High AST/ALT Ratio Is Associated with Cardiac Involvement in Acute COVID-19 Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Study Population
2.2. Echocardiography
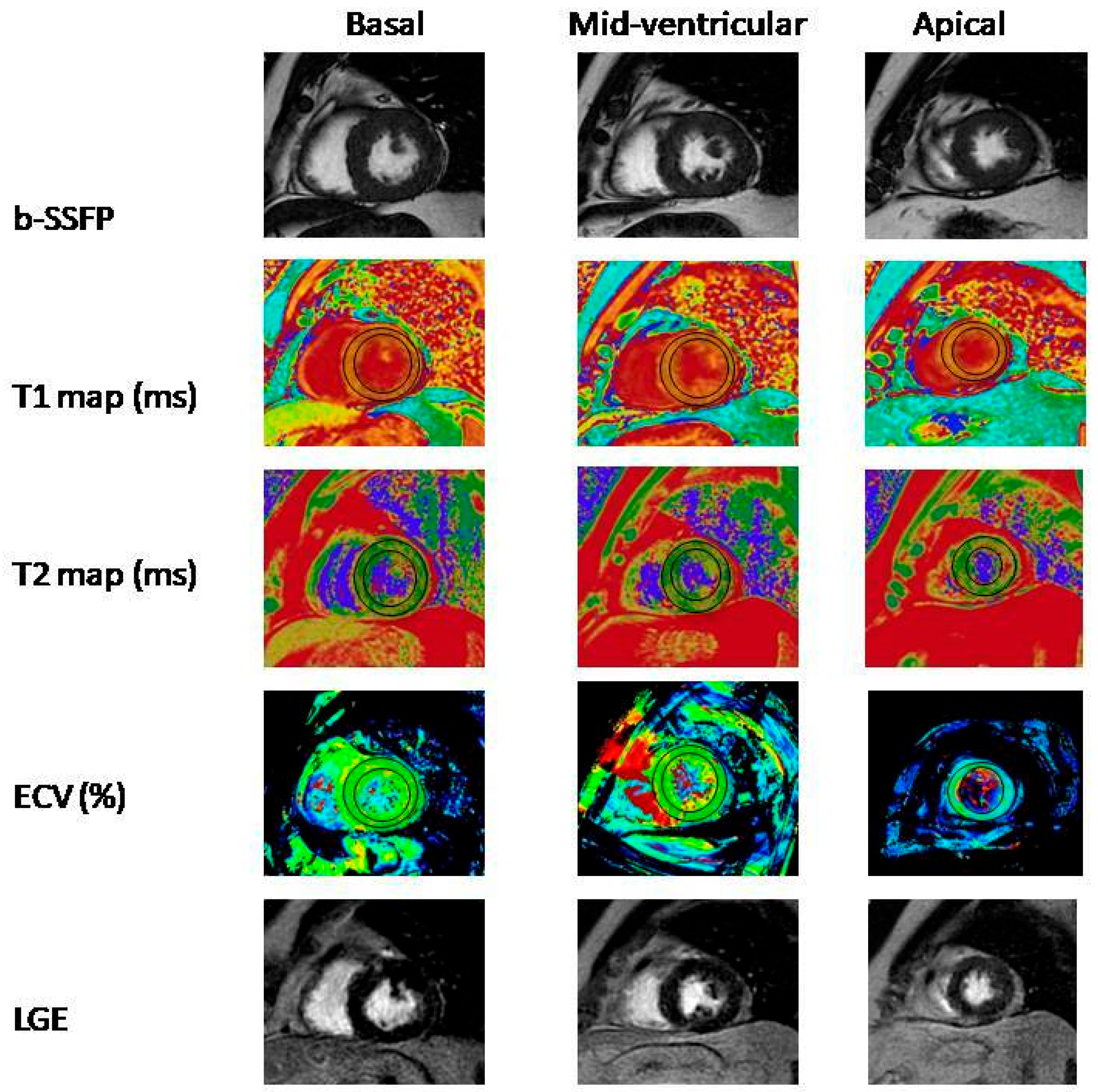
2.3. Cardiac Magnetic Resonance Imaging
2.4. Statistical Analysis
3. Results
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clerkin, K.J.; Fried, J.A.; Raikhelkar, J.; Sayer, G.; Griffin, J.M.; Masoumi, A.; Jain, S.S.; Burkhoff, D.; Kumaraiah, D.; Rabbani, L.R.; et al. COVID-19 and Cardiovascular Disease. Circulation 2020, 141, 1648–1655. [Google Scholar] [CrossRef] [Green Version]
- Azevedo, R.B.; Botelho, B.G.; de Hollanda, J.V.G.; Ferreira, L.V.L.; de Andrade, L.Z.J.; Oei, S.S.M.L.; Mello, T.D.S.; Muxfeldt, E.S. COVID-19 and the cardiovascular system: A comprehensive review. J. Hum. Hypertens. 2021, 35, 4–11. [Google Scholar] [CrossRef]
- Zou, L.; Ruan, F.; Huang, M.; Liang, L.; Huang, H.; Hong, Z.; Yu, J.; Kang, M.; Song, Y.; Xia, J.; et al. SARS-CoV-2 Viral Load in Upper Respiratory Specimens of Infected Patients. N. Engl. J. Med. 2020, 382, 1177–1179. [Google Scholar] [CrossRef]
- Jiang, F.; Deng, L.; Zhang, L.; Cai, Y.; Cheung, C.W.; Xia, Z. Review of the Clinical Characteristics of Coronavirus Disease 2019 (COVID-19). J. Gen. Intern. Med. 2020, 35, 1545–1549. [Google Scholar] [CrossRef] [Green Version]
- AlShahrani, I.; Hosmani, J.; Shankar, V.G.; AlShahrani, A.; Togoo, R.A.; Yassin, S.M.; Khan, S.; Patil, S. COVID-19 and cardiovascular system—A comprehensive review. Rev. Cardiovasc. Med. 2021, 22, 343–351. [Google Scholar] [CrossRef]
- Madjid, M.; Safavi-Naeini, P.; Solomon, S.D.; Vardeny, O. Potential Effects of Coronaviruses on the Cardiovascular System: A Review. JAMA Cardiol. 2020, 5, 831–840. [Google Scholar] [CrossRef] [Green Version]
- Inciardi, R.M.; Lupi, L.; Zaccone, G.; Italia, L.; Raffo, M.; Tomasoni, D.; Cani, D.S.; Cerini, M.; Farina, D.; Gavazzi, E.; et al. Cardiac Involvement in a Patient With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 819–824. [Google Scholar] [CrossRef] [Green Version]
- Siripanthong, B.; Nazarian, S.; Muser, D.; Deo, R.; Santangeli, P.; Khanji, M.Y.; Cooper, L.T.; Chahal, C.A.A. Recognizing COVID-19-related myocarditis: The possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm. 2020, 17, 1463–1471. [Google Scholar] [CrossRef]
