New Insights into the Fluid Management in Patients with Septic Shock
Abstract
:1. Introduction
2. Methods
3. Fluid Resuscitation in Sepsis
4. Balanced Crystalloids versus Normal Saline in Sepsis and Septic Shock
4.1. Unbalanced Solutions
4.2. Balanced Solutions
4.3. Studies Comparing BSs versus NS in Sepsis and Septic Shock
5. Liberal versus Restricted Fluid Administration in Sepsis and Septic Shock
6. Conclusions and Future Directions
- Data on the optimal type (balanced crystalloids versus normal saline) and volume (liberal versus restricted administration) of fluids in sepsis and septic shock patients are still controversial and elusive.
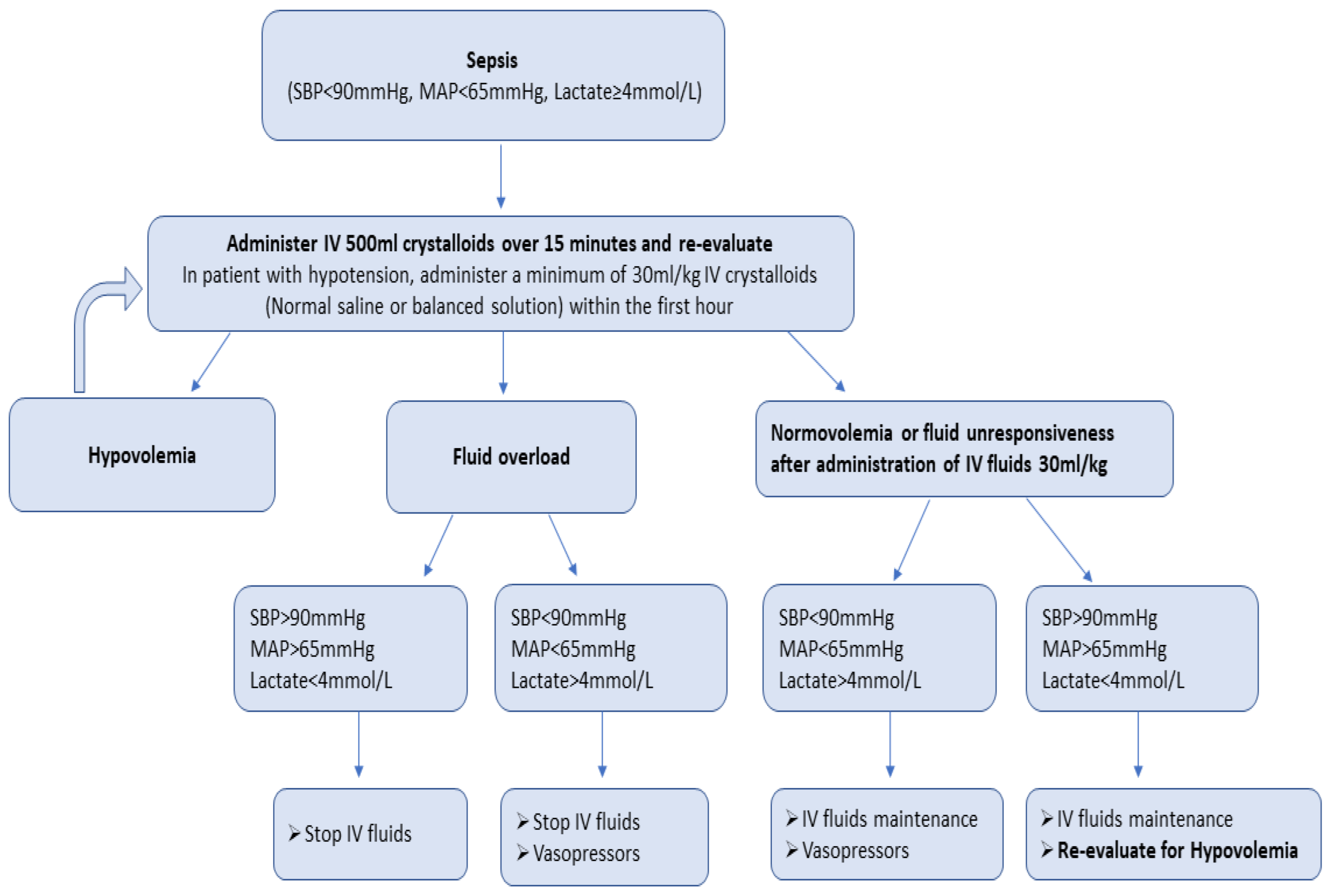
- Current SSC guidelines recommend the early administration of 30 mL/kg of IV crystalloid fluids for sepsis-related hypotension or a lactate ≥ 4 mmol/L, within the first 3 h of resuscitation. This is a weak recommendation and is based on low-quality evidence.
- Regarding the type of fluid administered during resuscitation, the majority of clinical trials demonstrated no significant difference between the balanced crystalloids and normal saline in the acute kidney injury and mortality.
- Excessive fluid administration during resuscitation can lead to worse outcomes in the septic patient.
- Fluid administration after initial resuscitation should be preferably guided by dynamic measures of fluid responsiveness.
- Fluid management in the critical patient can be optimized by the personalized, bedside, and dynamic assessment of macro- and microcirculation indices.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AKI | Acute kidney injury |
| ALBIOS | Albumin Italian Outcome Sepsis |
| APACHE | Acute Physiology and Chronic Health Evaluation |
| ARDS | Acute respiratory distress syndrome |
| BaSICS | Balanced Solution in Intensive Care Study |
| BS | Balanced Solution |
| CEUS | Contrast-enhanced ultrasound |
| CLASSIC | Conservative versus Liberal Approach to Fluid Therapy of Septic Shock in Intensive Care |
| CLOVERS | Crystalloid Liberal or Vasopressors Early Resuscitation in Sepsis |
| CO | Cardiac output |
| CRT | Capillary refill time |
| CVP | Central venous pressure |
| DAMPs | Danger-associated molecular patterns |
| ED | Emergency department |
| EGDT | Early goal-directed therapy |
| ESICM | European Society of Intensive Care Medicine |
| EVLW | Extravascular lung water |
| FEAST | Fluid Expansion as Supportive Therapy |
| FRESH | Fluid Responsiveness Evaluation in Sepsis-associated Hypotension |
| ICU | Intensive care unit |
| HES | Hydroxyethyl starch |
| HIV | Human immunodeficiency virus |
| HVM | Hand-held vital microscopes |
| IL-1β | Interleukin-1 beta |
| IL-6 | Interleukin-6 |
| IV | Intravenous |
| IVC | Inferior vena cava |
| JVP | Jugular venous pressure |
| MAP | Mean arterial pressure |
| MODS | Multiple Organ Dysfunction Score |
| MV | Mechanical ventilation |
| NS | Normal saline |
| PAMPs | Pathogen-associated molecular patterns |
| PLR | Passive leg raising |
| PLUS | Plasma-Lyte 148 versus Saline Study |
| PPV | Pulse pressure variation |
| ProCESS | Protocolized Care for Early Septic Shock |
| ProMISe | Protocolised Management in Sepsis |
| PRoMPT BOLUS | Pragmatic Pediatric trial of Balanced versus NS Fluid in sepsis |
| PRRs | Pattern recognition receptors |
| RCTs | Randomized controlled trials |
| ROSE | Resuscitation, optimization, stabilization, and evacuation |
| RR | Respiratory rate |
| RRT | Renal replacement therapy |
| SAFE | Saline versus Albumin Fluid Evaluation |
| SALT | Isotonic Solution Administration Logistical Testing |
| SAPS | Simplified Acute Physiology Score |
| ScvO2 | Central venous oxygen saturation |
| SIRS | Systemic inflammatory response syndrome |
| SMART | Isotonic Solutions and Major Adverse Renal Events Trial |
| SOC | Standard of care |
| SOFA | Sequential organ failure assessment |
| SPLIT | Saline vs. Plasma-Lyte 148 for ICU fluid Therapy |
| SpO2 | Oxygen saturation |
| SSC | Surviving Sepsis Campaign |
| SSSP | Simplified Severe Sepsis Protocol |
| SSSP-2 | Simplified Severe Sepsis Protocol 2 |
| SV | Stroke volume |
| SVR | Systemic vascular resistance |
| SVV | Stroke volume variation |
| TLRs | Toll-like receptors |
| TNF-a | Tumor necrosis factor alpha |
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensiv. Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, R.S.; Karl, I.E. The Pathophysiology and Treatment of Sepsis. N. Engl. J. Med. 2003, 348, 138–150. [Google Scholar] [CrossRef]
- Angus, D.C.; van der Poll, T. Severe Sepsis and Septic Shock. N. Engl. J. Med. 2013, 369, 840–851. [Google Scholar] [CrossRef]
- Cohen, J. The immunopathogenesis of sepsis. Nature 2002, 420, 885–891. [Google Scholar] [CrossRef]
- De Backer, D.; Cecconi, M.; Lipman, J.; Machado, F.; Myatra, S.N.; Ostermann, M.; Perner, A.; Teboul, J.-L.; Vincent, J.-L.; Walley, K.R. Challenges in the management of septic shock: A narrative review. Intensiv. Care Med. 2019, 45, 420–433. [Google Scholar] [CrossRef]
- Gavelli, F.; Castello, L.M.; Avanzi, G.C. Management of sepsis and septic shock in the emergency department. Intern. Emerg. Med. 2021, 16, 1649–1661. [Google Scholar] [CrossRef]
- Brown, R.M.; Semler, M.W. Fluid Management in Sepsis. J. Intensiv. Care Med. 2019, 34, 364–373. [Google Scholar] [CrossRef]
- Macdonald, S. Fluid Resuscitation in Patients Presenting with Sepsis: Current Insights. Open Access Emerg. Med. 2022, 14, 633–638. [Google Scholar] [CrossRef]
