Effects of Intravitreal Ranibizumab Injection on Peripheral Retinal Microcirculation and Cytokines in Branch Retinal Vein Occlusion with Macular Edema
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Routine Evaluations
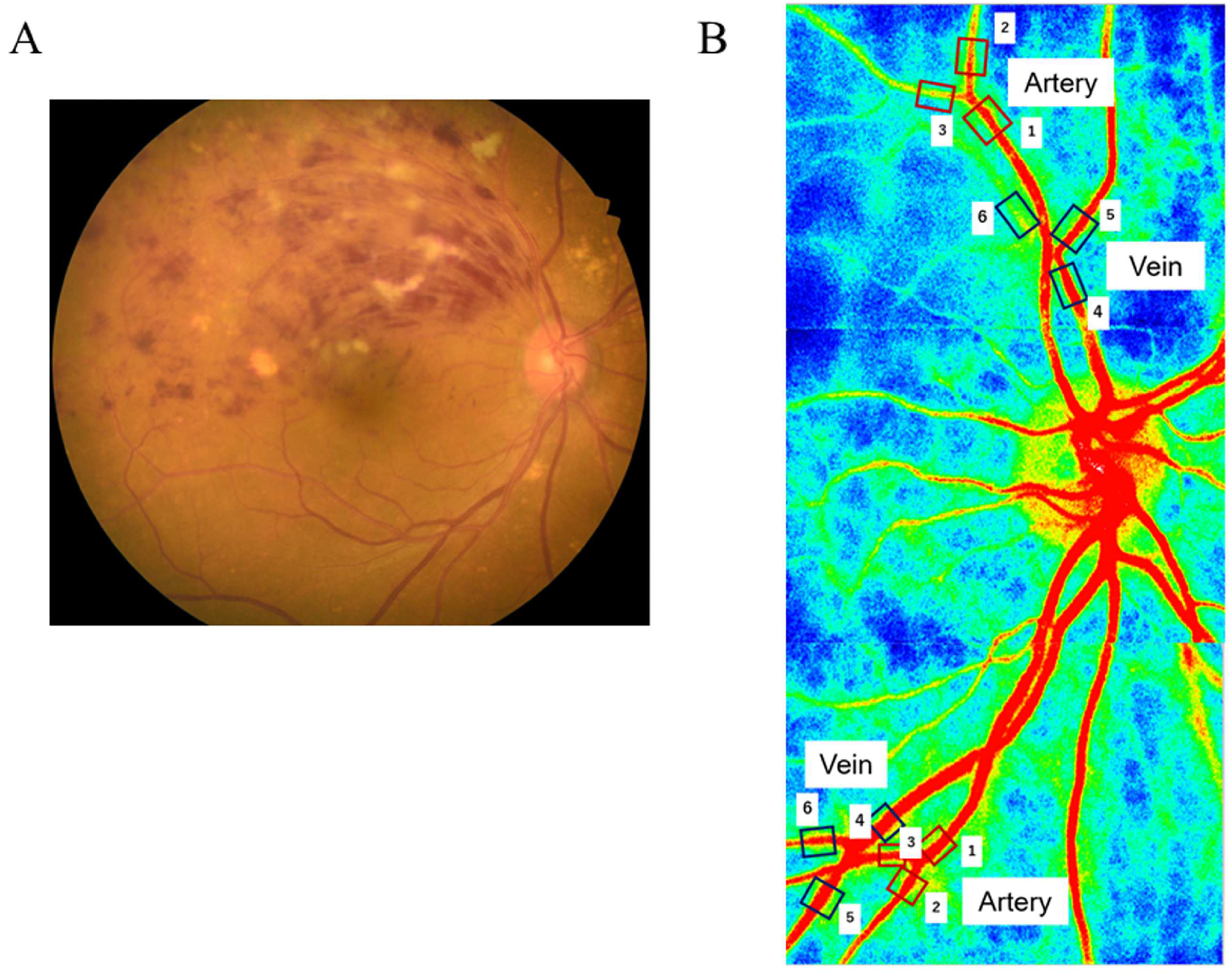
2.3. LSFG
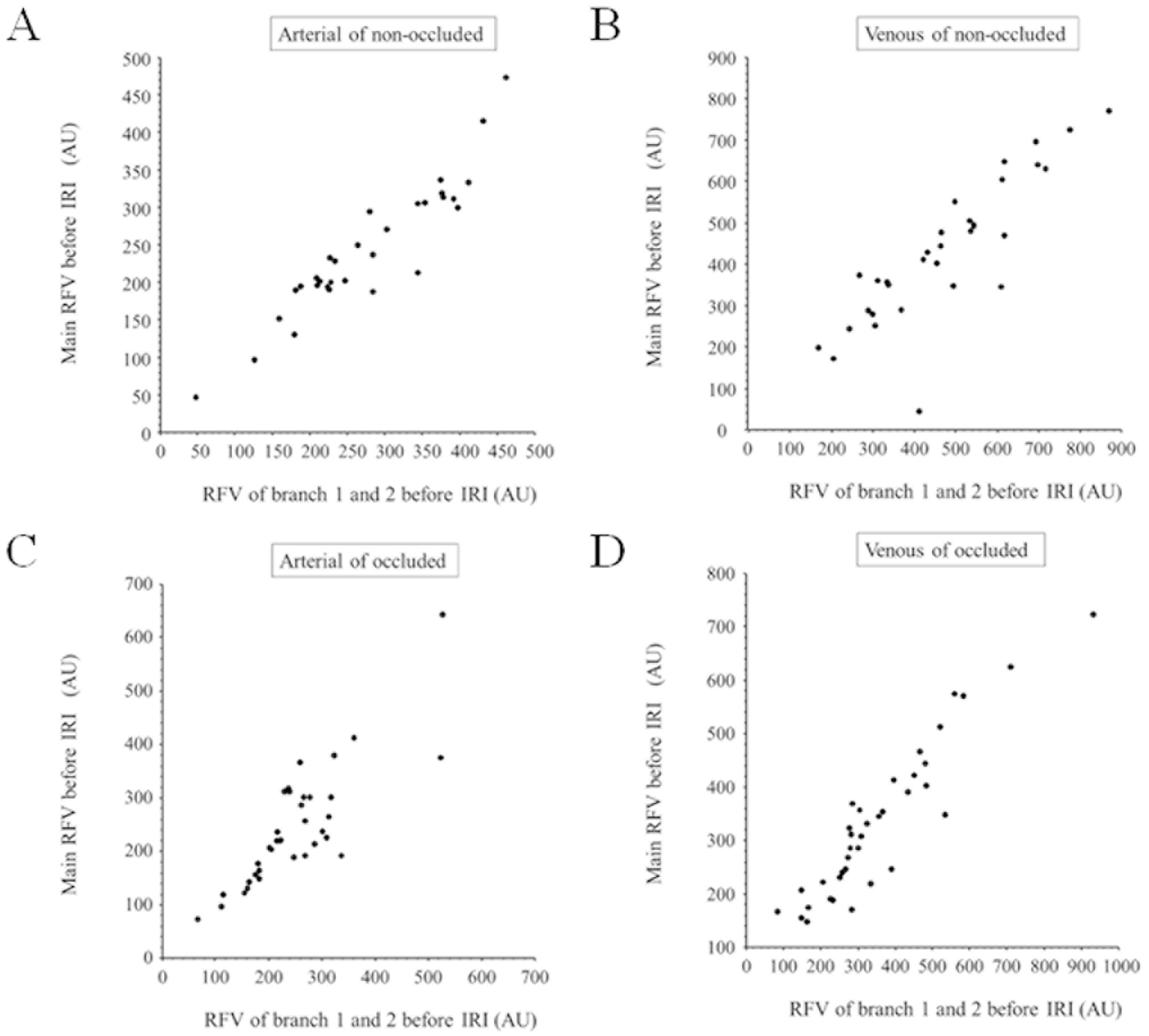
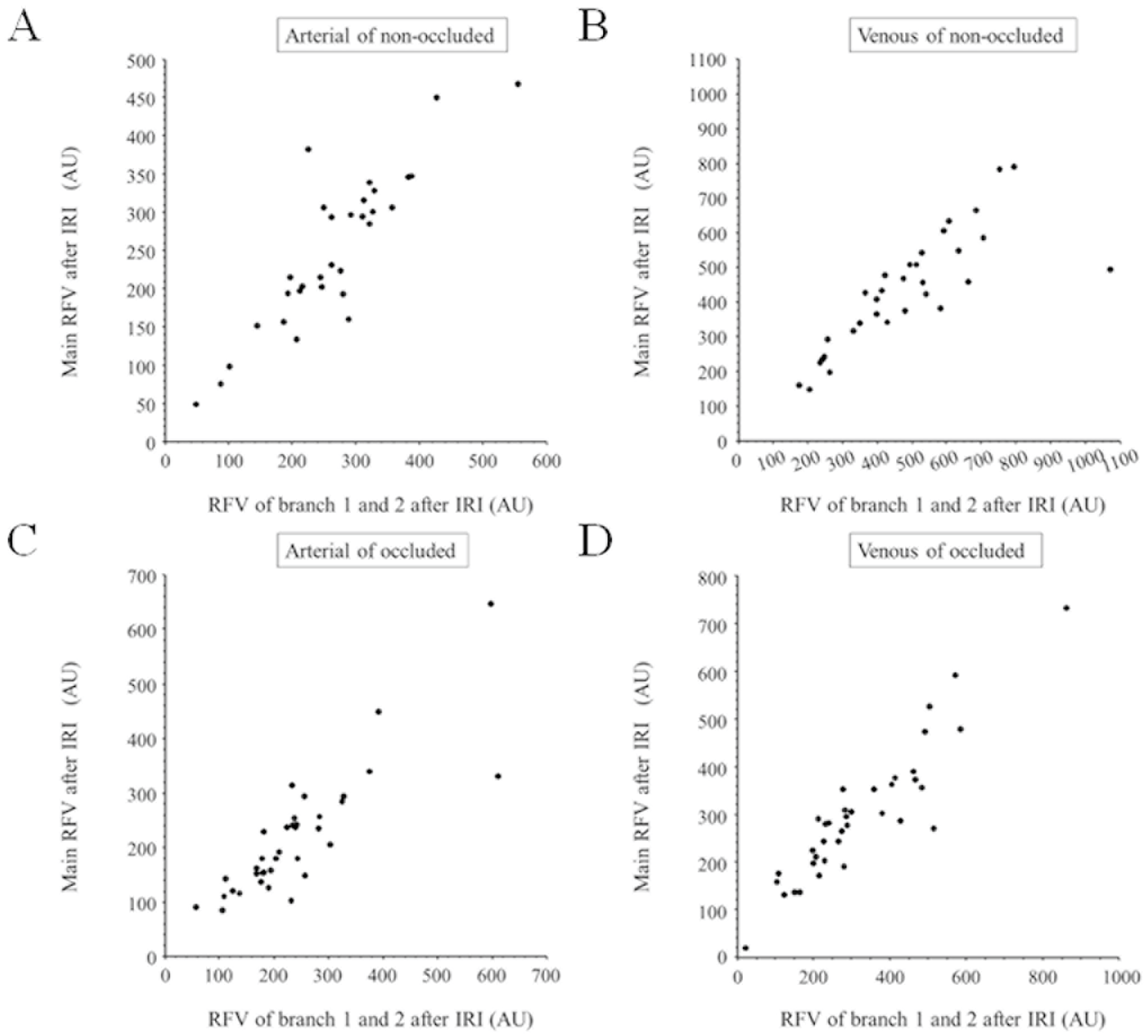
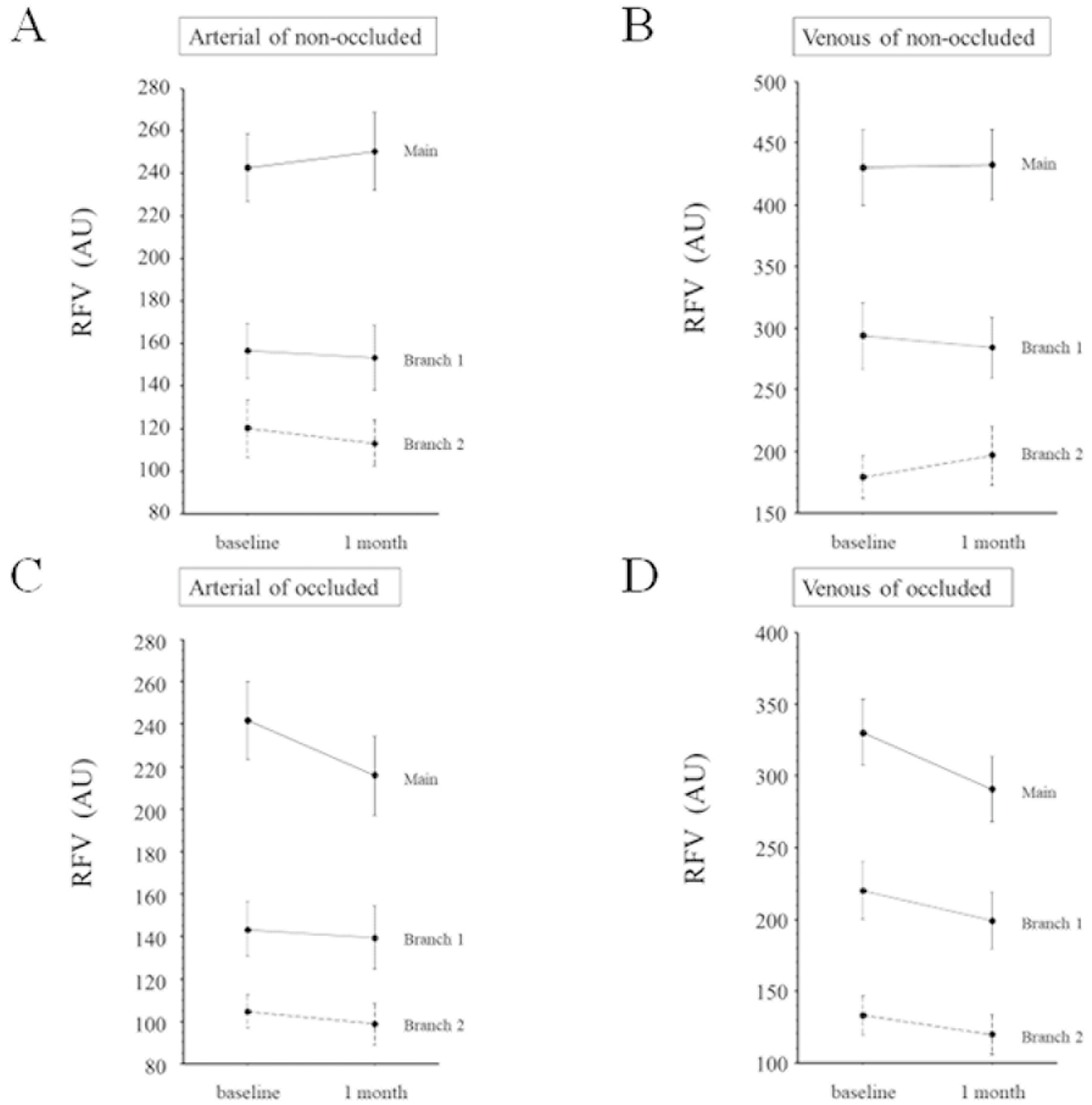
2.4. Hemodynamics
2.5. Cytokine Measurements
2.6. Statistical Analysis
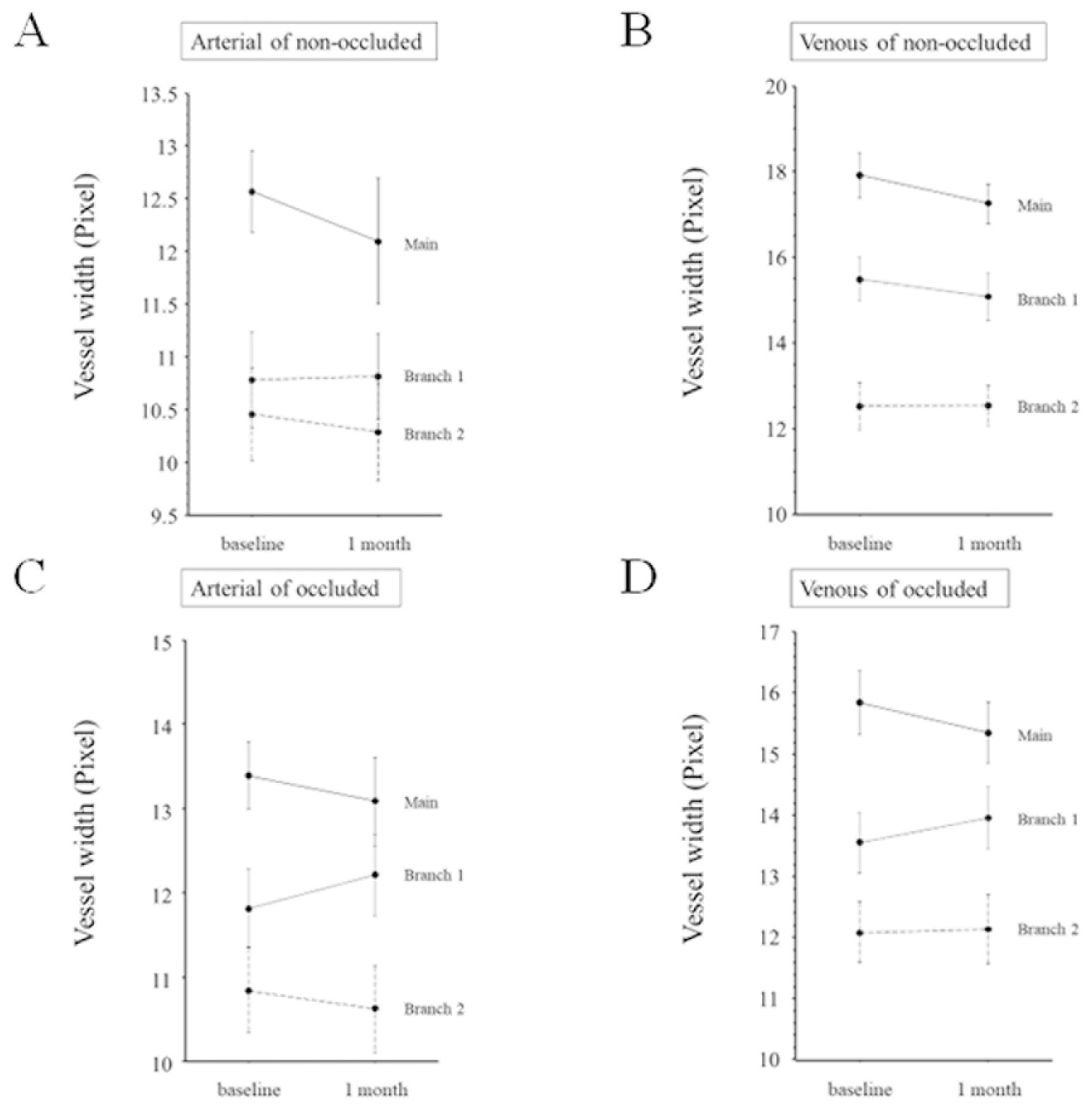
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rogers, S.L.; McIntosh, R.L.; Lim, L.; Mitchell, P.; Cheung, N.; Kowalski, J.W.; Nguyen, H.P.; Wang, J.J.; Wong, T.Y. Natural history of branch retinal vein occlusion: An evidence-based systematic review. Ophthalmology 2010, 117, 1094–1101. [Google Scholar] [CrossRef]
- Kiire, C.A.; Chong, N.V. Managing retinal vein occlusion. BMJ 2012, 22, e499. [Google Scholar] [CrossRef] [PubMed]
- Christoffersen, N.L.; Larsen, M. Pathophysiology and hemodynamics of branch retinal vein occlusion. Ophthalmology 1999, 106, 2054–2062. [Google Scholar] [CrossRef]
- Coscas, G.; Cunha-Vaz, J.; Soubrane, G. Macular Edema: Definition and Basic Concepts. Dev. Ophthalmol. 2017, 58, 1–9. [Google Scholar] [PubMed]
- Noma, H.; Funatsu, H.; Mimura, T.; Eguchi, S.; Hori, S. Soluble vascular endothelial growth factor receptor-2 and inflammatory factors in macular edema with branch retinal vein occlusion. Am. J. Ophthalmol. 2011, 152, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Noma, H.; Yasuda, K.; Shimura, M. Cytokines and the Pathogenesis of Macular Edema in Branch Retinal Vein Occlusion. J. Ophthalmol. 2019, 2019, 5185128. [Google Scholar] [CrossRef]
- Tamaki, Y.; Araie, M.; Kawamoto, E.; Eguchi, S.; Fujii, H. Noncontact, two-dimensional measurement of retinal microcirculation using laser speckle phenomenon. Investig. Ophthalmol. Vis. Sci. 1994, 35, 3825–3834. [Google Scholar]
- Sugiyama, T.; Araie, M.; Riva, C.E.; Schmetterer, L.; Orgul, S. Use of laser speckle flowgraphy in ocular blood flow research. Acta Ophthalmol. 2010, 88, 723–729. [Google Scholar] [CrossRef]
- Shiga, Y.; Asano, T.; Kunikata, H.; Nitta, F.; Sato, H.; Nakazawa, T.; Shimura, M. Relative flow volume, a novel blood flow index in the human retina derived from laser speckle flowgraphy. Investig. Ophthalmol. Vis. Sci. 2014, 55, 3899–3904. [Google Scholar] [CrossRef]
- Iwase, T.; Ra, E.; Yamamoto, K.; Kaneko, H.; Ito, Y.; Terasaki, H. Differences of Retinal Blood Flow Between Arteries and Veins Determined by Laser Speckle Flowgraphy in Healthy Subjects. Medicine 2015, 94, e1256. [Google Scholar] [CrossRef]
