Lower Extremity Muscle Activation during the Star Excursion Balance Test in Patients with Chronic Ankle Instability and Copers
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Instrumentation for Kinematics
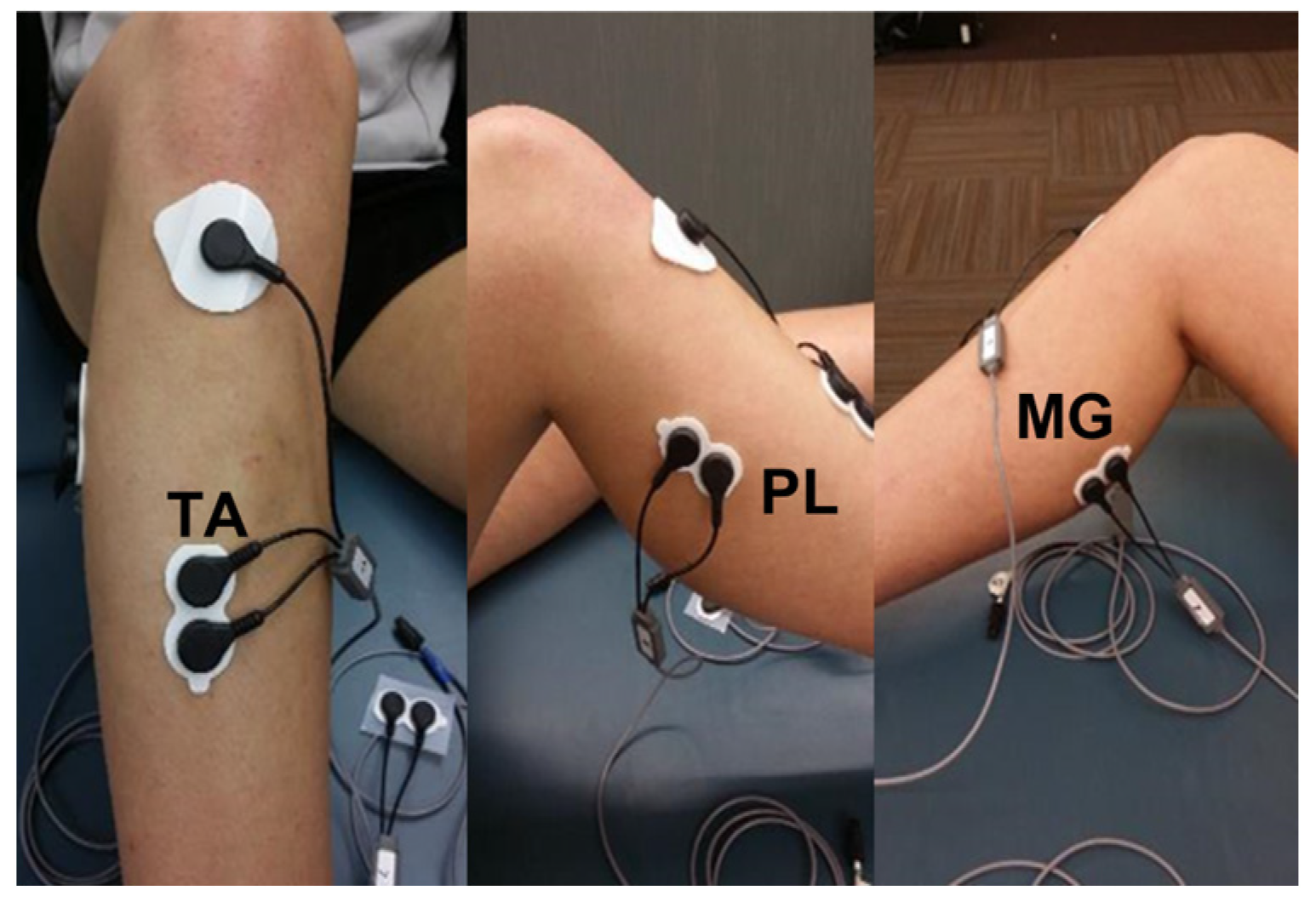
2.3. Instrumentation for Electromyography
2.4. Star Excursion Balance Test
2.5. Procedure
2.6. Statistics
3. Results
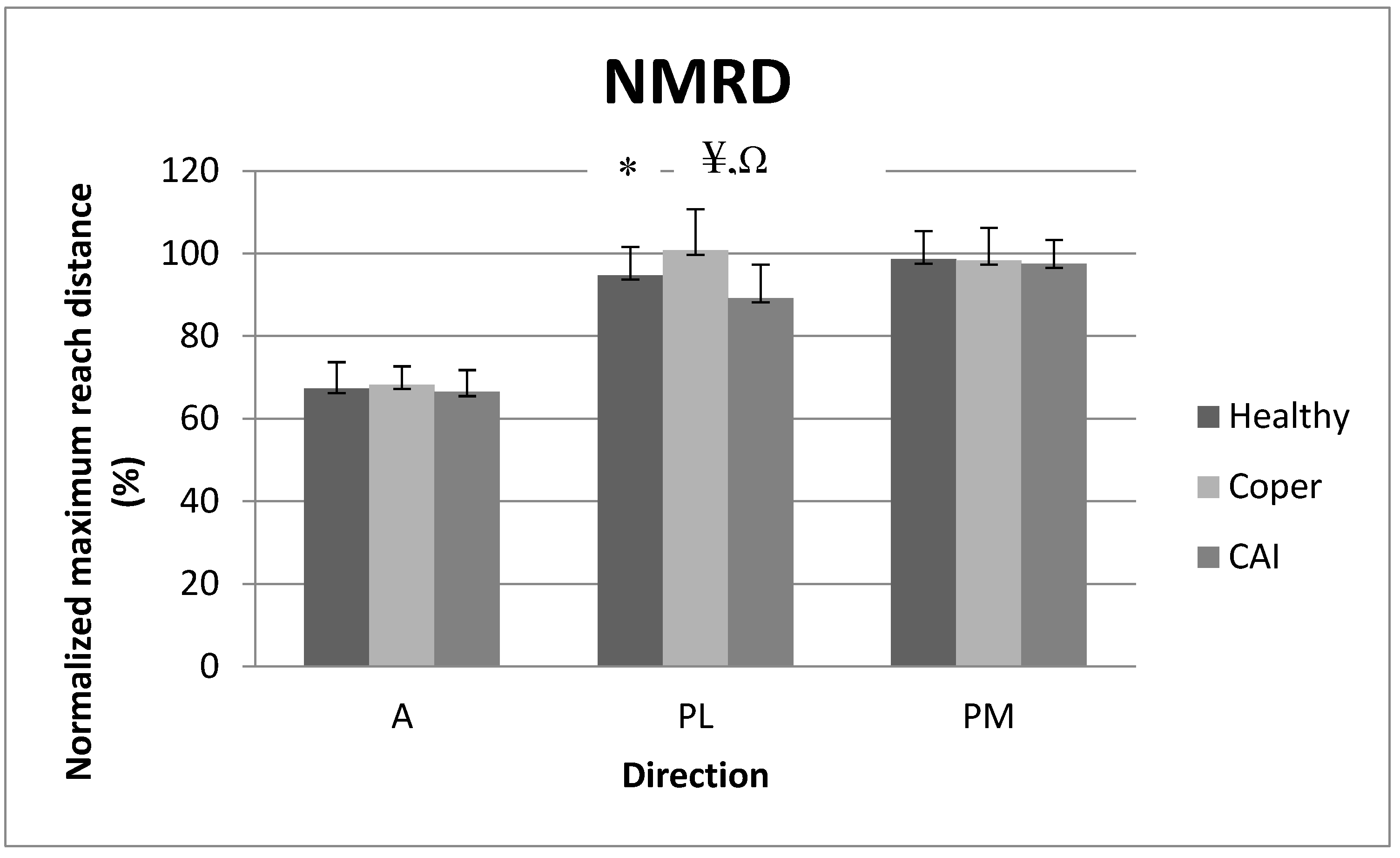
3.1. Normalized Maximum Reach Distance (NMRD)
3.2. Mean Amplitude of Tibialis Anterior (TA)
3.3. Mean Amplitude of Fibularis Longus (FL)
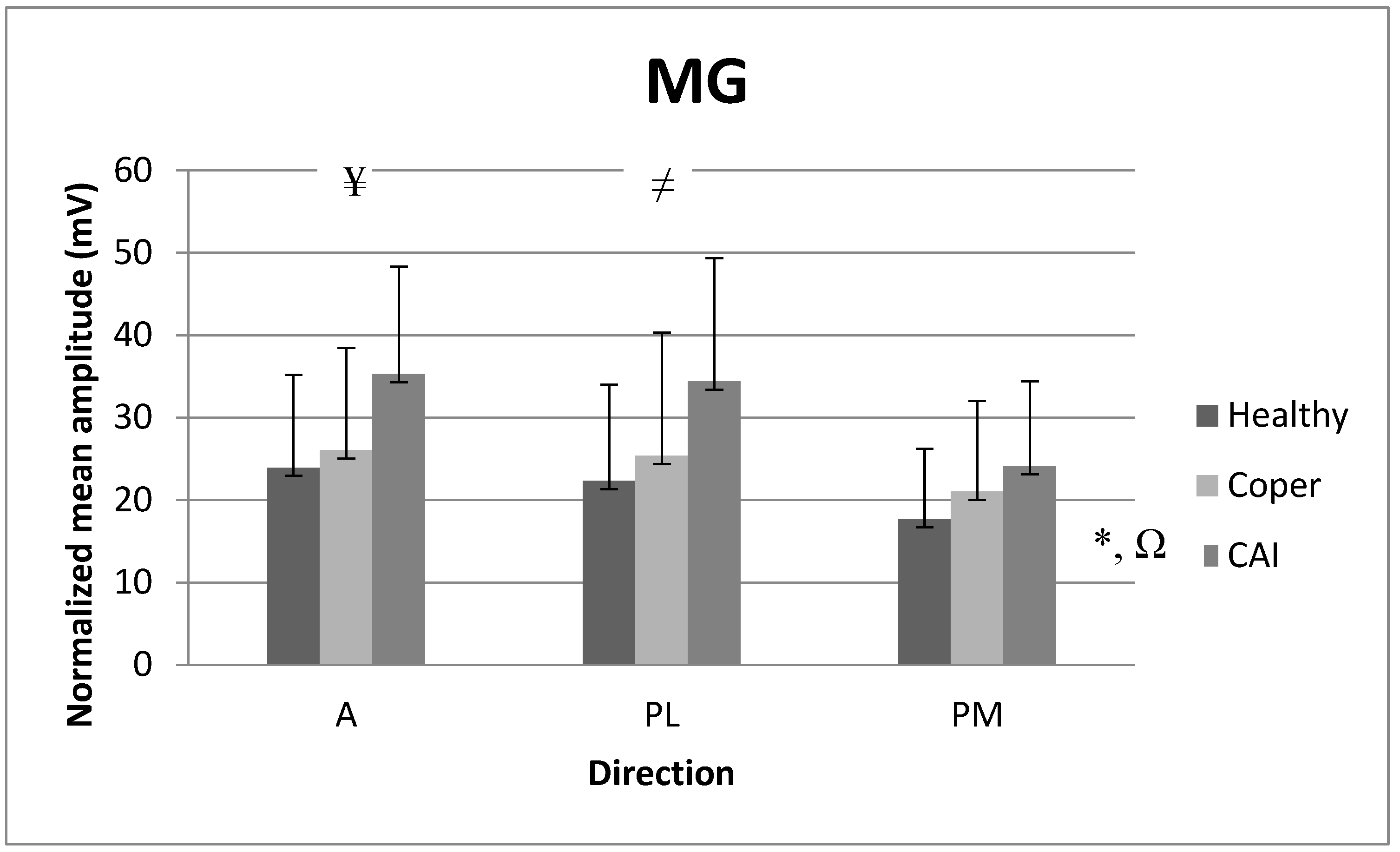
3.4. Mean Amplitude of Medial Gastrocnemius (MG)
4. Discussion
4.1. Reach Distance Analysis
4.2. EMG Analysis
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yeung, M.S.; Chan, K.M.; So, C.H.; Yuan, W.Y. An epidemiological survey on ankle sprain. Br. J. Sport. Med. 1994, 28, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Shakked, R.; Sheskier, S. Acute and Chronic Lateral Ankle Instability Diagnosis, Management, and New Concepts. Bull. Hosp. Jt. Dis. (2013) 2017, 75, 71–80. [Google Scholar] [PubMed]
- Al-Mohrej, O.A.; Al-Kenani, N.S. Chronic ankle instability: Current perspectives. Avicenna J. Med. 2016, 6, 103–108. [Google Scholar] [CrossRef]
- Hiller, C.E.; Kilbreath, S.L.; Refshauge, K.M. Chronic Ankle Instability: Evolution of the Model. J. Athl. Train. 2011, 46, 133–141. [Google Scholar] [CrossRef]
- Liu, W.; Jain, T.K.; Santos, M.; Heller, D.; Hiller, C. Important Issues Concerning Use of Term ‘Copers’ in Chronic Ankle Instability Research. Sport. Med. 2014, 44, 1775–1776. [Google Scholar] [CrossRef]
- Steib, S.; Hentschke, C.; Welsch, G.; Pfeifer, K.; Zech, A. Effects of fatiguing treadmill running on sensorimotor control in athletes with and without functional ankle instability. Clin. Biomech. 2013, 28, 790–795. [Google Scholar] [CrossRef] [PubMed]