- Ladzinski, A.T.; Thind, G.S.; Siuba, M.T. Rational Fluid Resuscitation in Sepsis for the Hospitalist: A Narrative Review. Mayo Clin. Proc. 2021, 96, 2464–2473. [Google Scholar] [CrossRef]
- Monnet, X.; Teboul, J.-L. My patient has received fluid. How to assess its efficacy and side effects? Ann. Intensiv. Care 2018, 8, 54. [Google Scholar] [CrossRef]
- Marik, P.; Bellomo, R. A rational approach to fluid therapy in sepsis. Br. J. Anaesth. 2016, 116, 339–349. [Google Scholar] [CrossRef]
- Bissell, B.D.; Mefford, B. Pathophysiology of Volume Administration in Septic Shock and the Role of the Clinical Pharmacist. Ann. Pharmacother. 2020, 54, 388–396. [Google Scholar] [CrossRef]
- Ueyama, H.; Kiyonaka, S. Predicting the Need for Fluid Therapy—Does Fluid Responsiveness Work? J. Intensiv. Care 2017, 5, 34. [Google Scholar] [CrossRef]
- Marik, P.E.; Lemson, J. Fluid responsiveness: An evolution of our understanding. Br. J. Anaesth. 2014, 112, 617–620. [Google Scholar] [CrossRef]
- Malbrain, M.L.N.G.; Van Regenmortel, N.; Saugel, B.; De Tavernier, B.; Van Gaal, P.-J.; Joannes-Boyau, O.; Teboul, J.-L.; Rice, T.W.; Mythen, M.; Monnet, X. Principles of fluid management and stewardship in septic shock: It is time to consider the four D’s and the four phases of fluid therapy. Ann. Intensiv. Care 2018, 8, 66. [Google Scholar] [CrossRef]
- Lundy, D.J.; Trzeciak, S. Microcirculatory Dysfunction in Sepsis. Crit. Care Clin. 2009, 25, 721–731. [Google Scholar] [CrossRef]
- Marik, P.E.; Byrne, L.; van Haren, F. Fluid resuscitation in sepsis: The great 30 mL per kg hoax. J. Thorac. Dis. 2020, 12, S37–S47. [Google Scholar] [CrossRef]
- Byrne, L.; Van Haren, F. Fluid resuscitation in human sepsis: Time to rewrite history? Ann. Intensiv. Care 2017, 7, 4. [Google Scholar] [CrossRef]
- Byrne, L.; Byrne, L.; Obonyo, N.G.; Obonyo, N.G.; Diab, S.D.; Diab, S.D.; Dunster, K.R.; Dunster, K.R.; Passmore, M.R.; Passmore, M.R.; et al. Unintended Consequences: Fluid Resuscitation Worsens Shock in an Ovine Model of Endotoxemia. Am. J. Respir. Crit. Care Med. 2018, 198, 1043–1054. [Google Scholar] [CrossRef] [PubMed]
- Maeder, M.; Fehr, T.; Rickli, H.; Ammann, P. Sepsis-associated myocardial dysfunction: Diagnostic and prognostic impact of cardiac troponins and natriuretic peptides. Chest 2006, 129, 1349–1366. [Google Scholar] [CrossRef] [PubMed]
- Bessière, F.; Khenifer, S.; Dubourg, J.; Durieu, I.; Lega, J.-C. Prognostic value of troponins in sepsis: A meta-analysis. Intensiv. Care Med. 2013, 39, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Marik, P.E.; Cavallazzi, R. Does the Central Venous Pressure Predict Fluid Responsiveness? An Updated Meta-Analysis and a Plea for Some Common Sense. Crit. Care Med. 2013, 41, 1774–1781. [Google Scholar] [CrossRef]
- Leisman, D.; Doerfler, M.E.; Schneider, S.M.; Masick, K.D.; D’amore, J.A.; D’angelo, J.K. Predictors, Prevalence, and Outcomes of Early Crystalloid Responsiveness Among Initially Hypotensive Patients With Sepsis and Septic Shock. Crit. Care Med. 2018, 46, 189–198. [Google Scholar] [CrossRef]
- Rhodes, A.; Lamb, F.J.; Malagon, I.; Newman, P.J.; Grounds, R.M.; Bennett, E.D. A prospective study of the use of a dobutamine stress test to identify outcome in patients with sepsis, severe sepsis, or septic shock. Crit. Care Med. 1999, 27, 2361–2366. [Google Scholar] [CrossRef]
- Losser, M.-R.; Forget, A.-P.; Payen, D. Nitric oxide involvement in the hemodynamic response to fluid resuscitation in endotoxic shock in rats. Crit. Care Med. 2006, 34, 2426–2431. [Google Scholar] [CrossRef]
- García, M.I.M.; González, P.G.; Romero, M.G.; Gil Cano, A.; Oscier, C.; Rhodes, A.; Grounds, R.M.; Cecconi, M. Effects of fluid administration on arterial load in septic shock patients. Intensiv. Care Med. 2015, 41, 1247–1255. [Google Scholar] [CrossRef]
- Pohl, U.; De Wit, C.; Gloe, T. Large arterioles in the control of blood flow: Role of endothelium-dependent dilation. Acta Physiol. Scand. 2000, 168, 505–510. [Google Scholar] [CrossRef]
- Malbrain, M.L.; Marik, P.E.; Witters, I.; Cordemans, C.; Kirkpatrick, A.W.; Roberts, D.J.; Van Regenmortel, N. Fluid overload, de-resuscitation, and outcomes in critically ill or injured patients: A systematic review with suggestions for clinical practice. Anaesthesiol. Intensiv. Ther. 2014, 46, 361–380. [Google Scholar] [CrossRef]
- Samoni, S.; Vigo, V.; Reséndiz, L.I.B.; Villa, G.; De Rosa, S.; Nalesso, F.; Ferrari, F.; Meola, M.; Brendolan, A.; Malacarne, P.; et al. Impact of hyperhydration on the mortality risk in critically ill patients admitted in intensive care units: Comparison between bioelectrical impedance vector analysis and cumulative fluid balance recording. Crit. Care 2016, 20, 95. [Google Scholar] [CrossRef]
- Landry, D.W.; Oliver, J.A. The Pathogenesis of Vasodilatory Shock. N. Engl. J. Med. 2001, 345, 588–595. [Google Scholar] [CrossRef]
- Sadaka, F.; Juarez, M.; Naydenov, S.; O’brien, J. Fluid resuscitation in septic shock: The effect of increasing fluid balance on mortality. J. Intensiv. Care Med. 2013, 29, 213–217. [Google Scholar] [CrossRef]
- Edwards, M.R.; Mythen, M.G. Fluid therapy in critical illness. Extrem. Physiol. Med. 2014, 3, 16. [Google Scholar] [CrossRef]
- Uchimido, R.; Schmidt, E.P.; Shapiro, N.I. The glycocalyx: A novel diagnostic and therapeutic target in sepsis. Crit. Care 2019, 23, 16. [Google Scholar] [CrossRef]
- Chappell, D.; Bruegger, D.; Potzel, J.; Jacob, M.; Brettner, F.; Vogeser, M.; Conzen, P.; Becker, B.F.; Rehm, M. Hypervolemia increases release of atrial natriuretic peptide and shedding of the endothelial glycocalyx. Crit. Care 2014, 18, 538. [Google Scholar] [CrossRef]