- Brown, D.M.; Campochiaro, P.A.; Bhisitkul, R.B.; Ho, A.C.; Gray, S.; Saroj, N.; Adamis, A.P.; Rubio, R.G.; Murahashi, W.Y. Sustained Benefits from Ranibizumab for Macular Edema Following Branch Retinal Vein Occlusion: 12-Month Outcomes of a Phase III Study. Ophthalmology 2011, 118, 1594–1602. [Google Scholar] [CrossRef] [PubMed]
- Heier, J.S.; Campochiaro, P.A.; Yau, L.; Li, Z.; Saroj, N.; Rubio, R.G.; Lai, P. Ranibizumab for macular edema due to retinal vein occlusions: Long-term follow-up in the HORIZON trial. Ophthalmology 2012, 119, 802–809. [Google Scholar] [CrossRef]
- Campochiaro, P.A.; Sophie, R.; Pearlman, J.; Brown, D.M.; Boyer, D.S.; Heier, J.S.; Marcus, D.M.; Feiner, L.; Patel, A. Long-term outcomes in patients with retinal vein occlusion treated with ranibizumab: The RETAIN study. Ophthalmology 2014, 121, 209–219. [Google Scholar] [CrossRef]
- Fukami, M.; Iwase, T.; Yamamoto, K.; Kaneko, H.; Yasuda, S.; Terasaki, H. Changes in Retinal Microcirculation After Intravitreal Ranibizumab Injection in Eyes with Macular Edema Secondary to Branch Retinal Vein Occlusion. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1246–1255. [Google Scholar] [CrossRef] [PubMed]
- Noma, H.; Yasuda, K.; Mimura, T.; Suganuma, N.; Shimura, M. Retinal Microcirculation and Cytokines as Predictors for Recurrence of Macular Edema after Intravitreal Ranibizumab Injection in Branch Retinal Vein Occlusion. J. Clin. Med. 2020, 10, 58. [Google Scholar] [CrossRef]
- Noma, H.; Yasuda, K.; Mimura, T.; Ofusa, A.; Shimura, M. Relationship between retinal blood flow and cytokines in central retinal vein occlusion. BMC Ophthalmol. 2020, 20, 215. [Google Scholar] [CrossRef]
- Riva, C.E.; Titze, P.; Hero, M.; Petrig, B.L. Effect of acute decreases of perfusion pressure on choroidal blood flow in humans. Investig. Ophthalmol. Vis. Sci. 1997, 38, 1752–1760. [Google Scholar]
- Noma, H.; Mimura, T.; Yasuda, K.; Shimura, M. Role of soluble vascular endothelial growth factor receptors-1 and -2, their ligands, and other factors in branch retinal vein occlusion with macular edema. Investig. Ophthalmol. Vis. Sci. 2014, 55, 3878–3885. [Google Scholar] [CrossRef]
- Noma, H.; Mimura, T.; Yasuda, K.; Motohashi, R.; Kotake, O.; Shimura, M. Aqueous Humor Levels of Soluble Vascular Endothelial Growth Factor Receptor and Inflammatory Factors in Diabetic Macular Edema. Ophthalmologica 2017, 238, 81–88. [Google Scholar] [CrossRef]
