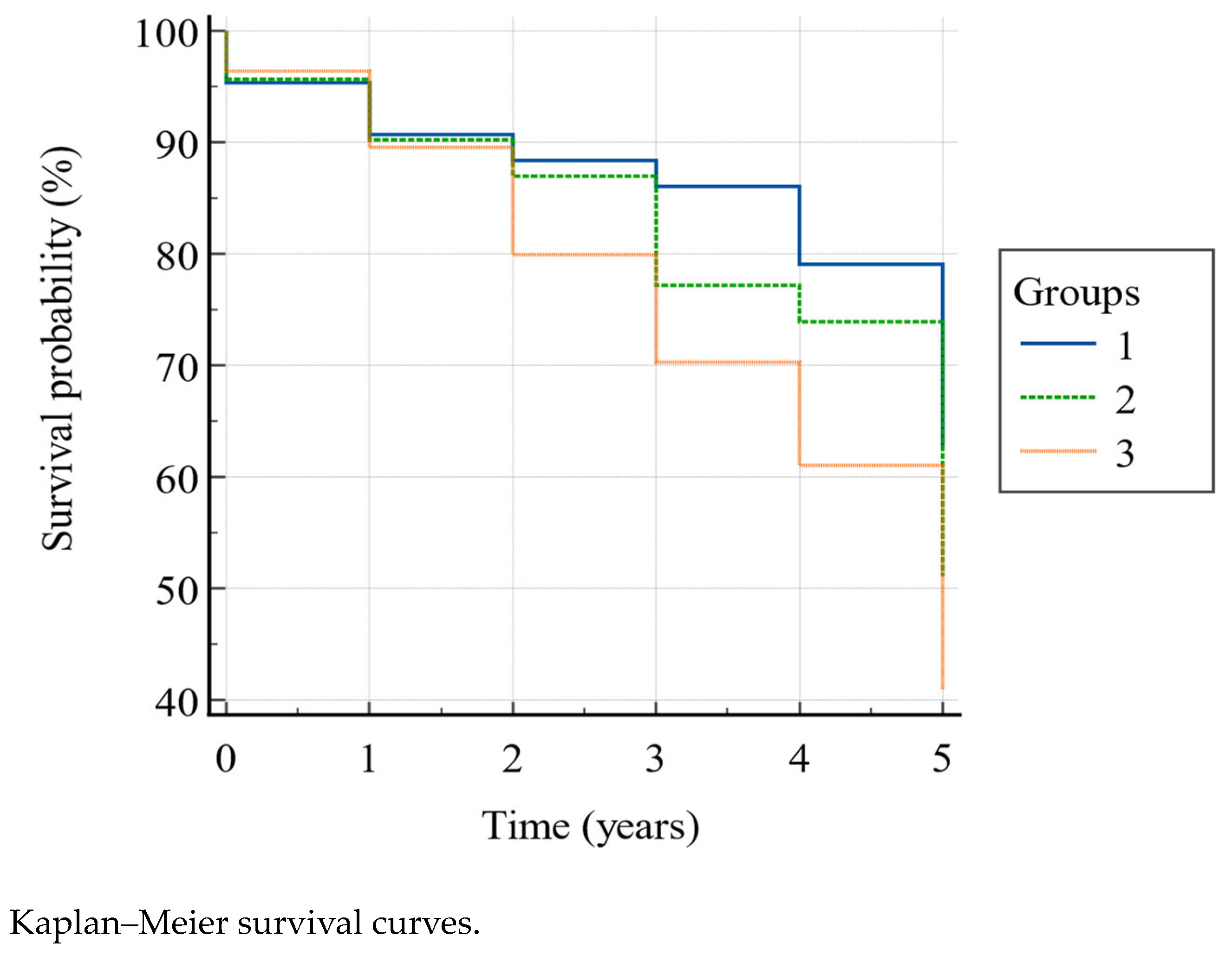
According to the Kaplan–Meier model (Figure 2) the overall 5-year mortality was 54.2%, and the survival rate was significantly higher (Log-rank χ2 = 8.78, p = 0.0124) in group 1 than in group 2 and 3, and in group 2 than in group 3, as shown in Table 4, in the second (88.4% vs. 87% vs. 79.9%), third (86% vs. 77.2% vs. 70.3%), fourth (79.1% vs. 73.9% vs. 61%), and fifth (62.8% vs. 51.1% vs. 41%) year. In particular, belonging to group 1 gave HR = 1.83 (95% CI: 1.20–2.79) for survival with respect to group 3. The other HRs showed a higher tendency to survive in group 1 vs. group 2, and in group 2 vs. group 3, though without reaching the statistical significance (Table 5).