- Jacob, M.; Saller, T.; Chappell, D.; Rehm, M.; Welsch, U.; Becker, B.F. Physiological levels of A-, B- and C-type natriuretic peptide shed the endothelial glycocalyx and enhance vascular permeability. Basic Res. Cardiol. 2013, 108, 347. [Google Scholar] [CrossRef]
- Bihari, S.; Prakash, S.; Bersten, A.D. Post resusicitation fluid boluses in severe sepsis or septic shock: Prevalence and efficacy (price study). Shock 2013, 40, 28–34. [Google Scholar] [CrossRef]
- Glassford, N.J.; Eastwood, G.M.; Bellomo, R. Physiological changes after fluid bolus therapy in sepsis: A systematic review of contemporary data. Crit. Care 2014, 18, 696. [Google Scholar] [CrossRef]
- Woodcock, T.E.; Woodcock, T.M. Revised Starling equation and the glycocalyx model of transvascular fluid exchange: An improved paradigm for prescribing intravenous fluid therapy. Br. J. Anaesth. 2012, 108, 384–394. [Google Scholar] [CrossRef]
- Van Regenmortel, N.; Verbrugghe, W.; Roelant, E.; Wyngaert, T.V.D.; Jorens, P.G. Maintenance fluid therapy and fluid creep impose more significant fluid, sodium, and chloride burdens than resuscitation fluids in critically ill patients: A retrospective study in a tertiary mixed ICU population. Intensiv. Care Med. 2018, 44, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Van Regenmortel, N.; Hendrickx, S.; Roelant, E.; Baar, I.; Dams, K.; Van Vlimmeren, K.; Embrecht, B.; Wittock, A.; Hendriks, J.M.; Lauwers, P.; et al. 154 compared to 54 mmol per liter of sodium in intravenous maintenance fluid therapy for adult patients undergoing major thoracic surgery (TOPMAST): A single-center randomized controlled double-blind trial. Intensiv. Care Med. 2019, 45, 1422–1432. [Google Scholar] [CrossRef] [PubMed]
- Dalfino, L.; Tullo, L.; Donadio, I.; Malcangi, V.; Brienza, N. Intra-abdominal hypertensionand acute renal failurein critically ill patients. Intensiv. Care Med. 2008, 34, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Diebel, L.N.; Wilson, R.F.; Dulchavsky, S.A.; Saxe, J. Effect of Increased Intra-Abdominal Pressure on Hepatic Arterial, Portal Venous, and Hepatic Microcirculatory Blood Flow. J. Trauma 1992, 33, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Scheeren, T.W.L.; Bakker, J.; De Backer, D.; Annane, D.; Asfar, P.; Boerma, E.C.; Cecconi, M.; Dubin, A.; Dünser, M.W.; Duranteau, J.; et al. Current use of vasopressors in septic shock. Ann. Intensiv. Care 2019, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.M.; Evans, L.E.; Rhodes, A. The Surviving Sepsis Campaign Bundle: 2018 update. Intensiv. Care Med. 2018, 44, 925–928. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, V.; Evans, L. Implementation of the Surviving Sepsis Campaign guidelines. Curr. Opin. Crit. Care 2017, 23, 412–416. [Google Scholar] [CrossRef]
- Cosnett, J. The Origins of Intravenous Fluid Therapy. Lancet 1989, 333, 768–771. [Google Scholar] [CrossRef]
- Daniels, R. Surviving the first hours in sepsis: Getting the basics right (an intensivist’s perspective). J. Antimicrob. Chemother. 2011, 66 (Suppl. 2), ii11–ii23. [Google Scholar] [CrossRef]
- Rivers, E.; Nguyen, B.; Havstad, S.; Ressler, J.; Muzzin, A.; Knoblich, B.; Peterson, E.; Tomlanovich, M. Early Goal-Directed Therapy in the Treatment of Severe Sepsis and Septic Shock. N. Engl. J. Med. 2001, 345, 1368–1377. [Google Scholar] [CrossRef]
- Angus, D.C.; Barnato, A.E.; Bell, D.; Bellomo, R.; Chong, C.-R.; Coats, T.J.; Davies, A.; Delaney, A.; Harrison, D.A.; Holdgate, A.; et al. A systematic review and meta-analysis of early goal-directed therapy for septic shock: The ARISE, ProCESS and ProMISe Investigators. Intensiv. Care Med. 2015, 41, 1549–1560. [Google Scholar] [CrossRef]
- Ehrman, R.R.; Gallien, J.Z.; Smith, R.K.; Akers, K.; Malik, A.N.; Harrison, N.; Welch, R.D.; Levy, P.D.; Sherwin, R.L. Resuscitation Guided by Volume Responsiveness Does Not Reduce Mortality in Sepsis: A Meta-Analysis. Crit. Care Explor. 2019, 1, e0015. [Google Scholar] [CrossRef]
- Smith, S.H.; Perner, A. Higher vs. lower fluid volume for septic shock: Clinical characteristics and outcome in unselected patients in a prospective, multicenter cohort. Crit. Care 2012, 16, R76. [Google Scholar] [CrossRef]
- McIntyre, L.; Rowe, B.H.; Walsh, T.S.; Gray, A.; Arabi, Y.; Perner, A.; Gordon, A.; Marshall, J.; Cook, D.; Fox-Robichaud, A.; et al. Multicountry survey of emergency and critical care medicine physicians’ fluid resuscitation practices for adult patients with early septic shock. BMJ Open 2016, 6, e010041. [Google Scholar] [CrossRef]
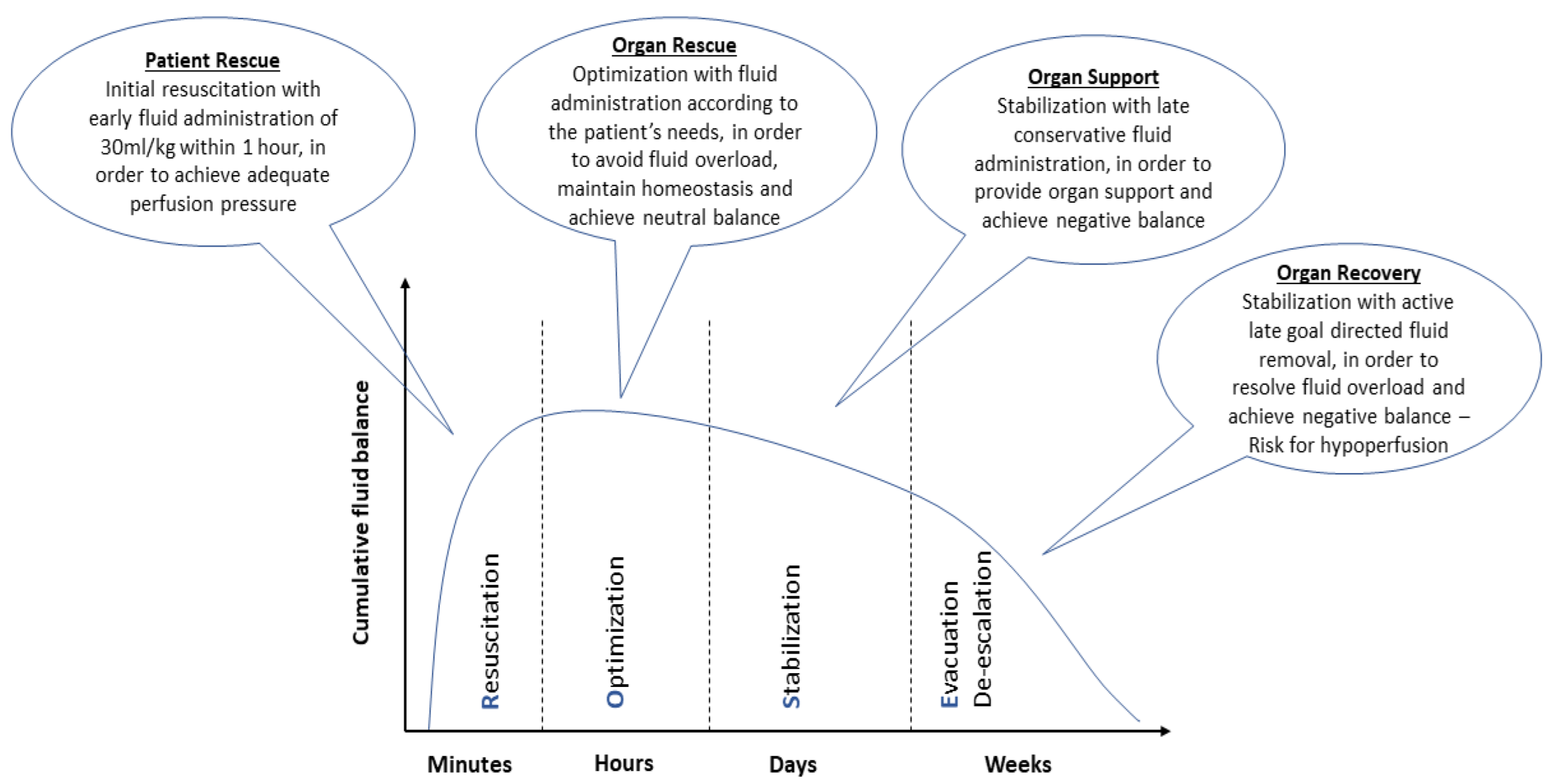
- Hoste, E.A.; Maitland, K.; Brudney, C.S.; Mehta, R.; Vincent, J.-L.; Yates, D.; Kellum, J.A.; Mythen, M.G.; Shaw, A.D. Four phases of intravenous fluid therapy: A conceptual model. Br. J. Anaesth. 2014, 113, 740–747. [Google Scholar] [CrossRef]
- Malbrain, M.L.N.G.; Van Regenmortel, N.; Owczuk, R. It is time to consider the four D’s of fluid management. Anaesthesiol. Intensiv. Ther. 2015, 47, s1–s5. [Google Scholar] [CrossRef]
- Brunkhorst, F.M.; Engel, C.; Bloos, F.; Meier-Hellmann, A.; Ragaller, M.; Weiler, N.; Moerer, O.; Gruendling, M.; Oppert, M.; Grond, S.; et al. Intensive Insulin Therapy and Pentastarch Resuscitation in Severe Sepsis. N. Engl. J. Med. 2008, 358, 125–139. [Google Scholar] [CrossRef]