- Noma, H.; Yasuda, K.; Shimura, M. Change of cytokines after intravitreal ranibizumab in patients with recurrent branch retinal vein occlusion and macular edema. Eur. J. Ophthalmol. 2021, 31, 204–210. [Google Scholar] [CrossRef]
- Utsumi, T.; Noma, H.; Yasuda, K.; Goto, H.; Shimura, M. Effects of ranibizumab on growth factors and mediators of inflammation in the aqueous humor of patients with diabetic macular edema. Graefes Arch. Clin. Exp. Ophthalmol. 2021, 259, 2597–2603. [Google Scholar] [CrossRef] [PubMed]
- Noma, H.; Yasuda, K.; Mimura, T.; Shimura, M. Relationship between Choroidal Findings and Growth Factors, Cytokines, and Other Inflammatory Mediators after Intravitreal Ranibizumab Injection in Patients with Macular Edema Secondary to Branch Retinal Vein Occlusion. Retina 2022, 42, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Shimura, M.; Yasuda, K. Macular ischaemia after intravitreal bevacizumab injection in patients with central retinal vein occlusion and a history of diabetes and vascular disease. Br. J. Ophthalmol. 2010, 94, 381–383. [Google Scholar] [CrossRef] [PubMed]
- Manousaridis, K.; Talks, J. Macular ischaemia: A contraindication for anti-VEGF treatment in retinal vascular disease? Br. J. Ophthalmol. 2012, 96, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Mejía, M.E.; Ríos, H.A.; Rosenstiehl, S.; Rodríguez, F.J. Optical coherence tomography angiography as predictor of visual outcomes in retinal vein occlusion treated with antiangiogenic therapy. Eur. J. Ophthalmol. 2023, 33, 434–440. [Google Scholar] [CrossRef]
- Clermont, A.C.; Aiello, L.P.; Mori, F.; Aiello, L.M.; Bursell, S.E. Vascular endothelial growth factor and severity of nonproliferative diabetic retinopathy mediate retinal hemodynamics in vivo: A potential role for vascular endothelial growth factor in the progression of nonproliferative diabetic retinopathy. Am. J. Ophthalmol. 1997, 124, 433–446. [Google Scholar] [CrossRef]
- Tilton, R.G.; Chang, K.C.; LeJeune, W.S.; Stephan, C.C.; Brock, T.A.; Williamson, J.R. Role for nitric oxide in the hyperpermeability and hemodynamic changes induced by intravenous VEGF. Investig. Ophthalmol. Vis. Sci. 1999, 40, 689–696. [Google Scholar]
- Nagaoka, T.; Sakamoto, T.; Mori, F.; Sato, E.; Yoshida, A. The effect of nitric oxide on retinal blood flow during hypoxia in cats. Investig. Ophthalmol. Vis. Sci. 2002, 43, 3037–3044. [Google Scholar]
- Papadopoulou, D.N.; Mendrinos, E.; Mangioris, G.; Donati, G.; Pournaras, C.J. Intravitreal ranibizumab may induce retinal arteriolar vasoconstriction in patients with neovascular age-related macular degeneration. Ophthalmology 2009, 116, 1755–1761. [Google Scholar] [CrossRef]