- Steib, S.; Zech, A.; Hentschke, C.; Pfeifer, K. Fatigue-Induced Alterations of Static and Dynamic Postural Control in Athletes with a History of Ankle Sprain. J. Athl. Train. 2013, 48, 203–208. [Google Scholar] [CrossRef]
- Ferber, R.; Davis, I.M.; Williams, D.S.; Laughton, C. A comparison of within- and between-day reliability of discrete 3D lower extremity variables in runners. J. Orthop. Res. 2002, 20, 1139–1145. [Google Scholar] [CrossRef]
- Gabriner, M.L.; Houston, M.N.; Kirby, J.L.; Hoch, M.C. Contributing factors to Star Excursion Balance Test performance in individuals with chronic ankle instability. Gait Posture 2015, 41, 912–916. [Google Scholar] [CrossRef]
- Pozzi, F.; Moffat, M.; Gutierrez, G. Neuromuscular control during performance of a dynamic balance task in subjects with and without ankle instability. Int. J. Sport. Phys. Ther. 2015, 10, 520–529. [Google Scholar]
- Arnold, B.L.; Linens, S.W.; de la Motte, S.J.; Ross, S.E. Concentric Evertor Strength Differences and Functional Ankle Instability: A Meta-Analysis. J. Athl. Train. 2009, 44, 653–662. [Google Scholar] [CrossRef]
- Wikstrom, E.A.; Hubbard-Turner, T.; McKeon, P.O. Understanding and treating lateral ankle sprains and their consequences: A constraints-based approach. Sport. Med. 2013, 43, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Ty Hopkins, J.; Coglianese, M.; Glasgow, P.; Reese, S.; Seeley, M.K. Alterations in evertor/invertor muscle activation and center of pressure trajectory in participants with functional ankle instability. J. Electromyogr. Kinesiol. 2012, 22, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.; Ross, S.; Mynark, R.; Guskiewicz, K. Assessing Functional Ankle Instability with Joint Position Sense, Time to Stabilization, and Electromyography. J. Sport Rehabil. 2004, 13, 122–134. [Google Scholar] [CrossRef]
