Giant Echinococcosis of the Liver with Suppuration: A Case Report and Review of the Literature
Abstract
1. Introduction
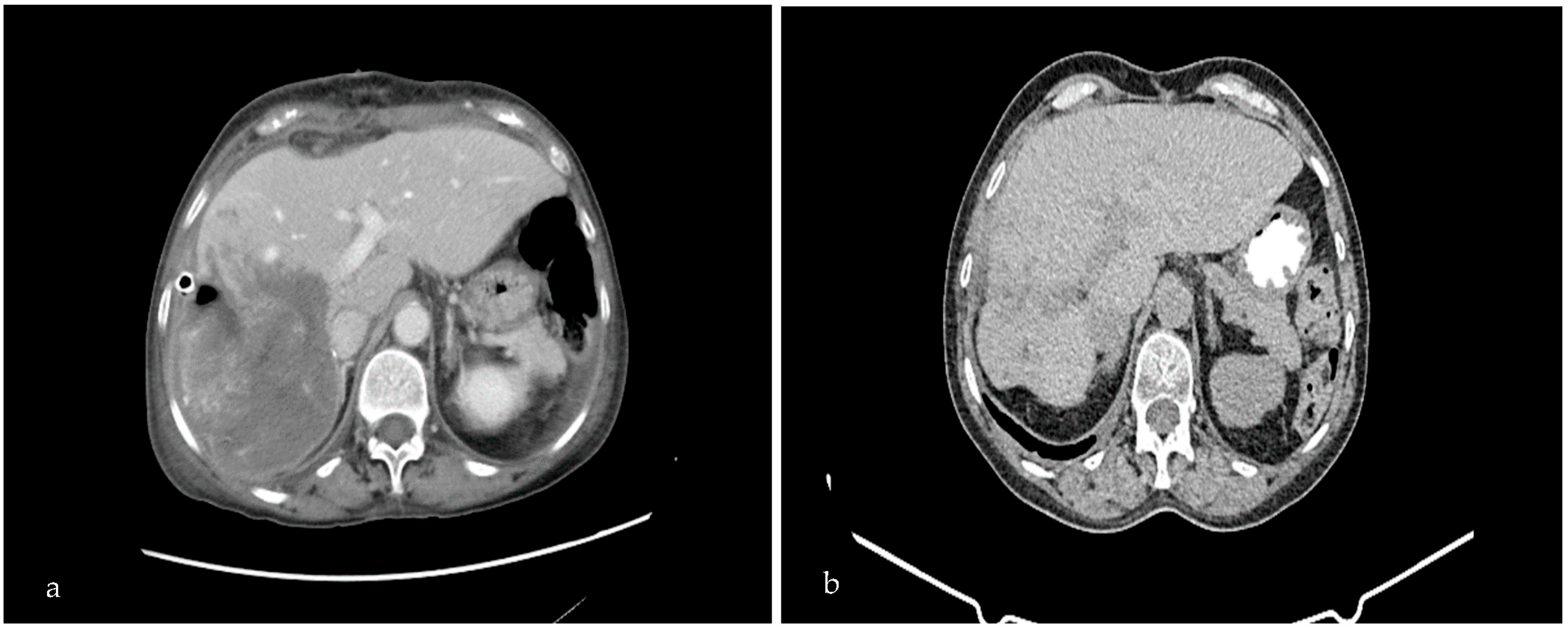
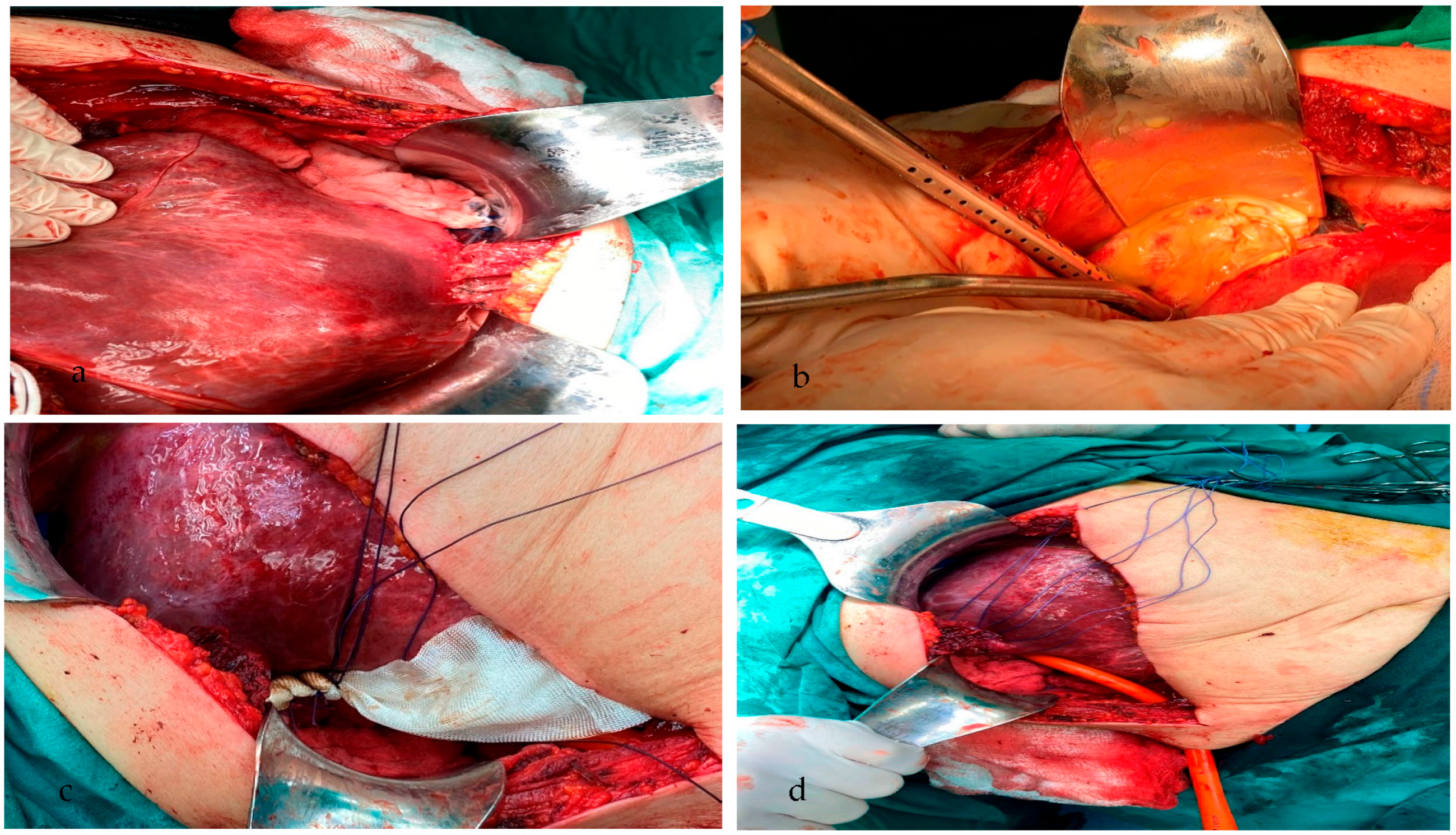
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Touma, D.; Sersté, T.; Ntounda, R.; Mulkay, J.P.; Buset, M.; Van Laethem, Y. The liver involvement of the hydatid disease: A systematic review designed for the hepato-gastroenterologist. Acta Gastro-Enterol. Belg. 2013, 76, 210–218. [Google Scholar]
- Chen, Y.C.; Yeh, T.S.; Tseng, J.H.; Huang, S.F.; Lin, D.Y. Hepatic hydatid cysts with superinfection in a non-endemic area in Taiwan. Am. J. Trop. Med. Hyg. 2002, 67, 524–527. [Google Scholar] [CrossRef] [PubMed]
- Buttenschoen, K.; Carli Buttenschoen, D. Echinococcus granulosus infection: The challenge of surgical treatment. Langenbeck’s Arch. Surg. 2003, 388, 218–230. [Google Scholar] [CrossRef] [PubMed]
- García, M.B.; Lledías, J.P.; Pérez, I.G.; Tirado, V.V.; Pardo, L.F.; Bellvís, L.M.; Varela, G.; Sánchez, M.C. Primary super-infection of hydatid cyst-clinical setting and microbiology in 37 cases. Am. J. Trop. Med. Hyg. 2010, 82, 376–378. [Google Scholar] [CrossRef] [PubMed]
- Prousalidis, J.; Kosmidis, C.; Anthimidis, G.; Fachantidis, E.; Harlaftis, N.; Aletras, H. Forty-four years’ experience (1963–2006) in the management of primarily infected hydatid cyst of the liver. Int. Hepato Pancreato Biliary Assoc. 2008, 10, 18–24. [Google Scholar] [CrossRef]
- Arslan, F.; Zengin, K.; Mert, A.; Ozaras, R.; Tabak, F. Pelvic and retroperitoneal hydatid cysts superinfected with Brucella sp. and review of infected hydatid cysts. Trop. Biomed. 2013, 30, 92–96. [Google Scholar]
- Craig, P.S.; McManus, D.P.; Lightowlers, M.W.; Chabalgoity, J.A.; Garcia, H.H.; Gavidia, C.M.; Gilman, R.H.; Gonzalez, A.E.; Lorca, M.; Naquira, C.; et al. Prevention and control of cystic echinococcosis. Lancet Infect. Dis. 2007, 7, 385–394. [Google Scholar] [CrossRef]