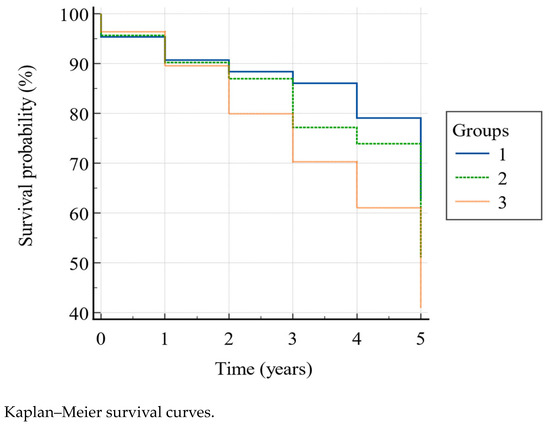
Figure 2.
Group 1, PPT ≥ 20 and POMA > 24; group 2, PPT between 11–19 and/or POMA between 20–24; group 3, PPT < 11 and/or POMA < 20.

Table 4.
Survival rates.

Table 5.
Hazard ratios with 95% CI (survival).
These data were deepened with a multivariate analysis, in which we considered death as a dependent variable, and age, CIRS, MMSE, GDS, ADL, IADL, MNA, and number of drugs taken as independent variables. With an AUC = 0.723 (standard error: 0.028, 95% CI: 0.67–0.77, p < 0.0001), the logistic regression considered age (OR: 1.08), GDS (OR: 0.89), and MNA (OR: 0.84) independently associated with the outcome. The other variables were excluded by the model (Table 6).

Table 6.
Logistic regression—stepwise (dependent variable: exitus).
4. Discussion
Global aging is a pressing issue and is expected to lead to an increasing number of social and health implications [1,8]. Poor physical performance and high risk of falling are associated with negative outcomes [19,20,21,22], especially in older people. Indeed, even if such risks and outcomes can also depend on cognitive-affective status, comorbidity burden, and polypharmacotherapy [24], several studies focus on the assessment of physical performance and risk of falling [25,26,27], based on balance, gait, and the execution of standardized physical tasks.
The primary aim of our study was to identify if physical performance and risk of falling are related to mortality. Its secondary aim was to establish if people with both severely impaired physical performance, and high risk of falling also presented impairment in other geriatric domains. We recruited 384 subjects aged 65 years or more, of whom 280 were women (72.7%), with a median age of 81 years, and followed them for five years. We decided to exclude people with CIRS > 30, meaning a significant comorbidity burden, in order to avoid potential confounding factors: it was in fact demonstrated that comorbidity burden is an independent risk factor for deaths [49]. We did not exclude any particular pathology, although there are several studies demonstrating the association between specific comorbidities and death, but they are usually mediated by a general burden, typical of the elderly age [50,51,52,53].
Our data showed a strong correlation between physical performance and risk of falling (rho = 0.828), emphasizing that in older subjects, various domains follow the same trend, and a person with the inability to carry out standardized activities also presents a higher risk of falling. Accordingly, we divided the sample into three groups: the first, made up of people without an augmented risk of falling and able to perform adequate physical activity, the second, made up of people with a moderate risk of falling and/or disability, and the third, made up of people with a severe risk of falling and/or disability. Our analysis suggested that people belonging to the third group, in addition to the worse physical abilities, also revealed higher comorbidities (though our sampling foresaw too high of a comorbidity burden, in order to avoid confounding factors), worse cognitive capacities, more deflected mood, reduced autonomies, and more deficient nutritional status, according to the above. Moreover, the prospective study showed that poor physical capacities and a high risk of falling were associated with an increased risk of death, with the survival curves spreading with increasing follow-up months, reaching 41% 5-year survival in severely impaired people, 51.1% survival in moderately impaired people, and 62.8% survival in people with adequate physical abilities and a falling risk. In order to deepen such results, we conducted a multivariate analysis to consider a possible relationship between death and impaired geriatric domains, highlighting age, mood, and nutritional status as significant regressors of the outcome. In particular, older age showed the lowest hazard ratio, while poorer nutritional status (116%) and better mood (111%) were more clearly independently associated with death. If the direct proportionality with age and worse nutritional status is consistent with the literature [54,55], the association with mood is not [56]. We believe that, although significative, it could not be associated with an effective clinical difference between the patients, owing to the fact that the GDS median score was moderately high (8 points), and the larger part of the sample (59.1%) had deflected mood, especially the most physically impaired subjects.