- Gribble, P.A.; Kelly, S.E.; Refshauge, K.M.; Hiller, C.E. Interrater reliability of the star excursion balance test. J. Athl. Train. 2013, 48, 621–626. [Google Scholar] [CrossRef]
- Hogan, K.K.; Powden, C.J.; Hoch, M.C. The influence of foot posture on dorsiflexion range of motion and postural control in those with chronic ankle instability. Clin. Biomech. 2016, 38, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.E.; Guskiewicz, K.M.; Gross, M.T.; Yu, B. Assessment tools for identifying functional limitations associated with functional ankle instability. J. Athl. Train. 2008, 43, 44–50. [Google Scholar] [CrossRef]
- Pionnier, R.; Découfour, N.; Barbier, F.; Popineau, C.; Simoneau-Buessinger, E. A new approach of the Star Excursion Balance Test to assess dynamic postural control in people complaining from chronic ankle instability. Gait Posture 2016, 45, 97–102. [Google Scholar] [CrossRef]
- Gribble, P.A.; Hertel, J.; Plisky, P. Using the Star Excursion Balance Test to Assess Dynamic Postural-Control Deficits and Outcomes in Lower Extremity Injury: A Literature and Systematic Review. J. Athl. Train. 2012, 47, 339–357. [Google Scholar] [CrossRef] [PubMed]
- Plante, J.E.; Wikstrom, E.A. Differences in clinician-oriented outcomes among controls, copers, and chronic ankle instability groups. Phys. Ther. Sport 2013, 14, 221–226. [Google Scholar] [CrossRef]
- Gribble, P.A.; Delahunt, E.; Bleakley, C.M.; Caulfield, B.; Docherty, C.L.; Fong, D.T.-P.; Fourchet, F.; Hertel, J.; Hiller, C.E.; Kaminski, T.W.; et al. Selection Criteria for Patients with Chronic Ankle Instability in Controlled Research: A Position Statement of the International Ankle Consortium. J. Athl. Train. 2014, 49, 121–127. [Google Scholar] [CrossRef]
- Wikstrom, E.A.; Brown, C.N. Minimum Reporting Standards for Copers in Chronic Ankle Instability Research. Sport. Med. 2014, 44, 251–268. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.J.; Arnold, B.L.; Ross, S.E.; Linens, S.W. Recalibration and Validation of the Cumberland Ankle Instability Tool Cutoff Score for Individuals with Chronic Ankle Instability. Arch. Phys. Med. Rehabil. 2014, 95, 1853–1859. [Google Scholar] [CrossRef] [PubMed]