- Guidet, B.; Martinet, O.; Boulain, T.; Philippart, F.; Poussel, J.; Maizel, J.; Forceville, X.; Feissel, M.; Hasselmann, M.; Heininger, A.; et al. Assessment of hemodynamic efficacy and safety of 6% hydroxyethylstarch 130/0.4 vs. 0.9% NaCl fluid replacement in patients with severe sepsis: The CRYSTMAS study. Crit. Care 2012, 16, R94. [Google Scholar] [CrossRef]
- Perner, A.; Haase, N.; Guttormsen, A.B.; Tenhunen, J.; Klemenzson, G.; Åneman, A.; Madsen, K.R.; Møller, M.H.; Elkjær, J.M.; Poulsen, L.M.; et al. Hydroxyethyl Starch 130/0.42 versus Ringer’s Acetate in Severe Sepsis. N. Engl. J. Med. 2012, 367, 124–134. [Google Scholar] [CrossRef]
- Myburgh, J.A.; Finfer, S.; Bellomo, R.; Billot, L.; Cass, A.; Gattas, D.; Glass, P.; Lipman, J.; Liu, B.; McArthur, C.; et al. Hydroxyethyl Starch or Saline for Fluid Resuscitation in Intensive Care. N. Engl. J. Med. 2012, 367, 1901–1911. [Google Scholar] [CrossRef]
- Zarychanski, R.; Abou-Setta, A.M.; Turgeon, A.F.; Houston, B.; McIntyre, L.; Marshall, J.C.; Fergusson, D. Association of Hydroxyethyl Starch Administration With Mortality and Acute Kidney Injury in Critically Ill Patients Requiring Volume Resuscitation: A systematic review and meta-analysis. JAMA 2013, 309, 678–688. [Google Scholar] [CrossRef] [PubMed]
- Moeller, C.; Fleischmann, C.; Thomas-Rueddel, D.; Vlasakov, V.; Rochwerg, B.; Theurer, P.; Gattinoni, L.; Reinhart, K.; Hartog, C.S. How safe is gelatin? A systematic review and meta-analysis of gelatin-containing plasma expanders vs crystalloids and albumin. J. Crit. Care 2016, 35, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Finfer, S.; McEvoy, S.; Bellomo, R.; McArthur, C.; Myburgh, J.; Norton, R. Impact of albumin compared to saline on organ function and mortality of patients with severe sepsis. Intensiv. Care Med. 2011, 37, 86–96. [Google Scholar] [CrossRef]
- Caironi, P.; Tognoni, G.; Masson, S.; Fumagalli, R.; Pesenti, A.; Romero, M.; Fanizza, C.; Caspani, L.; Faenza, S.; Grasselli, G.; et al. Albumin Replacement in Patients with Severe Sepsis or Septic Shock. N. Engl. J. Med. 2014, 370, 1412–1421. [Google Scholar] [CrossRef]
- Xu, J.-Y.; Chen, Q.-H.; Xie, J.-F.; Pan, C.; Liu, S.-Q.; Huang, L.-W.; Yang, C.-S.; Liu, L.; Huang, Y.-Z.; Guo, F.-M.; et al. Comparison of the effects of albumin and crystalloid on mortality in adult patients with severe sepsis and septic shock: A meta-analysis of randomized clinical trials. Crit. Care 2014, 18, 702. [Google Scholar] [CrossRef] [PubMed]
- Chien, S.-C.; Chen, C.-Y.; Lin, C.-F.; Yeh, H.-I. Critical appraisal of the role of serum albumin in cardiovascular disease. Biomark. Res. 2017, 5, 31. [Google Scholar] [CrossRef]
- Casey, J.D.; Brown, R.M.; Semler, M.W. Resuscitation fluids. Curr. Opin. Crit. Care 2018, 24, 512–518. [Google Scholar] [CrossRef]
- Winters, M.E.; Sherwin, R.; Vilke, G.M.; Wardi, G. What is the Preferred Resuscitation Fluid for Patients with Severe Sepsis and Septic Shock? J. Emerg. Med. 2017, 53, 928–939. [Google Scholar] [CrossRef]
- Seifter, J.L. Integration of Acid–Base and Electrolyte Disorders. N. Engl. J. Med. 2015, 372, 389–392. [Google Scholar] [CrossRef]
- Gruartmoner, G.; Mesquida, J.; Ince, C. Fluid therapy and the hypovolemic microcirculation. Curr. Opin. Crit. Care 2015, 21, 276–284. [Google Scholar] [CrossRef]
- Kozek-Langenecker, S.A. Fluids and coagulation. Curr. Opin. Crit. Care 2015, 21, 285–291. [Google Scholar] [CrossRef]
- Funke, B.E.; Jackson, K.E.; Self, W.H.; Collins, S.P.; Saunders, C.T.; Wang, L.; Blume, J.D.; Wickersham, N.; Brown, R.M.; Casey, J.D.; et al. Effect of balanced crystalloids versus saline on urinary biomarkers of acute kidney injury in critically ill adults. BMC Nephrol. 2021, 22, 54. [Google Scholar] [CrossRef]
- Zhou, F.; Peng, Z.-Y.; Bishop, J.V.B.; Cove, M.E.; Singbartl, K.; Kellum, J.A.M. Effects of Fluid Resuscitation With 0.9% Saline Versus a Balanced Electrolyte Solution on Acute Kidney Injury in a Rat Model of Sepsis. Crit. Care Med. 2014, 42, e270–e278. [Google Scholar] [CrossRef]
- Jaynes, M.P.; Murphy, C.V.; Ali, N.; Krautwater, A.; Lehman, A.; Doepker, B.A. Association between chloride content of intravenous fluids and acute kidney injury in critically ill medical patients with sepsis. J. Crit. Care 2018, 44, 363–367. [Google Scholar] [CrossRef]
- Myburgh, J.A.; Mythen, M.G. Resuscitation fluids. N. Engl. J. Med. 2013, 369, 1243–1251. [Google Scholar] [CrossRef]
- Morgan, T.J. The ideal crystalloid what is ‘balanced’? Curr. Opin. Crit. Care 2013, 19, 299–307. [Google Scholar] [CrossRef]
- Singh, S.; Kerndt, C.C.; Davis, D. Ringer’s Lactate. In Statpearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Rizoli, S. PlasmaLyte. J. Trauma 2011, 70, S17–S18. [Google Scholar] [CrossRef]
- Duffy, R.A.; Foroozesh, M.B.; Loflin, R.D.; Ie, S.R.; Icard, B.L.; Tegge, A.N.; Nogueira, J.R.; Kuehl, D.R.; Smith, D.C.; Loschner, A.L. Normal saline versus Normosol™-R in sepsis resuscitation: A retrospective cohort study. J. Intensiv. Care Soc. 2019, 20, 223–230. [Google Scholar] [CrossRef]
- Young, P.; Bailey, M.; Beasley, R.; Henderson, S.; Mackle, D.; McArthur, C.; McGuinness, S.; Mehrtens, J.; Myburgh, J.; Psirides, A.; et al. Effect of a Buffered Crystalloid Solution vs Saline on Acute Kidney Injury Among Patients in the Intensive Care Unit: The SPLIT Randomized Clinical Trial. JAMA 2015, 314, 1701–1710. [Google Scholar] [CrossRef]
- Semler, M.W.; Wanderer, J.P.; Ehrenfeld, J.M.; Stollings, J.L.; Self, W.H.; Siew, E.D.; Wang, L.; Byrne, D.W.; Shaw, A.D.; Bernard, G.R.; et al. Balanced Crystalloids versus Saline in the Intensive Care Unit. The SALT Randomized Trial. Am. J. Respir. Crit. Care Med. 2017, 195, 1362–1372. [Google Scholar] [CrossRef]