- Sacu, S.; Pemp, B.; Weigert, G.; Matt, G.; Garhofer, G.; Pruente, C.; Schmetterer, L.; Schmidt-Erfurth, U. Response of retinal vessels and retrobulbar hemodynamics to intravitreal anti-VEGF treatment in eyes with branch retinal vein occlusion. Investig. Ophthalmol. Vis. Sci. 2011, 52, 3046–3050. [Google Scholar] [CrossRef]
- Yoshida, S.; Yoshida, A.; Ishibashi, T.; Elner, S.G.; Elner, V.M. Role of MCP-1 and MIP-1alpha in retinal neovascularization during postischemic inflammation in a mouse model of retinal neovascularization. J. Leukoc. Biol. 2003, 73, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Ito, W.D.; Arras, M.; Winkler, B.; Scholz, D.; Schaper, J.; Schaper, W. Monocyte chemotactic protein-1 increases collateral and peripheral conductance after femoral artery occlusion. Circ. Res. 1997, 80, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Tesch, G.H. Role of macrophages in complications of type 2 diabetes. Clin. Exp. Pharmacol. Physiol. 2007, 34, 1016–1019. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Kubo, Y.; Kobayashi, Y.; Zhou, Y.; Nakama, T.; Yamaguchi, M.; Tachibana, T.; Ishikawa, K.; Arita, R.; Nakao, S.; et al. Increased vitreous concentrations of MCP-1 and IL-6 after vitrectomy in patients with proliferative diabetic retinopathy: Possible association with postoperative macular oedema. Br. J. Ophthalmol. 2015, 99, 960–966. [Google Scholar] [CrossRef]
- Karakurum, M.; Shreeniwas, R.; Chen, J.; Pinsky, D.; Yan, S.D.; Anderson, M.; Sunouchi, K.; Major, J.; Hamilton, T.; Kuwabara, K.; et al. Hypoxic induction of interleukin-8 gene expression in human endothelial cells. J. Clin. Investig. 1994, 93, 1564–1570. [Google Scholar] [CrossRef] [PubMed]
- Shono, T.; Ono, M.; Izumi, H.; Jimi, S.I.; Matsushima, K.; Okamoto, T.; Kohno, K.; Kuwano, M. Involvement of the transcription factor NF-kappaB in tubular morphogenesis of human microvascular endothelial cells by oxidative stress. Mol. Cell. Biol. 1996, 16, 4231–4239. [Google Scholar] [CrossRef] [PubMed]
- Taub, D.D.; Anver, M.; Oppenheim, J.J.; Longo, D.L.; Murphy, W.J. T lymphocyte recruitment by interleukin-8 (IL-8). IL-8-induced degranulation of neutrophils releases potent chemoattractants for human T lymphocytes both in vitro and in vivo. J. Clin. Investig. 1996, 97, 1931–1941. [Google Scholar] [CrossRef]