- Sawalha, K.; Abozenah, M.; Kadado, A.J.; Battisha, A.; Al-Akchar, M.; Salerno, C.; Hernandez-Montfort, J.; Islam, A.M. Systematic Review of COVID-19 Related Myocarditis: Insights on Management and Outcome. Cardiovasc. Revasc. Med. 2021, 23, 107–113. [Google Scholar] [CrossRef]
- Castiello, T.; Georgiopoulos, G.; Finocchiaro, G.; Claudia, M.; Gianatti, A.; Delialis, D.; Aimo, A.; Prasad, S. COVID-19 and myocarditis: A systematic review and overview of current challenges. Heart Fail Rev. 2022, 27, 251–261. [Google Scholar] [CrossRef]
- Gibson, P.G.; Qin, L.; Puah, S.H. COVID-19 acute respiratory distress syndrome (ARDS): Clinical features and differences from typical pre-COVID-19 ARDS. Med. J. Aust. 2020, 213, 54–56.e1. [Google Scholar] [CrossRef]
- Attaway, A.H.; Scheraga, R.G.; Bhimraj, A.; Biehl, M.; Hatipoğlu, U. Severe COVID-19 pneumonia: Pathogenesis and clinical management. BMJ 2021, 372, n436. [Google Scholar] [CrossRef]
- Batah, S.S.; Fabro, A.T. Pulmonary pathology of ARDS in COVID-19: A pathological review for clinicians. Respir. Med. 2021, 176, 106239. [Google Scholar] [CrossRef]
- Repessé, X.; Charron, C.; Vieillard-Baron, A. Right ventricular failure in acute lung injury and acute respiratory distress syndrome. Minerva Anestesiol. 2012, 78, 941–948. [Google Scholar]
- Paternoster, G.; Bertini, P.; Innelli, P.; Trambaiolo, P.; Landoni, G.; Franchi, F.; Scolletta, S.; Guarracino, F. Right Ventricular Dysfunction in Patients With COVID-19: A Systematic Review and Meta-analysis. J. Cardiothorac. Vasc. Anesth. 2021, 35, 3319–3324. [Google Scholar] [CrossRef]
- Lazzeri, C.; Bonizzoli, M.; Batacchi, S.; Peris, A. Echocardiographic assessment of the right ventricle in COVID-related acute respiratory syndrome. Intern. Emerg. Med. 2021, 16, 1–5. [Google Scholar] [CrossRef]
- Botros, M.; Sikaris, K.A. The de ritis ratio: The test of time. Clin. Biochem. Rev. 2013, 34, 117–130. [Google Scholar]
- Djakpo, D.K.; Wang, Z.Q.; Shrestha, M. The significance of transaminase ratio (AST/ALT) in acute myocardial infarction. Arch. Med. Sci. Atheroscler. Dis. 2020, 5, e279–e283. [Google Scholar] [CrossRef]