- Aminaka, N.; Gribble, P.A. Patellar taping, patellofemoral pain syndrome, lower extremity kinematics, and dynamic postural control. J. Athl. Train. 2008, 43, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Gribble, P.A.; Hertel, J.; Denegar, C.R. Chronic Ankle Instability and Fatigue Create Proximal Joint Alterations during Performance of the Star Excursion Balance Test. Int. J. Sports Med. 2007, 28, 236–242. [Google Scholar] [CrossRef]
- Kwon, Y.U.; Blaise Williams, D.S., 3rd. The effect of variable rest intervals and chronic ankle instability on triplanar ankle motion during performance of the Star Excursion Balance Test. Hum. Mov. Sci. 2017, 52, 143–150. [Google Scholar] [CrossRef]
- Gribble, P.A.; Hertel, J. Considerations for Normalizing Measures of the Star Excursion Balance Test. Meas. Phys. Educ. Exerc. Sci. 2003, 7, 89–100. [Google Scholar] [CrossRef]
- Robinson, R.H.; Gribble, P.A. Support for a Reduction in the Number of Trials Needed for the Star Excursion Balance Test. Arch. Phys. Med. Rehabil. 2008, 89, 364–370. [Google Scholar] [CrossRef]
- Doherty, C.; Bleakley, C.; Hertel, J.; Caulfield, B.; Ryan, J.; Delahunt, E. Dynamic balance deficits in individuals with chronic ankle instability compared to ankle sprain copers 1 year after a first-time lateral ankle sprain injury. Knee Surg. Sport. Traumatol. Arthrosc. 2016, 24, 1086–1095. [Google Scholar] [CrossRef]
- Koldenhoven, R.M.; Feger, M.A.; Fraser, J.J.; Saliba, S.; Hertel, J. Surface electromyography and plantar pressure during walking in young adults with chronic ankle instability. Knee Surg. Sport. Traumatol. Arthrosc. 2016, 24, 1060–1070. [Google Scholar] [CrossRef]