- Detmers, P.A.; Lo, S.K.; Olsen-Egbert, E.; Walz, A.; Baggiolini, M.; Cohn, Z.A. Neutrophil-activating protein 1/interleukin 8 stimulates the binding activity of the leukocyte adhesion receptor CD11b/CD18 on human neutrophils. J. Exp. Med. 1990, 171, 1155–1162. [Google Scholar] [CrossRef]
- Paccaud, J.P.; Schifferli, J.A.; Baggiolini, M. NAP-1/IL-8 induces up-regulation of CR1 receptors in human neutrophil leukocytes. Biochem. Biophys. Res. Commun. 1990, 166, 187–192. [Google Scholar] [CrossRef]
- Tsujikawa, A.; Ogura, Y.; Hiroshiba, N.; Miyamoto, K.; Kiryu, J.; Honda, Y. In vivo evaluation of leukocyte dynamics in retinal ischemia reperfusion injury. Investig. Ophthalmol. Vis. Sci. 1998, 39, 793–800. [Google Scholar]
- Wang, B.; Zhang, X.; Chen, H.; Koh, A.; Zhao, C.; Chen, Y. A Review of Intraocular Biomolecules in Retinal Vein Occlusion: Toward Potential Biomarkers for Companion Diagnostics. Front. Pharmacol. 2022, 13, 859951. [Google Scholar] [CrossRef]
- Lindahl, P.; Johansson, B.R.; Levéen, P.; Betsholtz, C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science 1997, 277, 242–245. [Google Scholar] [CrossRef]
- Hirschi, K.K.; Rohovsky, S.A.; D’Amore, P.A. PDGF, TGF-beta, and heterotypic cell-cell interactions mediate endothelial cell-induced recruitment of 10T1/2 cells and their differentiation to a smooth muscle fate. J. Cell Biol. 1998, 141, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Hellström, M.; Kalén, M.; Lindahl, P.; Abramsson, A.; Betsholtz, C. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development 1999, 126, 3047–3055. [Google Scholar] [CrossRef] [PubMed]
- Tallquist, M.D.; French, W.J.; Soriano, P. Additive effects of PDGF receptor beta signaling pathways in vascular smooth muscle cell development. PLoS Biol. 2003, 1, 17. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, B.; Wenzel, U.O.; Marra, F.; Abboud, H.E. A nuclear protein in mesangial cells that binds to the promoter region of the platelet-derived growth factor-A chain gene. Induction by phorbol ester. J. Biol. Chem. 1995, 270, 5541–5548. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P. Mechanisms of angiogenesis and arteriogenesis. Nat. Med. 2000, 6, 389–395. [Google Scholar] [CrossRef]
- Betsholtz, C. Role of platelet-derived growth factors in mouse development. Int. J. Dev. Biol. 1995, 39, 817–825. [Google Scholar]
- Lindblom, P.; Gerhardt, H.; Liebner, S.; Abramsson, A.; Enge, M.; Hellstrom, M.; Backstrom, G.; Fredriksson, S.; Landegren, U.; Nystrom, H.C.; et al. Endothelial PDGF-B retention is required for proper investment of pericytes in the microvessel wall. Genes Dev. 2003, 17, 1835–1840. [Google Scholar] [CrossRef]