The strengths of our study are represented by the 5-year follow-up, and the fact that it was performed using common and easy-to-administer screening tools, also easily reproducible in everyday clinical practice. It did not take into account some variables, such as fear of falling [57], sarcopenia [58], and lack of exercise [59], which were indeed reported to influence the results, and this is its main limitation. Future prospective studies are needed to confirm our results, and public health intervention is necessary to reduce the risk of falling among old subjects.
5. Conclusions
In conclusion, our study tries to address the necessity of screening for risk of falling [60,61,62], continuing along the path traced by other studies, demonstrating that poor physical performance combined with a high risk of falling, correlated with each other, are associated with a higher mortality and impairment in multiple domains in older adults.
Author Contributions
F.S. and A.M. contributed to the study design, performed the data analyses and the interpretation of the findings; F.S. contributed to the data collection, and wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of the University of Cagliari (protocol code NP/2022/1382, 30 March 2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data and materials used and/or analyzed during the current study are not publicly available. The are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
ADL, Activities of Daily Living; CIRS, Cumulative Illness Rating Scale; GDS, Geriatric Depression Scale; IADL, Instrumental Activities of Daily Living; MMSE, Mini Mental State Examination; MNA, Mini Nutritional Assessment; POMA, Performance Oriented Mobility Assessment; PPT, Physical Performance Test
References
- Brivio, P.; Paladini, M.S.; Racagni, G.; Riva, M.A.; Calabrese, F.; Molteni, R. From Healthy Aging to Frailty: In Search of the Underlying Mechanisms. Curr. Med. Chem. 2019, 26, 3685–3701. [Google Scholar] [CrossRef] [PubMed]
- ISTAT. Indicatori Demografici 2022. Available online: https://www.istat.it/it/archivio/indicatori+demografici (accessed on 25 April 2023).
- Partridge, L.; Deelen, J.; Slagboom, P.E. Facing up to the global challenges of ageing. Nature 2018, 561, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.; Pester, J.; Vera, L.; Jeanmonod, D.; Jeanmonod, R. Elderly fall patients triaged to the trauma bay: Age, injury patterns, and mortality risk. Am. J. Emerg. Med. 2015, 33, 1635–1638. [Google Scholar] [CrossRef] [PubMed]
- North, B.J.; Sinclair, D.A. The intersection between aging and cardiovascular disease. Circ. Res. 2012, 110, 1097–1108. [Google Scholar] [CrossRef]
- Seraji-Bzorgzad, N.; Paulson, H.; Heidebrink, J. Neurologic examination in the elderly. Handb. Clin. Neurol. 2019, 167, 73–88. [Google Scholar] [CrossRef]
- Norman, K.; Haß, U.; Pirlich, M. Malnutrition in Older Adults-Recent Advances and Remaining Challenges. Nutrients 2021, 13, 2764. [Google Scholar] [CrossRef]
- Nikolova, S.; Heaven, A.; Hulme, C.; West, R.; Pendleton, N.; Humphrey, S.; Cundill, B.; Clegg, A. Social care costs for community-dwelling older people living with frailty. Health Soc. Care Community 2022, 30, e804–e811. [Google Scholar] [CrossRef]
- Klotz, U. Pharmacokinetics and drug metabolism in the elderly. Drug Metab. Rev. 2009, 41, 67–76. [Google Scholar] [CrossRef]
- Salis, F.; Palimodde, A.; Rundeddu, S.; Mandas, A. STOPP/START Anti-aggregation and Anticoagulation Alerts in Atrial Fibrillation. Curr. Vasc. Pharmacol. 2023. [Google Scholar] [CrossRef]