- Alghofaily, K.A.; Saeedan, M.B.; Aljohani, I.M.; Alrasheed, M.; McWilliams, S.; Aldosary, A.; Neimatallah, M. Hepatic hydatid disease complications: Review of imaging findings and clinical implications. Abdom. Radiol. 2017, 42, 199–210. [Google Scholar] [CrossRef]
- Mandal, S.; Mandal, M.D. Human cystic echinococcosis: Epidemiologic, zoonotic, clinical, diagnostic and therapeutic aspects. Asian Pac. J. Trop. Med. 2012, 5, 253–260. [Google Scholar] [CrossRef]
- Grosso, G.; Gruttadauria, S.; Biondi, A.; Marventano, S.; Mistretta, A. Worldwide epidemiology of liver hydatidosis including the Mediterranean area. World J. Gastroenterol. 2012, 18, 1425–1437. [Google Scholar] [CrossRef]
- Wen, H.; Vuitton, L.; Tuxun, T.; Li, J.; Vuitton, D.A.; Zhang, W.; McManus, D.P. Echinococcosis: Advances in the 21st Century. Clin. Microbiol. Rev. 2019, 32, e00075-18. [Google Scholar] [CrossRef]
- Agudelo Higuita, N.I.; Brunetti, E.; McCloskey, C. Cystic Echinococcosis. J. Clin. Microbiol. 2016, 54, 518–523. [Google Scholar] [CrossRef]
- Otero-Abad, B.; Torgerson, P.R. A systematic review of the epidemiology of echinococcosis in domestic and wild animals. PLoS Negl. Trop. Dis. 2013, 7, e2249. [Google Scholar] [CrossRef]
- Amahmid, O.; El Guamri, Y.; Zenjari, K.; Bouhout, S.; Ait Moh, M.; Boraam, F.; Ait Melloul, A.; Benfaida, H.; Bouhoum, K.; Belghyti, D. Epidemiology and clinical features of human cystic echinococcosis in adults from an endemic area (Morocco). Clin. Epidemiol. Glob. Health 2020, 8, 606–611. [Google Scholar] [CrossRef]
- McManus, D.P.; Zhang, W.; Li, J.; Bartley, P.B. Echinococcosis. Lancet 2003, 362, 1295–1304. [Google Scholar] [CrossRef]
- Farooq, S.M.; Liu, W. Intrabiliary rupture of hepatic echinococcosis. Radiol. Infect. Dis. 2022, 9, 145–151. [Google Scholar] [CrossRef]
- Manzano-Román, R.; Sánchez-Ovejero, C.; Hernández-González, A.; Casulli, A.; Siles-Lucas, M. Serological Diagnosis and Follow-Up of Human Cystic Echinococcosis: A New Hope for the Future? BioMed Res. Int. 2015, 2015, 428205. [Google Scholar] [CrossRef]