- Bjarnegård, M.; Enge, M.; Norlin, J.; Gustafsdottir, S.; Fredriksson, S.; Abramsson, A.; Takemoto, M.; Gustafsson, E.; Fässler, R.; Betsholtz, C. Endothelium-specific ablation of PDGFB leads to pericyte loss and glomerular, cardiac and placental abnormalities. Development 2004, 131, 1847–1857. [Google Scholar] [CrossRef]
- Nyström, H.C.; Lindblom, P.; Wickman, A.; Andersson, I.; Norlin, J.; Fäldt, J.; Lindahl, P.; Skøtt, O.; Bjarnegård, M.; Fitzgerald, S.M.; et al. Platelet-derived growth factor B retention is essential for development of normal structure and function of conduit vessels and capillaries. Cardiovasc. Res. 2006, 71, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Abramsson, A.; Kurup, S.; Busse, M.; Yamada, S.; Lindblom, P.; Schallmeiner, E.; Stenzel, D.; Sauvaget, D.; Ledin, J.; Ringvall, M.; et al. Defective N-sulfation of heparan sulfate proteoglycans limits PDGF-BB binding and pericyte recruitment in vascular development. Genes. Dev. 2007, 21, 316–331. [Google Scholar] [CrossRef] [PubMed]
- Caporarello, N.; D’Angeli, F.; Cambria, M.T.; Candido, S.; Giallongo, C.; Salmeri, M.; Lombardo, C.; Longo, A.; Giurdanella, G.; Anfuso, C.D.; et al. Pericytes in Microvessels: From “Mural” Function to Brain and Retina Regeneration. Int. J. Mol. Sci. 2019, 20, 6351. [Google Scholar] [CrossRef] [PubMed]
- Stratman, A.N.; Schwindt, A.E.; Malotte, K.M.; Davis, G.E. Endothelial-derived PDGF-BB and HB-EGF coordinately regulate pericyte recruitment during vasculogenic tube assembly and stabilization. Blood 2010, 116, 4720–4730. [Google Scholar] [CrossRef]
| Findings | BRVO (n = 37) |
|---|---|
| Age (years) | 65.7 ± 8.36 ‡ |
| Gender (female/male) | 19/18 |
| Duration of macular edema (days) | 51.1 ± 44.9 ‡ |
| Hypertension | 26 (70.3%) |
| Systolic Blood pressure (mmHg) | 141 ± 19 |
| Diastolic Blood pressure (mmHg) | 82 ± 13 |
| Hyperlipidemia | 16 (43.2%) |
| Baseline BCVA (logMAR) | 0.43 ± 0.29 ‡ |
| Baseline CMT (μm) | 622 ± 180 ‡ |
| MBP (mmHg) | 102 ± 14 ‡ |
| OPP (mmHg) | 55 ± 9.0 ‡ |
| Aqueous Factors/Cytokines | VEGF (pg/mL) | PlGF (pg/mL) | PDGF-AA (pg/mL) | sICAM-1 (pg/mL) | MCP-1 (pg/mL) | IL-6 (pg/mL) | IL-8 (pg/mL) | IP-10 (pg/mL) |
|---|---|---|---|---|---|---|---|---|
| Variable | r p value | r p value | r p value | r p value | r p value | r p value | r p value | r p value |
| Arterial of the non-occluded region: main | −0.01 0.966 | −0.01 0.965 | −0.04 0.829 | 0.17 0.330 | −0.26 0.141 | −0.03 0.877 | −0.05 0.798 | −0.08 0.701 |
| Arterial of the non-occluded region: branch 1 | 0.08 0.644 | 0.03 0.824 | 0.11 0.538 | 0.42 0.011 | 0.14 0.416 | 0.09 0.611 | 0.27 0.124 | 0.22 0.270 |