- Wastesson, J.W.; Morin, L.; Tan, E.C.K.; Johnell, K. An update on the clinical consequences of polypharmacy in older adults: A narrative review. Expert Opin. Drug Saf. 2018, 17, 1185–1196. [Google Scholar] [CrossRef]
- Guaraldo, L.; Cano, F.G.; Damasceno, G.S.; Rozenfeld, S. Inappropriate medication use among the elderly: A systematic review of administrative databases. BMC Geriatr. 2011, 11, 79. [Google Scholar] [CrossRef]
- Lee, J.; Negm, A.; Peters, R.; Wong, E.K.C.; Holbrook, A. Deprescribing fall-risk increasing drugs (FRIDs) for the prevention of falls and fall-related complications: A systematic review and meta-analysis. BMJ Open 2021, 11, e035978. [Google Scholar] [CrossRef]
- Parker, S.G.; McCue, P.; Phelps, K.; McCleod, A.; Arora, S.; Nockels, K.; Kennedy, S.; Roberts, H.; Conroy, S. What is Comprehensive Geriatric Assessment (CGA)? An umbrella review. Age Ageing 2018, 47, 149–155. [Google Scholar] [CrossRef]
- Briggs, R.; McDonough, A.; Ellis, G.; Bennett, K.; O’Neill, D.; Robinson, D. Comprehensive Geriatric Assessment for community-dwelling, high-risk, frail, older people. Cochrane Database Syst. Rev. 2022, 5, CD012705. [Google Scholar] [CrossRef]
- Salis, F.; Loddo, S.; Zanda, F.; Peralta, M.M.; Serchisu, L.; Mandas, A. Comprehensive Geriatric Assessment: Application and correlations in a real-life cross-sectional study. Front. Med. 2022, 9, 984046. [Google Scholar] [CrossRef]
- Rozzini, R.; Frisoni, G.B.; Bianchetti, A.; Zanetti, O.; Trabucchi, M. Physical Performance Test and Activities of Daily Living scales in the assessment of health status in elderly people. J. Am. Geriatr. Soc. 1993, 41, 1109–1113. [Google Scholar] [CrossRef]
- Tinetti, M.E. Performance-oriented assessment of mobility problems in elderly patients. J. Am. Geriatr. Soc. 1986, 34, 119–126. [Google Scholar] [CrossRef]
- Ang, G.C.; Low, S.L.; How, C.H. Approach to falls among the elderly in the community. Singap. Med. J. 2020, 61, 116–121. [Google Scholar] [CrossRef]
- Moreland, B.; Kakara, R.; Henry, A. Trends in Nonfatal Falls and Fall-Related Injuries among Adults Aged ≥65 Years—United States, 2012–2018. MMWR Morb. Mortal. Wkly Rep. 2020, 69, 875–881. [Google Scholar] [CrossRef]
- Finlayson, M.L.; Peterson, E.W. Falls, aging, and disability. Phys. Med. Rehabil. Clin. N. Am. 2010, 21, 357–373. [Google Scholar] [CrossRef]
- Fuller, G.F. Falls in the elderly. Am. Fam. Physician 2000, 61, 2159–2168, 2173–2174. [Google Scholar] [PubMed]
- Cuevas-Trisan, R. Balance Problems and Fall Risks in the Elderly. Clin. Geriatr. Med. 2019, 35, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Callis, N. Falls prevention: Identification of predictive fall risk factors. Appl. Nurs. Res. 2016, 29, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Marzetti, E.; Calvani, R.; Tosato, M.; Cesari, M.; Di Bari, M.; Cherubini, A.; Broccatelli, M.; Savera, G.; D’Elia, M.; Pahor, M.; et al. Physical activity and exercise as countermeasures to physical frailty and sarcopenia. Aging Clin. Exp. Res. 2017, 29, 35–42. [Google Scholar] [CrossRef]
- Varesco, G.; Hunter, S.K.; Rozand, V. Physical activity and aging research: Opportunities abound. Appl. Physiol. Nutr. Metab. 2021, 46, 1004–1006. [Google Scholar] [CrossRef]
- Thomas, E.; Battaglia, G.; Patti, A.; Brusa, J.; Leonardi, V.; Palma, A.; Bellafiore, M. Physical activity programs for balance and fall prevention in elderly: A systematic review. Medicine 2019, 98, e16218. [Google Scholar] [CrossRef]
- Eckstrom, E.; Neukam, S.; Kalin, L.; Wright, J. Physical Activity and Healthy Aging. Clin. Geriatr. Med. 2020, 36, 671–683. [Google Scholar] [CrossRef]