- Gao, F.; Chen, C.; Lu, J.; Zheng, J.; Ma, X.-C.; Yuan, X.-Y.; Huo, K.; Han, J.-F. De Ritis ratio (AST/ALT) as an independent predictor of poor outcome in patients with acute ischemic stroke. Neuropsychiatr. Dis. Treat. 2017, 13, 1551–1557. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Ding, C.; Hu, L.; Li, M.; Zhou, W.; Wang, T.; Zhu, L.; Bao, H.; Cheng, X. The association between AST/ALT ratio and all-cause and cardiovascular mortality in patients with hypertension. Medicine 2021, 100, e26693. [Google Scholar] [CrossRef]
- Steininger, M.; Winter, M.-P.; Reiberger, T.; Koller, L.; El-Hamid, F.; Forster, S.; Schnaubelt, S.; Hengstenberg, C.; Distelmaier, K.; Goliasch, G.; et al. De-Ritis Ratio Improves Long-Term Risk Prediction after Acute Myocardial Infarction. J. Clin. Med. 2018, 7, 474. [Google Scholar] [CrossRef] [Green Version]
- Lu, Z.; Ma, G.; Chen, L. De-Ritis Ratio Is Associated with Mortality after Cardiac Arrest. Dis. Mrk. 2020, 2020, 8826318. [Google Scholar] [CrossRef]
- Zinellu, A.; Arru, F.; De Vito, A.; Sassu, A.; Valdes, G.; Scano, V.; Zinellu, E.; Perra, R.; Madeddu, G.; Carru, C.; et al. The De Ritis ratio as prognostic biomarker of in-hospital mortality in COVID-19 patients. Eur. J. Clin. Invest. 2021, 51, e13427. [Google Scholar] [CrossRef]
- Pranata, R.; Huang, I.; Lim, M.A.; Yonas, E.; Vania, R.; Lukito, A.A.; Nasution, S.A.; Siswanto, B.B.; Kuswardhani, R.A.T. Elevated De Ritis Ratio Is Associated With Poor Prognosis in COVID-19: A Systematic Review and Meta-Analysis. Front. Med. 2021, 8, 676581. [Google Scholar] [CrossRef]
- Yazar, H.; Kayacan, Y.; Ozdin, M. De Ritis ratio and biochemical parameters in COVID-19 patients. Arch. Physiol. Biochem. 2022, 128, 1676–1680. [Google Scholar] [CrossRef]
- Chang, W.T.; Toh, H.S.; Liao, C.T.; Yu, W.L. Cardiac Involvement of COVID-19: A Comprehensive Review. Am. J. Med. Sci. 2021, 361, 14–22. [Google Scholar] [CrossRef]
- Eipel, C.; Abshagen, K.; Vollmar, B. Regulation of hepatic blood flow: The hepatic arterial buffer response revisited. World J. Gastroenterol. 2010, 16, 6046–6057. [Google Scholar] [CrossRef]
- Naeem, A.; Tabassum, S.; Gill, S.; Khan, M.Z.; Mumtaz, N.; Qaiser, Q.; Karamat, M.; Arif, M.; Naeem, F.; Afifi, A.; et al. COVID-19 and Cardiovascular Diseases: A Literature Review From Pathogenesis to Diagnosis. Cureus 2023, 15, e35658. [Google Scholar] [CrossRef]
- Greenway, C.V.; Stark, R.D. Hepatic vascular bed. Physiol. Rev. 1971, 51, 23–65. [Google Scholar] [CrossRef]