| Arterial of the non-occluded region: branch 2 | 0.03 0.828 | −0.10 0.567 | −0.01 0.956 | −0.08 0.646 | −0.32 0.068 | −0.12 0.497 | −0.18 0.297 | −0.22 0.268 |
| Arterial of the occluded region: main | −0.01 0.958 | −0.22 0.191 | −0.37 0.025 | −0.04 0.799 | −0.37 0.026 | −0.22 0.181 | −0.32 0.059 | −0.30 0.102 |
| Arterial of the occluded region: branch 1 | −0.03 0.860 | −0.22 0.181 | −0.21 0.210 | −0.08 0.627 | −0.25 0.132 | −0.15 0.375 | −0.20 0.226 | −0.12 0.552 |
| Arterial of the occluded region: branch 2 | −0.02 0.902 | −0.09 0.590 | −0.24 0.156 | 0.24 0.152 | −0.21 0.206 | −0.18 0.290 | −0.22 0.178 | −0.18 0.296 |
| Venous of the non-occluded region: main | 0.10 0.550 | 0.15 0.369 | −0.11 0.541 | −0.31 0.065 | −0.02 0.882 | 0.11 0.538 | 0.13 0.465 | −0.14 0.484 |
| Venous of the non-occluded region: branch 1 | 0.02 0.907 | 0.14 0.409 | 0.09 0.593 | −0.10 0.552 | 0.05 0.781 | 0.15 0.376 | 0.09 0.594 | 0.16 0.423 |
| Venous of the non-occluded region: branch 2 | 0.21 0.204 | 0.04 0.794 | −0.13 0.427 | 0.15 0.370 | −0.06 0.702 | −0.12 0.468 | 0.12 0.466 | −0.20 0.320 |
| Venous of the occluded region: main | −0.20 0.224 | −0.28 0.096 | −0.55 <0.001 | −0.08 0.629 | −0.44 0.006 | −0.38 0.019 | −0.48 0.003 | −0.23 0.170 |
| Venous of the occluded region: branch 1 | −0.14 0.400 | −0.35 0.030 | 0.30 0.064 | −0.11 0.495 | −0.36 0.028 | −0.37 0.023 | −0.52 <0.001 | −0.20 0.237 |
| Venous of the occluded region: branch 2 | −0.28 0.085 | −0.05 0.736 | −0.43 0.007 | −0.07 0.655 | −0.16 0.331 | −0.19 0.237 | −0.12 0.467 | −0.03 0.844 |
| Aqueous Factors/Cytokines | VEGF (pg/mL) | PlGF (pg/mL) | PDGF-AA (pg/mL) | sICAM-1 (pg/mL) | MCP-1 (pg/mL) | IL-6 (pg/mL) | IL-8 (pg/mL) | IP-10 (pg/mL) |
|---|---|---|---|---|---|---|---|---|
| Variable | r p value | r p value | r p value | r p value | r p value | r p value | r p value | r p value |
| Arterial of the non-occluded region: main | 0.11 0.504 | −0.17 0.322 | 0.35 0.038 | 0.15 0.376 | −0.04 0.799 | −0.11 0.501 | 0.02 0.894 | 0.03 0.852 |
| Arterial of the non-occluded region: branch 1 | 0.15 0.369 | −0.08 0.641 | 0.26 0.124 | 0.23 0.182 | 0.11 0.531 | −0.02 0.890 | 0.13 0.449 | 0.15 0.436 |
| Arterial of the non-occluded region: branch 2 | 0.22 0.198 | −0.15 0.372 | 0.24 0.166 | −0.01 0.986 | −0.18 0.293 | −0.24 0.159 | −0.13 0.454 | −0.24 0.168 |
| Arterial of the occluded region: main | 0.08 0.616 | 0.05 0.763 | −0.03 0.827 | −0.10 0.554 | −0.11 0.499 | 0.09 0.581 | 0.01 0.975 | −0.19 0.243 |