- Pinheiro, M.B.; Oliveira, J.S.; Baldwin, J.N.; Hassett, L.; Costa, N.; Gilchrist, H.; Wang, B.; Kwok, W.; Albuquerque, B.S.; Pivotto, L.R.; et al. Impact of physical activity programs and services for older adults: A rapid review. Int. J. Behav. Nutr. Phys. Act. 2022, 19, 87. [Google Scholar] [CrossRef]
- Izquierdo, M.; Duque, G.; Morley, J.E. Physical activity guidelines for older people: Knowledge gaps and future directions. Lancet Healthy Longev. 2021, 2, e380–e383. [Google Scholar] [CrossRef]
- de Souto Barreto, P.; Rolland, Y.; Vellas, B.; Maltais, M. Association of Long-term Exercise Training with Risk of Falls, Fractures, Hospitalizations, and Mortality in Older Adults: A Systematic Review and Meta-analysis. JAMA Intern. Med. 2019, 179, 394–405. [Google Scholar] [CrossRef]
- Ferriolli, E.; Pessanha, F.P.; Marchesi, J.C. Diabetes and exercise in the elderly. Med. Sport Sci. 2014, 60, 122–129. [Google Scholar] [CrossRef]
- Schroeder, E.C.; Franke, W.D.; Sharp, R.L.; Lee, D.C. Comparative effectiveness of aerobic, resistance, and combined training on cardiovascular disease risk factors: A randomized controlled trial. PLoS ONE 2019, 14, e0210292. [Google Scholar] [CrossRef]
- Karssemeijer, E.G.A.; Aaronson, J.A.; Bossers, W.J.; Smits, T.; Olde Rikkert, M.G.M.; Kessels, R.P.C. Positive effects of combined cognitive and physical exercise training on cognitive function in older adults with mild cognitive impairment or dementia: A meta-analysis. Ageing Res. Rev. 2017, 40, 75–83. [Google Scholar] [CrossRef]
- Vagetti, G.C.; Barbosa Filho, V.C.; Moreira, N.B.; Oliveira Vd Mazzardo, O.; Campos, W.d. Association between physical activity and quality of life in the elderly: A systematic review, 2000–2012. Braz. J. Psychiatry 2014, 36, 76–88. [Google Scholar] [CrossRef]
- de Oliveira, L.D.S.S.C.B.; Souza, E.C.; Rodrigues, R.A.S.; Fett, C.A.; Piva, A.B. The effects of physical activity on anxiety, depression, and quality of life in elderly people living in the community. Trends Psychiatry Psychother. 2019, 41, 36–42. [Google Scholar] [CrossRef]
- Franco, M.R.; Tong, A.; Howard, K.; Sherrington, C.; Ferreira, P.H.; Pinto, R.Z.; Ferreira, M.L. Older people’s perspectives on participation in physical activity: A systematic review and thematic synthesis of qualitative literature. Br. J. Sports Med. 2015, 49, 1268–1276. [Google Scholar] [CrossRef]
- McPhee, J.S.; French, D.P.; Jackson, D.; Nazroo, J.; Pendleton, N.; Degens, H. Physical activity in older age: Perspectives for healthy ageing and frailty. Biogerontology 2016, 17, 567–580. [Google Scholar] [CrossRef]
- Althoff, T.; Sosič, R.; Hicks, J.L.; King, A.C.; Delp, S.L.; Leskovec, J. Large-scale physical activity data reveal worldwide activity inequality. Nature 2017, 547, 336–339. [Google Scholar] [CrossRef]
- Venturelli, M.; Schena, F.; Richardson, R.S. The role of exercise capacity in the health and longevity of centenarians. Maturitas 2012, 73, 115–120. [Google Scholar] [CrossRef]
- Parmelee, P.A.; Thuras, P.D.; Katz, I.R.; Lawton, M.P. Validation of the Cumulative Illness Rating Scale in a geriatric residential population. J. Am. Geriatr. Soc. 1995, 43, 130–137. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Salis, F.; Costaggiu, D.; Mandas, A. Mini-Mental State Examination: Optimal Cut-Off Levels for Mild and Severe Cognitive Impairment. Geriatrics 2023, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, J.I.; Yesavage, J.A. Geriatric depression scale (GDS): Recent evidence and development of a shorter version. Clin. Gerontol. J. Aging Ment. Health 1986, 5, 165–173. [Google Scholar]