- Ewid, M.; Sherif, H.; Allihimy, A.S.; Alharbi, S.A.; Aldrewesh, D.A.; Alkuraydis, S.A.; Abazid, R. AST/ALT ratio predicts the functional severity of chronic heart failure with reduced left ventricular ejection fraction. BMC Res. Notes 2020, 13, 178. [Google Scholar] [CrossRef]
- Liu, F.; Liu, F.; Wang, L. COVID-19 and cardiovascular diseases. J. Mol. Cell Biol. 2021, 13, 161–167. [Google Scholar] [CrossRef]
- Garrido, I.; Liberal, R.; Macedo, G. Review article: COVID-19 and liver disease-what we know on 1st May 2020. Aliment. Pharm. Ther. 2020, 52, 267–275. [Google Scholar] [CrossRef]
- Ali, N. Relationship Between COVID-19 Infection and Liver Injury: A Review of Recent Data. Front. Med. 2020, 7, 458. [Google Scholar] [CrossRef]
- Li, H.; Zhu, H.; Yang, Z.; Tang, D.; Huang, L.; Xia, L. Tissue Characterization by Mapping and Strain Cardiac MRI to Evaluate Myocardial Inflammation in Fulminant Myocarditis. J. Magn. Reson. Imaging 2020, 52, 930–938. [Google Scholar] [CrossRef]
- Dolan, R.S.; Rahsepar, A.A.; Blaisdell, J.; Suwa, K.; Ghafourian, K.; Wilcox, J.E.; Khan, S.; Vorovich, E.E.; Rich, J.D.; Anderson, A.; et al. Multiparametric Cardiac Magnetic Resonance Imaging Can Detect Acute Cardiac Allograft Rejection After Heart Transplantation. JACC Cardiovasc. Imaging 2019, 12 Pt 2, 1632–1641. [Google Scholar] [CrossRef]
- Hinojar, R.; Varma, N.; Child, N.; Goodman, B.; Jabbour, A.; Yu, C.-Y.; Gebker, R.; Doltra, A.; Kelle, S.; Khan, S.; et al. T1 Mapping in Discrimination of Hypertrophic Phenotypes: Hypertensive Heart Disease and Hypertrophic Cardiomyopathy: Findings From the International T1 Multicenter Cardiovascular Magnetic Resonance Study. Circ. Cardiovasc. Imaging 2015, 8, e003285. [Google Scholar] [CrossRef] [Green Version]
- Puntmann, V.O.; Carerj, M.L.; Wieters, I.; Fahim, M.; Arendt, C.; Hoffmann, J.; Shchendrygina, A.; Escher, F.; Vasa-Nicotera, M.; Zeiher, A.M.; et al. Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered From Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 1265–1273, Erratum in JAMA Cardiol. 2020, 5, 1308. [Google Scholar] [CrossRef]
- Tangen, J.; Aukrust, P.; Barratt-Due, A.; Skulstad, H.; Edvardsen, T. Reduced Cardiac Function by Echocardiography in a Minority of COVID-19 Patients 3 Months after Hospitalization. J. Am. Soc. Echocardiogr. 2022, 35, 243–244. [Google Scholar] [CrossRef]