| Arterial of the occluded region: branch 1 | 0.05 0.747 | −0.04 0.785 | 0.17 0.313 | −0.03 0.842 | −0.10 0.549 | 0.05 0.740 | 0.06 0.688 | −0.10 0.571 |
| Arterial of the occluded region: branch 2 | −0.03 0.824 | 0.31 0.060 | −0.03 0.850 | 0.08 0.616 | 0.17 0.304 | 0.28 0.095 | 0.17 0.313 | 0.18 0.308 |
| Venous of the non-occluded region: main | 0.12 0.472 | 0.27 0.118 | 0.12 0.461 | 0.08 0.633 | 0.12 0.457 | 0.08 0.631 | 0.27 0.108 | 0.01 0.941 |
| Venous of the non-occluded region: branch 1 | 0.12 0.479 | 0.25 0.137 | 0.29 0.084 | −0.01 0.953 | 0.10 0.552 | 0.18 0.297 | 0.23 0.176 | 0.18 0.301 |
| Venous of the non-occluded region: branch 2 | 0.05 0.774 | 0.11 0.507 | −0.15 0.387 | 0.08 0.637 | −0.20 0.233 | −0.16 0.345 | 0.01 0.946 | −0.30 0.073 |
| Venous of the occluded region: main | 0.04 0.781 | −0.01 0.931 | −0.56 <0.001 | −0.09 0.570 | −0.33 0.044 | −0.15 0.370 | −0.21 0.200 | −0.11 0.508 |
| Venous of the occluded region: branch 1 | −0.15 0.363 | −0.38 0.017 | −0.36 0.024 | −0.15 0.357 | −0.27 0.098 | −0.29 0.077 | −0.47 0.003 | −0.22 0.194 |
| Venous of the occluded region: branch 2 | −0.13 0.421 | 0.05 0.748 | −0.33 0.042 | −0.09 0.562 | −0.15 0.359 | −0.14 0.379 | −0.06 0.716 | −0.01 0.925 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yasuda, K.; Noma, H.; Mimura, T.; Nonaka, R.; Sasaki, S.; Suganuma, N.; Shimura, M. Effects of Intravitreal Ranibizumab Injection on Peripheral Retinal Microcirculation and Cytokines in Branch Retinal Vein Occlusion with Macular Edema. Medicina 2023, 59, 1053. https://doi.org/10.3390/medicina59061053
Yasuda K, Noma H, Mimura T, Nonaka R, Sasaki S, Suganuma N, Shimura M. Effects of Intravitreal Ranibizumab Injection on Peripheral Retinal Microcirculation and Cytokines in Branch Retinal Vein Occlusion with Macular Edema. Medicina. 2023; 59(6):1053. https://doi.org/10.3390/medicina59061053
Chicago/Turabian StyleYasuda, Kanako, Hidetaka Noma, Tatsuya Mimura, Ryota Nonaka, Shotaro Sasaki, Noboru Suganuma, and Masahiko Shimura. 2023. "Effects of Intravitreal Ranibizumab Injection on Peripheral Retinal Microcirculation and Cytokines in Branch Retinal Vein Occlusion with Macular Edema" Medicina 59, no. 6: 1053. https://doi.org/10.3390/medicina59061053
APA StyleYasuda, K., Noma, H., Mimura, T., Nonaka, R., Sasaki, S., Suganuma, N., & Shimura, M. (2023). Effects of Intravitreal Ranibizumab Injection on Peripheral Retinal Microcirculation and Cytokines in Branch Retinal Vein Occlusion with Macular Edema. Medicina, 59(6), 1053. https://doi.org/10.3390/medicina59061053