- Pashmdarfard, M.; Azad, A. Assessment tools to evaluate Activities of Daily Living (ADL) and Instrumental Activities of Daily Living (IADL) in older adults: A systematic review. Med. J. Islam. Repub. Iran 2020, 34, 33. [Google Scholar] [CrossRef]
- Vellas, B.; Guigoz, Y.; Garry, P.J.; Nourhashemi, F.; Bennahum, D.; Lauque, S.; Albarede, J.L. The Mini Nutritional Assessment (MNA) and its use in grading the nutritional state of elderly patients. Nutrition 1999, 15, 116–122. [Google Scholar] [CrossRef]
- Loddo, S.; Salis, F.; Rundeddu, S.; Serchisu, L.; Peralta, M.M.; Mandas, A. Nutritional Status and Potentially Inappropriate Medications in Elderly. J. Clin. Med. 2022, 11, 3465. [Google Scholar] [CrossRef]
- Salis, F.; Locci, G.; Mura, B.; Mandas, A. Anemia in Elderly Patients—The Impact of Hemoglobin Cut-Off Levels on Geriatric Domains. Diagnostics 2023, 13, 191. [Google Scholar] [CrossRef]
- Åhlund, K.; Ekerstad, N.; Bäck, M.; Karlson, B.W.; Öberg, B. Preserved physical fitness is associated with lower 1-year mortality in frail elderly patients with a severe comorbidity burden. Clin. Interv. Aging 2019, 14, 577–586. [Google Scholar] [CrossRef]
- Hayashi, M.; Shimizu, W.; Albert, C.M. The spectrum of epidemiology underlying sudden cardiac death. Circ. Res. 2015, 116, 1887–1906. [Google Scholar] [CrossRef]
- Williams, G.R.; Deal, A.M.; Lund, J.L.; Chang, Y.; Muss, H.B.; Pergolotti, M.; Guerard, E.J.; Shachar, S.S.; Wang, Y.; Kenzik, K.; et al. Patient-Reported Comorbidity and Survival in Older Adults with Cancer. Oncologist 2018, 23, 433–439. [Google Scholar] [CrossRef]
- Salis, F.; Palimodde, A.; Demelas, G.; Scionis, M.I.; Mandas, A. Frailty and comorbidity burden in Atrial Fibrillation. Front. Public Health 2023, 11, 1134453. [Google Scholar] [CrossRef]
- Barceló, M.; Torres, O.H.; Mascaró, J.; Casademont, J. Hip fracture and mortality: Study of specific causes of death and risk factors. Arch. Osteoporos. 2021, 16, 15, Erratum in Arch. Osteoporos. 2021, 16, 53. [Google Scholar] [CrossRef]
- Kaegi-Braun, N.; Mueller, M.; Schuetz, P.; Mueller, B.; Kutz, A. Evaluation of Nutritional Support and In-Hospital Mortality in Patients with Malnutrition. JAMA Netw. Open 2021, 4, e2033433. [Google Scholar] [CrossRef]
- Serón-Arbeloa, C.; Labarta-Monzón, L.; Puzo-Foncillas, J.; Mallor-Bonet, T.; Lafita-López, A.; Bueno-Vidales, N.; Montoro-Huguet, M. Malnutrition Screening and Assessment. Nutrients 2022, 14, 2392. [Google Scholar] [CrossRef]
- Takeida, K.; Nishi, M.; Miyake, H. Mental depression and death in elderly persons. J. Epidemiol. 1997, 7, 210–213. [Google Scholar] [CrossRef]
- Kruschke, C.; Butcher, H.K. Evidence-Based Practice Guideline: Fall Prevention for Older Adults. J. Gerontol. Nurs. 2017, 43, 15–21. [Google Scholar] [CrossRef]
- Morris, R.; Lewis, A. Falls and Cancer. Clin. Oncol. (R Coll. Radiol.) 2020, 32, 569–578. [Google Scholar] [CrossRef]
- Karinkanta, S.; Piirtola, M.; Sievänen, H.; Uusi-Rasi, K.; Kannus, P. Physical therapy approaches to reduce fall and fracture risk among older adults. Nat. Rev. Endocrinol. 2010, 6, 396–407. [Google Scholar] [CrossRef]
- Khow, K.S.F.; Visvanathan, R. Falls in the Aging Population. Clin. Geriatr. Med. 2017, 33, 357–368. [Google Scholar] [CrossRef]
- Blain, H.; Miot, S.; Bernard, P.L. How Can We Prevent Falls? 2020 Aug 21. In Orthogeriatrics: The Management of Older Patients with Fragility Fractures, 2nd ed.; Falaschi, P., Marsh, D., Eds.; Springer: Cham, Switzerland, 2021; chapter 16. [Google Scholar]
- Ambrose, A.F.; Paul, G.; Hausdorff, J.M. Risk factors for falls among older adults: A review of the literature. Maturitas 2013, 75, 51–61. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).