- Semler, M.W.; Self, W.H.; Wanderer, J.P.; Ehrenfeld, J.M.; Wang, L.; Byrne, D.W.; Stollings, J.L.; Kumar, A.B.; Hughes, C.G.; Hernandez, A.; et al. Balanced Crystalloids versus Saline in Critically Ill Adults. N. Engl. J. Med. 2018, 378, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.M.; Wang, L.; Coston, T.D.; Krishnan, N.I.; Casey, J.D.; Wanderer, J.P.; Ehrenfeld, J.M.; Byrne, D.W.; Stollings, J.L.; Siew, E.D.; et al. Balanced Crystalloids versus Saline in Sepsis. A Secondary Analysis of the SMART Clinical Trial. Am. J. Respir. Crit. Care Med. 2019, 200, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Zampieri, F.G.; Machado, F.R.; Biondi, R.S.; Freitas, F.G.R.; Veiga, V.C.; Figueiredo, R.C.; Lovato, W.J.; Amêndola, C.P.; Serpa-Neto, A.; Paranhos, J.L.R.; et al. Effect of Intravenous Fluid Treatment With a Balanced Solution vs 0.9% Saline Solution on Mortality in Critically Ill Patients: The BaSICS Randomized Clinical Trial. JAMA 2021, 326, 818–829. [Google Scholar] [CrossRef] [PubMed]
- Zampieri, F.G.; Machado, F.R.; Biondi, R.S.; Freitas, F.G.R.; Veiga, V.C.; Figueiredo, R.C.; Lovato, W.J.; Amêndola, C.P.; Serpa-Neto, A.; Paranhos, J.L.R.; et al. Association between Type of Fluid Received Prior to Enrollment, Type of Admission, and Effect of Balanced Crystalloid in Critically Ill Adults: A Secondary Exploratory Analysis of the BaSICS Clinical Trial. Am. J. Respir. Crit. Care Med. 2022, 205, 1419–1428. [Google Scholar] [CrossRef] [PubMed]
- Finfer, S.; Micallef, S.; Hammond, N.; Navarra, L.; Bellomo, R.; Billot, L.; Delaney, A.; Gallagher, M.; Gattas, D.; Li, Q.; et al. Balanced Multielectrolyte Solution versus Saline in Critically Ill Adults. N. Engl. J. Med. 2022, 386, 815–826. [Google Scholar] [CrossRef] [PubMed]
- Limapichat, T.; Pattanapong, K. Normal Saline Solution or Lactated Ringer’s Solution to Enhance Lactate Clearance in Septic Patients After Initial Resuscitation in the ED: A Retrospective Cohort Trial. Open Access Emerg. Med. 2021, 13, 511–519. [Google Scholar] [CrossRef]
- Raghunathan, K.; Shaw, A.; Nathanson, B.; Stürmer, T.; Brookhart, A.; Stefan, M.; Setoguchi, S.; Beadles, C.; Lindenauer, P.K. Association Between the Choice of IV Crystalloid and In-Hospital Mortality Among Critically Ill Adults With Sepsis. Crit. Care Med. 2014, 42, 1585–1591. [Google Scholar] [CrossRef]
- Corrêa, T.D.; Cavalcanti, A.B.; De Assunção, M.S.C. Balanced crystalloids for septic shock resuscitation. Rev. Bras. Ter. Intensiv. 2016, 28, 463–471. [Google Scholar] [CrossRef]
- Schindler, A.W.; Marx, G. Evidence-based fluid management in the ICU. Curr. Opin. Anaesthesiol. 2016, 29, 158–165. [Google Scholar] [CrossRef]
- Rochwerg, B.; Alhazzani, W.; Gibson, A.; Ribic, C.M.; Sindi, A.; Heels-Ansdell, D.; Thabane, L.; Fox-Robichaud, A.; Mbuagbaw, L.; Szczeklik, W.; et al. Fluid type and the use of renal replacement therapy in sepsis: A systematic review and network meta-analysis. Intensiv. Care Med. 2015, 41, 1561–1571. [Google Scholar] [CrossRef]
- Xue, M.; Zhang, X.; Liu, F.; Chang, W.; Xie, J.; Xu, J.; Yang, Y.; Qiu, H. Effects of chloride content of intravenous crystalloid solutions in critically ill adult patients: A meta-analysis with trial sequential analysis of randomized trials. Ann. Intensiv. Care 2019, 9, 30. [Google Scholar] [CrossRef]
- Zhu, Y.; Guo, N.M.; Song, M.M.; Xia, F.M.; Wu, Y.; Wang, X.M.; Chen, T.M.; Yang, Z.M.; Yang, S.M.; Zhang, Y.M.; et al. Balanced crystalloids versus saline in critically ill patients: The PRISMA study of a meta-analysis. Medicine 2021, 100, e27203. [Google Scholar] [CrossRef]
- Hammond, D.A.; Lam, S.W.; Rech, M.A.; Smith, M.N.; Westrick, J.; Trivedi, A.P.; Balk, R.A. Balanced Crystalloids Versus Saline in Critically Ill Adults: A Systematic Review and Meta-analysis. Ann. Pharmacother. 2020, 54, 5–13. [Google Scholar] [CrossRef]
- Beran, A.; Altorok, N.; Srour, O.; Malhas, S.-E.; Khokher, W.; Mhanna, M.; Ayesh, H.; Aladamat, N.; Abuhelwa, Z.; Srour, K.; et al. Balanced Crystalloids versus Normal Saline in Adults with Sepsis: A Comprehensive Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 1971. [Google Scholar] [CrossRef]
- De Castro, R.E.V.; Medeiros, D.N.M.; Prata-Barbosa, A.; de Magalhães-Barbosa, M.C. Surviving Sepsis Campaign International Guidelines for the Management of Septic Shock and Sepsis-Associated Organ Dysfunction in Children. Pediatr. Crit. Care Med. 2020, 21, 924–925. [Google Scholar] [CrossRef]
- Weiss, S.L.; Balamuth, F.; Long, E.; Thompson, G.C.; Hayes, K.L.; Katcoff, H.; Cook, M.; Tsemberis, E.; Hickey, C.P.; Williams, A.; et al. PRagMatic Pediatric Trial of Balanced vs nOrmaL Saline FlUid in Sepsis: Study protocol for the PRoMPT BOLUS randomized interventional trial. Trials 2021, 22, 776. [Google Scholar] [CrossRef]
- Boyd, J.H.; Forbes, J.; Nakada, T.-A.; Walley, K.R.; Russell, J.A. Fluid resuscitation in septic shock: A positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit. Care Med. 2011, 39, 259–265. [Google Scholar] [CrossRef]
- Wiedemann, H.P.; Wheeler, A.P.; Bernard, G.R.; Thompson, B.T.; Hayden, D.; Deboisblanc, B.; Connors, A.F.J.; Hite, R.D.; Harabin, A.L. Comparison of Two Fluid-Management Strategies in Acute Lung Injury. N. Engl. J. Med. 2006, 354, 2564–2575. [Google Scholar] [CrossRef]
- Prowle, J.R.; Kirwan, C.J.; Bellomo, R. Fluid management for the prevention and attenuation of acute kidney injury. Nat. Rev. Nephrol. 2014, 10, 37–47. [Google Scholar] [CrossRef]