- Moro, P.; Schantz, P.M. Echinococcosis: A review. International journal of infectious diseases. Int. Soc. Infect. Dis. 2009, 13, 125–133. [Google Scholar] [CrossRef]
- Mihmanli, M.; Idiz, U.O.; Kaya, C.; Demir, U.; Bostanci, O.; Omeroglu, S.; Bozkurt, E. Current status of diagnosis and treatment of hepatic echinococcosis. World J. Hepatol. 2016, 8, 1169–1181. [Google Scholar] [CrossRef]
- Greco, S.; Cannella, R.; Giambelluca, D.; Pecoraro, G.; Battaglia, E.; Midiri, M.; Brancatelli, G.; Vernuccio, F. Complications of hepatic echinococcosis: Multimodality imaging approach. Insights Into Imaging. 2019, 10, 1–12. [Google Scholar] [CrossRef]
- Sachar, S.; Goyal, S.; Goyal, S.; Sangwan, S. Uncommon locations and presentations of hydatid cyst. Ann. Med. Health Sci. Res. 2014, 4, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Salamone, G.; Licari, L.; Randisi, B.; Falco, N.; Tutino, R.; Vaglica, A.; Gullo, R.; Porello, C.; Cocorullo, G.; Gulotta, G. Uncommon localizations of hydatid cyst. Rev. Lit. Il G. Di Chir. 2016, 37, 180–185. [Google Scholar] [CrossRef]
- Hammami, A.; Hellara, O.; Mnari, W.; Loussaief, C.; Bedioui, F.; Safer, L.; Golli, M.; Chakroun, M.; Saffar, H. Unusual presentation of severely disseminated and rapidly progressive hydatic cyst: Malignant hydatidosis. World J. Hepatol. 2015, 7, 633–637. [Google Scholar] [CrossRef] [PubMed]
- Sura, B.K. A Study of Some Biochemical Changes in Hydatid Cyst Patients Before And After Surgical removal of hydatid cyst. l Mustansiriyah J. Pharm. Sci. 2013, 13, 103–108. [Google Scholar] [CrossRef]
- Sen, P.; Demirdal, T.; Nemli, S.A. Evaluation of clinical, diagnostic and treatment aspects in hydatid disease: Analysis of an 8-year experience. Afr. Health Sci. 2019, 19, 2431–2438. [Google Scholar] [CrossRef]
- Goja, S.; Saha, S.K.; Yadav, S.K.; Tiwari, A.; Soin, A.S. Surgical approaches to hepatic hydatidosis ranging from partial cystectomy to liver transplantation. Ann. Hepato-Biliary-Pancreat. Surg. 2018, 22, 208–215. [Google Scholar] [CrossRef]