- Willems, T.; Witvrouw, E.; Verstuyft, J.; Vaes, P.; De Clercq, D. Proprioception and Muscle Strength in Subjects With a History of Ankle Sprains and Chronic Instability. J. Athl. Train. 2002, 37, 487–493. [Google Scholar]
- Bowker, S.; Terada, M.; Thomas, A.C.; Pietrosimone, B.G.; Hiller, C.E.; Gribble, P.A. Neural Excitability and Joint Laxity in Chronic Ankle Instability, Coper, and Control Groups. J. Athl. Train. 2016, 51, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Hertel, J. Sensorimotor Deficits with Ankle Sprains and Chronic Ankle Instability. Clin. Sport. Med. 2008, 27, 353–370. [Google Scholar] [CrossRef]
- Dundas, M.A.; Gutierrez, G.M.; Pozzi, F. Neuromuscular control during stepping down in continuous gait in individuals with and without ankle instability. J. Sport. Sci. 2014, 32, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Delahunt, E.; Monaghan, K.; Caulfield, B. Altered Neuromuscular Control and Ankle Joint Kinematics during Walking in Subjects with Functional Instability of the Ankle Joint. Am. J. Sport. Med. 2006, 34, 1970–1976. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.; Padua, D.; Marshall, S.W.; Guskiewicz, K. Individuals with mechanical ankle instability exhibit different motion patterns than those with functional ankle instability and ankle sprain copers. Clin. Biomech. 2008, 23, 822–831. [Google Scholar] [CrossRef] [PubMed]
- Linens, S.W.; Ross, S.E.; Arnold, B.L.; Gayle, R.; Pidcoe, P. Postural-Stability Tests That Identify Individuals with Chronic Ankle Instability. J. Athl. Train. 2014, 49, 15–23. [Google Scholar] [CrossRef]
- Docherty, C.L.; Arnold, B.L.; Gansneder, B.M.; Hurwitz, S.; Gieck, J. Functional-Performance Deficits in Volunteers With Functional Ankle Instability. J. Athl. Train. 2005, 40, 30–34. [Google Scholar]
- Palmieri, R.M.; Ingersoll, C.D.; Stone, M.B.; Krause, B.A. Center-of-Pressure Parameters Used in the Assessment of Postural Control. J. Sport Rehabil. 2002, 11, 51–66. [Google Scholar] [CrossRef]