- Peake, S.L.; Delaney, A.; Bailey, M.; Bellomo, R.; Cameron, P.A.; Cooper, D.J.; Higgins, A.M.; Holdgate, A.; Howe, B.D.; Webb, S.A.; et al. Goal-directed resuscitation for patients with early septic shock. N. Engl. J. Med. 2014, 371, 1496–1506. [Google Scholar] [CrossRef]
- Yealy, D.M.; Kellum, J.A.; Huang, D.T.; Barnato, A.E.; Weissfeld, L.A.; Pike, F.; Terndrup, T.; Wang, H.E.; Hou, P.C.; LoVecchio, F.; et al. A randomized trial of protocol-based care for early septic shock. N. Engl. J. Med. 2014, 370, 1683–1693. [Google Scholar] [CrossRef]
- Mouncey, P.R.; Osborn, T.M.; Power, G.S.; Harrison, D.A.; Sadique, M.Z.; Grieve, R.D.; Jahan, R.; Harvey, S.E.; Bell, D.; Bion, J.F.; et al. Trial of Early, Goal-Directed Resuscitation for Septic Shock. N. Engl. J. Med. 2015, 372, 1301–1311. [Google Scholar] [CrossRef]
- Semler, M.W.; Rice, T.W. Sepsis Resuscitation: Fluid Choice and Dose. Clin. Chest Med. 2016, 37, 241–250. [Google Scholar] [CrossRef]
- Maitland, K.; Kiguli, S.; Opoka, R.O.; Engoru, C.; Olupot-Olupot, P.; Akech, S.O.; Nyeko, R.; Mtove, G.; Reyburn, H.; Lang, T.; et al. Mortality after Fluid Bolus in African Children with Severe Infection. N. Engl. J. Med. 2011, 364, 2483–2495. [Google Scholar] [CrossRef]
- Andrews, B.; Muchemwa, L.; Kelly, P.; Lakhi, S.; Heimburger, D.C.; Bernard, G.R. Simplified Severe Sepsis Protocol: A randomized controlled trial of modified early goal-directed therapy in Zambia. Crit. Care Med. 2014, 42, 2315–2324. [Google Scholar] [CrossRef]
- Andrews, B.; Semler, M.W.; Muchemwa, L.; Kelly, P.; Lakhi, S.; Heimburger, D.C.; Mabula, C.; Bwalya, M.; Bernard, G.R. Effect of an Early Resuscitation Protocol on In-hospital Mortality Among Adults With Sepsis and Hypotension: A Randomized Clinical Trial. JAMA 2017, 318, 1233–1240. [Google Scholar] [CrossRef]
- Tigabu, B.M.; Davari, M.; Kebriaeezadeh, A.; Mojtahedzadeh, M. Fluid volume, fluid balance and patient outcome in severe sepsis and septic shock: A systematic review. J. Crit. Care 2018, 48, 153–159. [Google Scholar] [CrossRef]
- Lewin, J.; Maconochie, I. Capillary refill time in adults. Emerg. Med. J. 2008, 25, 325–326. [Google Scholar] [CrossRef]
- La Via, L.; Sanfilippo, F.; Continella, C.; Triolo, T.; Messina, A.; Robba, C.; Astuto, M.; Hernandez, G.; Noto, A. Agreement between Capillary Refill Time measured at Finger and Earlobe sites in different positions: A pilot prospective study on healthy volunteers. BMC Anesthesiol. 2023, 23, 30. [Google Scholar] [CrossRef]
- Hernández, G.; Ospina-Tascón, G.A.; Damiani, L.P.; Estenssoro, E.; Dubin, A.; Hurtado, J.; Friedman, G.; Castro, R.; Alegría, L.; Teboul, J.-L.; et al. Effect of a Resuscitation Strategy Targeting Peripheral Perfusion Status vs Serum Lactate Levels on 28-Day Mortality Among Patients with Septic Shock: The Andromeda-Shock Randomized Clinical Trial. JAMA 2019, 321, 654–664. [Google Scholar] [CrossRef]
- Kattan, E.; Bakker, J.; Estenssoro, E.; Ospina-Tascón, G.A.; Cavalcanti, A.B.; De Backer, D.; Vieillard-Baron, A.; Teboul, J.-L.; Castro, R.; Hernández, G. Hemodynamic phenotype-based, capillary refill time-targeted resuscitation in early septic shock: The ANDROMEDA-SHOCK-2 Randomized Clinical Trial study protocol. Rev. Bras. Ter. Intensiv. 2022, 34, 96–106. [Google Scholar] [CrossRef]
- Meyhoff, T.S.; Hjortrup, P.B.; Wetterslev, J.; Sivapalan, P.; Laake, J.H.; Cronhjort, M.; Jakob, S.M.; Cecconi, M.; Nalos, M.; Ostermann, M.; et al. Restriction of Intravenous Fluid in ICU Patients with Septic Shock. N. Engl. J. Med. 2022, 386, 2459–2470. [Google Scholar] [CrossRef]
- Shapiro, N.I.; Douglas, I.S.; Brower, R.G.; Brown, S.M.; Exline, M.C.; Ginde, A.A.; Gong, M.N.; Grissom, C.K.; Hayden, D.; Hough, C.I.; et al. Early Restrictive or Liberal Fluid Management for Sepsis-Induced Hypotension. N. Engl. J. Med. 2023, 388, 499–510. [Google Scholar] [CrossRef]
- Reynolds, P.M.; Stefanos, S.; MacLaren, R. Restrictive resuscitation in patients with sepsis and mortality: A systematic review and meta-analysis with trial sequential analysis. Pharmacotherapy 2023, 43, 104–114. [Google Scholar] [CrossRef]
- Silversides, J.A.; Major, E.; Ferguson, A.J.; Mann, E.E.; McAuley, D.F.; Marshall, J.C.; Blackwood, B.; Fan, E. Conservative fluid management or deresuscitation for patients with sepsis or acute respiratory distress syndrome following the resuscitation phase of critical illness: A systematic review and meta-analysis. Intensiv. Care Med. 2017, 43, 155–170. [Google Scholar] [CrossRef]
- Michard, F.; Teboul, J.-L. Predicting Fluid Responsiveness in ICU Patients: A critical analysis of the evidence. Chest 2002, 121, 2000–2008. [Google Scholar] [CrossRef]
- Cherpanath, T.G.V.; Geerts, B.F.; Lagrand, W.K.; Schultz, M.J.; Groeneveld, A.B.J. Basic concepts of fluid responsiveness. Neth. Hear. J. 2013, 21, 530–536. [Google Scholar] [CrossRef]
- Monnet, X.; Lai, C.; Teboul, J.-L. How I personalize fluid therapy in septic shock? Crit. Care 2023, 27, 123. [Google Scholar] [CrossRef]
- Sevransky, J.E. Dynamic Measures to Determine Volume Responsiveness: Logical, Biologically Plausible, and Unproven. Crit. Care Med. 2016, 44, 1923–1926. [Google Scholar] [CrossRef]
- Monnet, X.; Marik, P.; Teboul, J.-L. Passive leg raising for predicting fluid responsiveness: A systematic review and meta-analysis. Intensiv. Care Med. 2016, 42, 1935–1947. [Google Scholar] [CrossRef]
- Douglas, I.S.; Alapat, P.M.; Corl, K.; Exline, M.C.; Forni, L.G.; Holder, A.L.; Kaufman, D.A.; Khan, A.; Levy, M.M.; Martin, G.S.; et al. Fluid Response Evaluation in Sepsis Hypotension and Shock: A Randomized Clinical Trial. Chest 2020, 158, 1431–1445. [Google Scholar] [CrossRef] [PubMed]