- Baruch, G.; Rothschild, E.; Sadon, S.; Szekely, Y.; Lichter, Y.; Kaplan, A.; Taieb, P.; Banai, A.; Hochstadt, A.; Merdler, I.; et al. Evolution of right and left ventricle routine and speckle-tracking echocardiography in patients recovering from coronavirus disease 2019: A longitudinal study. Eur. Heart J. Cardiovasc. Imaging 2022, 23, 1055–1065. [Google Scholar] [CrossRef]
| Low AST/ALT Ratio Group (n = 43) | High AST/ALT Ratio Group (n = 44) | p Value | |
|---|---|---|---|
| Patient characteristics | |||
| Age (years) | 43.7 ± 10.4 | 40.9 ± 9.3 | 0.224 |
| Gender | 0.599 | ||
| Male (n, %) | 25 (58.1%) | 28 (63.6%) | |
| Female (n, %) | 18 (41.9%) | 16 (36.4%) | |
| BMI (kg/m2) | 26.60 ± 3.73 | 25.51 ± 4.78 | 0.304 |
| Systolic arterial pressure (mmHg) | 116.5 ± 13.3 | 119.5 ± 10.6 | 0.198 |
| Diastolic arterial pressure (mmHg) | 75.0 ± 7.2 | 76.8 ± 7.4 | 0.340 |
| Laboratory Data | |||
| Hemoglobin, g/dL | 12.6 ± 2.32 | 13.7 ± 2.0 | 0.037 |
| Hematocrit (%) | 37.3 ± 6.7 | 39.2 ± 8.1 | 0.120 |
| White blood cell count (103/μL) | 7.7 ± 4.2 | 7.3 ± 2.5 | 0.840 |
| Neutrophil (103/μL) | 5.4 ± 3.8 | 5.1 ± 2.5 | 0.557 |
| Lymphocyte (103/μL) | 1.7 ± 0.9 | 1.7 ± 0.8 | 0.734 |
| Platelet (103/μL) | 310.0 ± 128.2 | 287.4 ± 94.5 | 0.376 |
| Serum creatinine (mg/dL) | 0.81 ± 0.14 | 0.87 ± 0.51 | 0.429 |
| Glucose (mg/dL) | 139.8 ± 75.0 | 151.8 ± 87.5 | 0.516 |
| Sodium (mEq/L) | 134.3 ± 21.7 | 138.3 ± 2.8 | 0.260 |
| Potassium (mEq/L) | 4.6 ± 0.5 | 4.5 ± 0.3 | 0.907 |
| AST (unit/L) | 22.8 ± 6.5 | 64.4 ± 43.1 | <0.001 |
| ALT (unit/L) | 53.8 ± 19.6 | 55.4 ± 26.9 | 0.749 |
| AST/ALT | 0.46 ± 0.14 | 1.14 ± 0.41 | <0.001 |
| Bilirubin (mg/dL) | 0.44 ± 0.26 | 0.40 ± 0.29 | 0.634 |
| Troponin-T (ng/L) | 1.59 ± 4.6 | 0.71 ± 1.24 | 0.809 |
| Albumin (g/L) | 38.0 ± 7.7 | 39.3 ± 4.2 | 0.410 |
| Total Protein (g/L) | 66.9 ± 4.7 | 75.2 ± 3.6 | <0.001 |
| C-reactive protein (CRP) (mg/dL) | 7.5 ± 34.1 | 13.9 ± 24.4 | <0.001 |
| D-Dimer (ug/mL) | 0.28 ± 0.15 | 1.05 ± 0.72 | <0.001 |
| Fibrinogen (mg/dL) | 406.9 ± 93.2 | 655.5 ± 102.3 | <0.001 |
| Prokalsitonin (ng/mL) | 0.04 ± 0.05 | 0.32 ± 1.34 | 0.120 |
| Lactate dehydrogenase (LDH) (unit/L) | 245.3 ± 60.3 | 256.5 ± 67.3 | 0.457 |
| Thyroid stimulating hormone (TSH) (mlU/L) | 1.54 ± 0.69 | 1.67 ± 0.86 | 0.700 |
| Low AST/ALT Ratio Group (n = 43) | High AST/ALT Ratio Group (n = 44) | p Value | |
|---|---|---|---|
| LVEDD (mm) | 45.4 ± 2.9 | 45.4 ± 3.1 | 0.917 |