- Prousalidis, J.; Kosmidis, C.; Anthimidis, G.; Kapoutzis, K.; Karamanlis, E.; Fachantidis, E. Postoperative recurrence of cystic hydatidosis. Canadian journal of surgery. J. Can. De Chir. 2012, 55, 15–20. [Google Scholar] [CrossRef]
- Cirenei, A.; Bertoldi, I. Evolution of surgery for liver hydatidosis from 1950 to today: Analysis of a personal experience. World J. Surg. 2001, 25, 87–92. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosmidis, C.S.; Papadopoulos, K.; Mystakidou, C.M.; Sevva, C.; Koulouris, C.; Varsamis, N.; Mantalovas, S.; Lagopoulos, V.; Magra, V.; Theodorou, V.; et al. Giant Echinococcosis of the Liver with Suppuration: A Case Report and Review of the Literature. Medicina 2023, 59, 1070. https://doi.org/10.3390/medicina59061070
Kosmidis CS, Papadopoulos K, Mystakidou CM, Sevva C, Koulouris C, Varsamis N, Mantalovas S, Lagopoulos V, Magra V, Theodorou V, et al. Giant Echinococcosis of the Liver with Suppuration: A Case Report and Review of the Literature. Medicina. 2023; 59(6):1070. https://doi.org/10.3390/medicina59061070
Chicago/Turabian StyleKosmidis, Christoforos S., Konstantinos Papadopoulos, Chrysi Maria Mystakidou, Christina Sevva, Charilaos Koulouris, Nikolaos Varsamis, Stylianos Mantalovas, Vasileios Lagopoulos, Vasiliki Magra, Vasiliki Theodorou, and et al. 2023. "Giant Echinococcosis of the Liver with Suppuration: A Case Report and Review of the Literature" Medicina 59, no. 6: 1070. https://doi.org/10.3390/medicina59061070
APA StyleKosmidis, C. S., Papadopoulos, K., Mystakidou, C. M., Sevva, C., Koulouris, C., Varsamis, N., Mantalovas, S., Lagopoulos, V., Magra, V., Theodorou, V., Ouzouni, S., Iason Katsios, N., Axi, P., Sapalidis, K., & Kesisoglou, I. (2023). Giant Echinococcosis of the Liver with Suppuration: A Case Report and Review of the Literature. Medicina, 59(6), 1070. https://doi.org/10.3390/medicina59061070