- Keren, H.; Burkhoff, D.; Squara, P. Evaluation of a noninvasive continuous cardiac output monitoring system based on thoracic bioreactance. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H583–H589. [Google Scholar] [CrossRef] [PubMed]
- Raval, N.Y.; Squara, P.; Cleman, M.; Yalamanchili, K.; Winklmaier, M.; Burkhoff, D. Multicenter Evaluation of Noninvasive Cardiac Output Measurement by Bioreactance Technique. J. Clin. Monit. Comput. 2008, 22, 113–119. [Google Scholar] [CrossRef]
- Michard, F.; Boussat, S.; Chemla, D.; Anguel, N.; Mercat, A.; Lecarpentier, Y.; Richard, C.; Pinsky, M.R.; Teboul, J.-L. Relation between Respiratory Changes in Arterial Pulse Pressure and Fluid Responsiveness in Septic Patients with Acute Circulatory Failure. Am. J. Respir. Crit. Care Med. 2000, 162, 134–138. [Google Scholar] [CrossRef]
- Marik, P.E.; Cavallazzi, R.; Vasu, T.; Hirani, A. Dynamic changes in arterial waveform derived variables and fluid responsiveness in mechanically ventilated patients: A systematic review of the literature. Crit. Care Med. 2009, 37, 2642–2647. [Google Scholar] [CrossRef]
- Jozwiak, M.; Monnet, X.; Teboul, J.-L. Pressure Waveform Analysis. Anesth. Analg. 2018, 126, 1930–1933. [Google Scholar] [CrossRef]
- Marik, P.E.; Monnet, X.; Teboul, J.-L. Hemodynamic parameters to guide fluid therapy. Ann. Intensiv. Care 2011, 1, 1. [Google Scholar] [CrossRef]
- Monnet, X.; Marik, P.E.; Teboul, J.-L. Prediction of fluid responsiveness: An update. Ann. Intensiv. Care 2016, 6, 111. [Google Scholar] [CrossRef]
- Vignon, P.; Repessé, X.; Bégot, E.; Léger, J.; Jacob, C.; Bouferrache, K.; Slama, M.; Prat, G.; Vieillard-Baron, A. Comparison of Echocardiographic Indices Used to Predict Fluid Responsiveness in Ventilated Patients. Am. J. Respir. Crit. Care Med. 2017, 195, 1022–1032. [Google Scholar] [CrossRef]
- La Via, L.; Astuto, M.; Dezio, V.; Muscarà, L.; Palella, S.; Zawadka, M.; Vignon, P.; Sanfilippo, F. Agreement between subcostal and transhepatic longitudinal imaging of the inferior vena cava for the evaluation of fluid responsiveness: A systematic review. J. Crit. Care 2022, 71, 154108. [Google Scholar] [CrossRef]
- Monnet, X.; Shi, R.; Teboul, J.-L. Prediction of fluid responsiveness. What’s new? Ann. Intensiv. Care 2022, 12, 46. [Google Scholar] [CrossRef] [PubMed]
- Jozwiak, M.; Teboul, J.-L.; Monnet, X. Extravascular lung water in critical care: Recent advances and clinical applications. Ann. Intensiv. Care 2015, 5, 38. [Google Scholar] [CrossRef]
- Bednarczyk, J.M.; Fridfinnson, J.A.; Kumar, A.; Blanchard, L.; Rabbani, R.; Bell, D.; Funk, D.; Turgeon, A.F.; Abou-Setta, A.M.; Zarychanski, R. Incorporating Dynamic Assessment of Fluid Responsiveness into Goal-Directed Therapy: A Systematic Review and Meta-Analysis. Crit. Care Med. 2017, 45, 1538–1545. [Google Scholar] [CrossRef]
- Richard, J.-C.; Bayle, F.; Bourdin, G.; Leray, V.; Debord, S.; Delannoy, B.; Stoian, A.C.; Wallet, F.; Yonis, H.; Guerin, C. Preload dependence indices to titrate volume expansion during septic shock: A randomized controlled trial. Crit. Care 2015, 19, 5. [Google Scholar] [CrossRef]
- Ince, C.; Boerma, E.C.; Cecconi, M.; De Backer, D.; Shapiro, N.I.; Duranteau, J.; Pinsky, M.R.; Artigas, A.; Teboul, J.-L.; Reiss, I.K.M.; et al. Second consensus on the assessment of sublingual microcirculation in critically ill patients: Results from a task force of the European Society of Intensive Care Medicine. Intensive Care Med. 2018, 44, 281–299. [Google Scholar] [CrossRef] [PubMed]
- Edul, V.K.; Gutierrez, F.J. Devices for assessing microcirculation. Curr. Opin. Crit. Care 2023, 29, 236–243. [Google Scholar] [CrossRef]
- Schneider, A.G.; Goodwin, M.D.; Schelleman, A.; Bailey, M.; Johnson, L.; Bellomo, R. Contrast-enhanced ultrasonography to evaluate changes in renal cortical microcirculation induced by noradrenaline: A pilot study. Crit. Care 2014, 18, 653. [Google Scholar] [CrossRef]
- Lima, A.M.; van Rooij, T.; Ergin, B.; Sorelli, M.M.; Ince, Y.M.; Specht, P.A.C.B.; Mik, E.G.M.; Bocchi, L.; Kooiman, K.; de Jong, N.; et al. Dynamic Contrast-Enhanced Ultrasound Identifies Microcirculatory Alterations in Sepsis-Induced Acute Kidney Injury. Crit. Care Med. 2018, 46, 1284–1292. [Google Scholar] [CrossRef]
- Mongkolpun, W.; Gardette, M.; Orbegozo, D.; Vincent, J.-L.; Creteur, J. An increase in skin blood flow induced by fluid challenge is associated with an increase in oxygen consumption in patients with circulatory shock. J. Crit. Care 2022, 69, 153984. [Google Scholar] [CrossRef] [PubMed]
- Mongkolpun, W.; Orbegozo, D.; Cordeiro, C.P.R.; Franco, C.J.C.S.; Vincent, J.-L.M.; Creteur, J. Alterations in Skin Blood Flow at the Fingertip Are Related to Mortality in Patients With Circulatory Shock. Crit. Care Med. 2020, 48, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Duranteau, J.; De Backer, D.; Donadello, K.; Shapiro, N.I.; Hutchings, S.D.; Rovas, A.; Legrand, M.; Harrois, A.; Ince, C. The future of intensive care: The study of the microcirculation will help to guide our therapies. Crit. Care 2023, 27, 190. [Google Scholar] [CrossRef] [PubMed]
| Balanced Solutions versus Normal Saline in Sepsis | ||||||
|---|---|---|---|---|---|---|
| Study ID | Year | Sample Size | Population | Intervention | Comparison | Outcome |
| SPLIT [64] | 2015 | 2262 | ICU patients | Plasma-Lyte148 (median volume 2000 mL) N = 1152 | NS (median volume 2000 mL) N = 1110 | No significant difference in the AKI and mortality within 90 days. |