| LVESD (mm) | 30.3 ± 2.0 | 30.5 ± 2.7 | 0.436 |
| LAD-AP (mm) | 30.6 ± 4.2 | 30.9 ± 3.7 | 0.598 |
| LAV maximum | 27.3 ± 12.1 | 44.7 ± 8.3 | <0.001 |
| LVEF (%) | 63.9 ± 4.1 | 58.9 ± 3.0 | <0.001 |
| IVS (mm) | 9.5 ± 1.1 | 9.2 ± 1.1 | 0.293 |
| PW (mm) | 9.4 ± 1.1 | 9.2 ± 1.1 | 0.303 |
| E/A ratio | 1.17 ± 0.64 | 1.15 ± 0.55 | 0.839 |
| Em lateral (cm/s) | 0.16 ± 0.05 | 0.14 ± 0.04 | 0.097 |
| Am lateral (cm/s) | 0.13 ± 0.04 | 0.12 ± 0.04 | 0.683 |
| IVRT (ms) | 80.5 ± 8.0 | 83.0 ± 7.3 | 0.172 |
| IVCT (ms) | 75.0 ± 7.3 | 76.6 ± 9.2 | 0.683 |
| DT (ms) | 180.4 ± 36.8 | 183.4 ± 38.0 | 0.466 |
| TAPSE (mm) | 25.3 ± 3.8 | 22.4 ± 2.4 | <0.001 |
| S’ (cm/s) | 0.18 ± 0.05 | 0.12 ± 0.03 | <0.001 |
| FAC (%) | 41.9 ± 7.6 | 34.8 ± 1.5 | <0.001 |
| LV-LS 4 chamber (%) | −19.2 ± 1.9 | −16.8 ± 1.2 | <0.001 |
| LV-LS 2 chamber (%) | −20.7 ± 2.8 | −17.9 ± 2.1 | <0.001 |
| LV-LS 3 chamber (%) | −18.9 ± 2.6 | −16.9 ± 1.4 | <0.001 |
| LV-GLS (%) | −19.4 ± 1.7 | −17.5 ± 1.0 | <0.001 |
| Low AST/ALT Ratio Group (n = 43) | High AST/ALT Ratio Group (n = 44) | p Value | |
|---|---|---|---|
| RVEDV | 140.2 ± 25.6 | 149.6 ± 35.0 | 0.240 |
| RVESV | 63.37 ± 15.98 | 74.91 ± 18.74 | 0.002 |
| RVSV | 55.11 ± 8.75 | 48.01 ± 4.97 | <0.001 |
| RVEF | 55.11 ± 8.75 | 48.01 ± 4.97 | <0.001 |
| T1 MAP Native | 1034.59 ± 48.60 | 1086.49 ± 32.38 | <0.001 |
| T2 MAP Native | 81.61 ± 23.11 | 99.95 ± 18.09 | 0.001 |
| ECV | 17.3 ± 5.0 | 36.2 ± 14.0 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karatas, M.; Keles, N.; Parsova, K.E.; Ciftci, H.O.; Ozkok, S.; Kahraman, E.; Durak, F.; Kocogullari, C.U.; Yiyit, N. High AST/ALT Ratio Is Associated with Cardiac Involvement in Acute COVID-19 Patients. Medicina 2023, 59, 1163. https://doi.org/10.3390/medicina59061163
Karatas M, Keles N, Parsova KE, Ciftci HO, Ozkok S, Kahraman E, Durak F, Kocogullari CU, Yiyit N. High AST/ALT Ratio Is Associated with Cardiac Involvement in Acute COVID-19 Patients. Medicina. 2023; 59(6):1163. https://doi.org/10.3390/medicina59061163
Chicago/Turabian StyleKaratas, Mesut, Nursen Keles, Kemal Emrecan Parsova, Hatice Ozge Ciftci, Sercin Ozkok, Erkan Kahraman, Furkan Durak, Cevdet Ugur Kocogullari, and Nurettin Yiyit. 2023. "High AST/ALT Ratio Is Associated with Cardiac Involvement in Acute COVID-19 Patients" Medicina 59, no. 6: 1163. https://doi.org/10.3390/medicina59061163