- Eltoukhy, M.; Kuenze, C.; Oh, J.; Wooten, S.; Signorile, J. Kinect-based assessment of lower limb kinematics and dynamic postural control during the star excursion balance test. Gait Posture 2017, 58, 421–427. [Google Scholar] [CrossRef]
- Hegedus, E.J.; McDonough, S.M.; Bleakley, C.; Baxter, D.; Cook, C.E. Clinician-friendly lower extremity physical performance tests in athletes: A systematic review of measurement properties and correlation with injury. Part 2—The tests for the hip, thigh, foot and ankle including the star excursion balance test. Br. J. Sport. Med. 2015, 49, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Jaber, H.; Lohman, E.; Daher, N.; Bains, G.; Nagaraj, A.; Mayekar, P.; Shanbhag, M.; Alameri, M. Neuromuscular control of ankle and hip during performance of the star excursion balance test in subjects with and without chronic ankle instability. PLoS ONE 2018, 13, e0201479. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, Y.U. Lower Extremity Muscle Activation during the Star Excursion Balance Test in Patients with Chronic Ankle Instability and Copers. Medicina 2023, 59, 1040. https://doi.org/10.3390/medicina59061040
Kwon YU. Lower Extremity Muscle Activation during the Star Excursion Balance Test in Patients with Chronic Ankle Instability and Copers. Medicina. 2023; 59(6):1040. https://doi.org/10.3390/medicina59061040
Chicago/Turabian StyleKwon, Yong Ung. 2023. "Lower Extremity Muscle Activation during the Star Excursion Balance Test in Patients with Chronic Ankle Instability and Copers" Medicina 59, no. 6: 1040. https://doi.org/10.3390/medicina59061040
APA StyleKwon, Y. U. (2023). Lower Extremity Muscle Activation during the Star Excursion Balance Test in Patients with Chronic Ankle Instability and Copers. Medicina, 59(6), 1040. https://doi.org/10.3390/medicina59061040