| SALT [65] | 2017 | 974 | ICU adult patients | BS (median volume 1617 mL) N = 520 | NS (median volume 1424 mL) N = 454 | No significant difference in the AKI and mortality within 30 days. More major kidney events in the NS group. |
| SMART [66] | 2019 | 1641 | ICU adult patients | BS Plasma-Lyte A and Lactated Ringer’s (mean volume 2967 mL) N = 824 | NS (mean volume 3454 mL) N = 817 | Lower incidence of mortality and major adverse kidney events within 30 days in the BS group. Greater number of vasopressor-free days and renal replacement therapy-free days in the BS group. |
| BaSICS [69,70] | 2021 | 10,520 | ICU adult patients | BS Plasma-Lyte (median volume 1500 mL) N = 5230 | NS (median volume 1500 mL) N = 5290 | No significant difference in the AKI and mortality within 90 days. Higher 90-day survival in the subgroup of septic patients receiving balanced crystalloids. |
| PLUS [71] | 2022 | 5037 | ICU adult patients | Plasma-Lyte 148 (median volume 3900 mL) N = 2515 | NS (median volume 3700 mL) N = 2522 | No significant difference in the AKI and mortality within 90 days. |
| PRoMPTBOLUS [72] | Ongoing | Estimated size: 8800 | Pediatric patients with sepsis | BS | NS | In progress |
| Liberal versus Restricted Fluid Administration in Sepsis | ||||||
|---|---|---|---|---|---|---|
| Study ID | Year | Sample Size | Population | Intervention | Comparison | Outcome |
| EGDT [40] | 2001 | 263 | Adults with sepsis in the ED | Early goal-directed therapy: CVP ≥ 8–12 mmHg, MAP ≥ 65 mmHg, urine ≥ 0.5 mL/kg/h, ScvO2 ≥ 70% N = 130 | SOC: CVP ≥ 8–12 mmHg, MAP ≥ 65 mmHg, urine ≥ 0.5 mL/kg/h N = 133 | Significantly lower in-hospital mortality, APACHE II, SAPS II, and MODS in the EGDT group. Patients in EGDT group received more initial fluids, blood transfusions and inotropic support. |
| FEAST [91] | 2011 | 3141 | Children with febrile illness and impaired perfusion | Albumin bolus group N = 1050 Saline bolus group N = 1047 | No bolus group N = 1044 | Recruitment was halted due to higher 48 h mortality in the intervention arms, and also, higher 4-week mortality in the bolus groups. |
| ARISE [87] | 2014 | 1588 | Adults with early septic shock in the ED | EGDT N = 796 | SOC N = 792 | No difference in 90-day mortality. More patients in the EGDT group received vasopressors, but no other significant differences were observed. |
| ProCESS [88] | 2014 | 902 | Adults in the ED with SIRS and refractory hypotension or hyperlactemia | EGDT N = 456 | SOC N = 446 | No difference in 60-day, 90-day, 1-year mortality, or need for organ support |
| ProMISe [89] | 2015 | 1260 | Adults >6 h in the ED with infection, refractory hypotension or hyperlactemia | EGDT N = 630 | SOC N = 630 | No difference in 90-daysmortality. Significantly higher cardiovascular support and length of ICU stay in the EGDT group. |
| SSSP-2 [93] | 2017 | 212 | Adults in ED with suspected sepsis and hypotension | Fluid administration guided by SpO2, RR, and JVP (total up to 4 L) N = 107 | Usual care N = 105 | Intervention arm received more fluids and vasopressors. Higher in-hospital, 28-day mortality and worsening hypoxemia in the intervention group. |
| FRESH [104] | 2020 | 124 | Adults with sepsis-associated hypotension in ED | Assessment of fluid responsiveness before fluid administration PLR test, SV change ≥ 10% N = 83 | Usual care N = 41 | Similar volume of resuscitation fluids and ICU length of stay in the two arms. Significantly less positive fluid balance, RRT, and MV in the intervention group. |
| CLASSIC [95] | 2022 | 1554 | Adults with septic shock in ICU | Restrictive fluid group Fluids guided by lac, MAP, urine output, mottling, losses, dehydration, and electrolyte disturbances N = 770 | Liberal fluids according to SOC N = 784 | No difference in 90-day mortality or serious adverse events between the two group. |
| CLOVERS [96] | 2023 | 1563 | Adults with infection and refractory hypotension | Restrictive fluid group Vasopressor prioritization Only “rescue fluids” for prespecified indications N = 782 | Liberal fluids according to SOC N = 781 | No difference in mortality before discharge home by day 90 between the two arms. Similar frequency of adverse events. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moschopoulos, C.D.; Dimopoulou, D.; Dimopoulou, A.; Dimopoulou, K.; Protopapas, K.; Zavras, N.; Tsiodras, S.; Kotanidou, A.; Fragkou, P.C. New Insights into the Fluid Management in Patients with Septic Shock. Medicina 2023, 59, 1047. https://doi.org/10.3390/medicina59061047
Moschopoulos CD, Dimopoulou D, Dimopoulou A, Dimopoulou K, Protopapas K, Zavras N, Tsiodras S, Kotanidou A, Fragkou PC. New Insights into the Fluid Management in Patients with Septic Shock. Medicina. 2023; 59(6):1047. https://doi.org/10.3390/medicina59061047
Chicago/Turabian StyleMoschopoulos, Charalampos D., Dimitra Dimopoulou, Anastasia Dimopoulou, Konstantina Dimopoulou, Konstantinos Protopapas, Nikolaos Zavras, Sotirios Tsiodras, Anastasia Kotanidou, and Paraskevi C. Fragkou. 2023. "New Insights into the Fluid Management in Patients with Septic Shock" Medicina 59, no. 6: 1047. https://doi.org/10.3390/medicina59061047
APA StyleMoschopoulos, C. D., Dimopoulou, D., Dimopoulou, A., Dimopoulou, K., Protopapas, K., Zavras, N., Tsiodras, S., Kotanidou, A., & Fragkou, P. C. (2023). New Insights into the Fluid Management in Patients with Septic Shock. Medicina, 59(6), 1047. https://doi.org/10.3390/medicina59061047










