New Options for Systemic Therapies in Intrahepatic Cholangiocarcinoma (iCCA)
Abstract
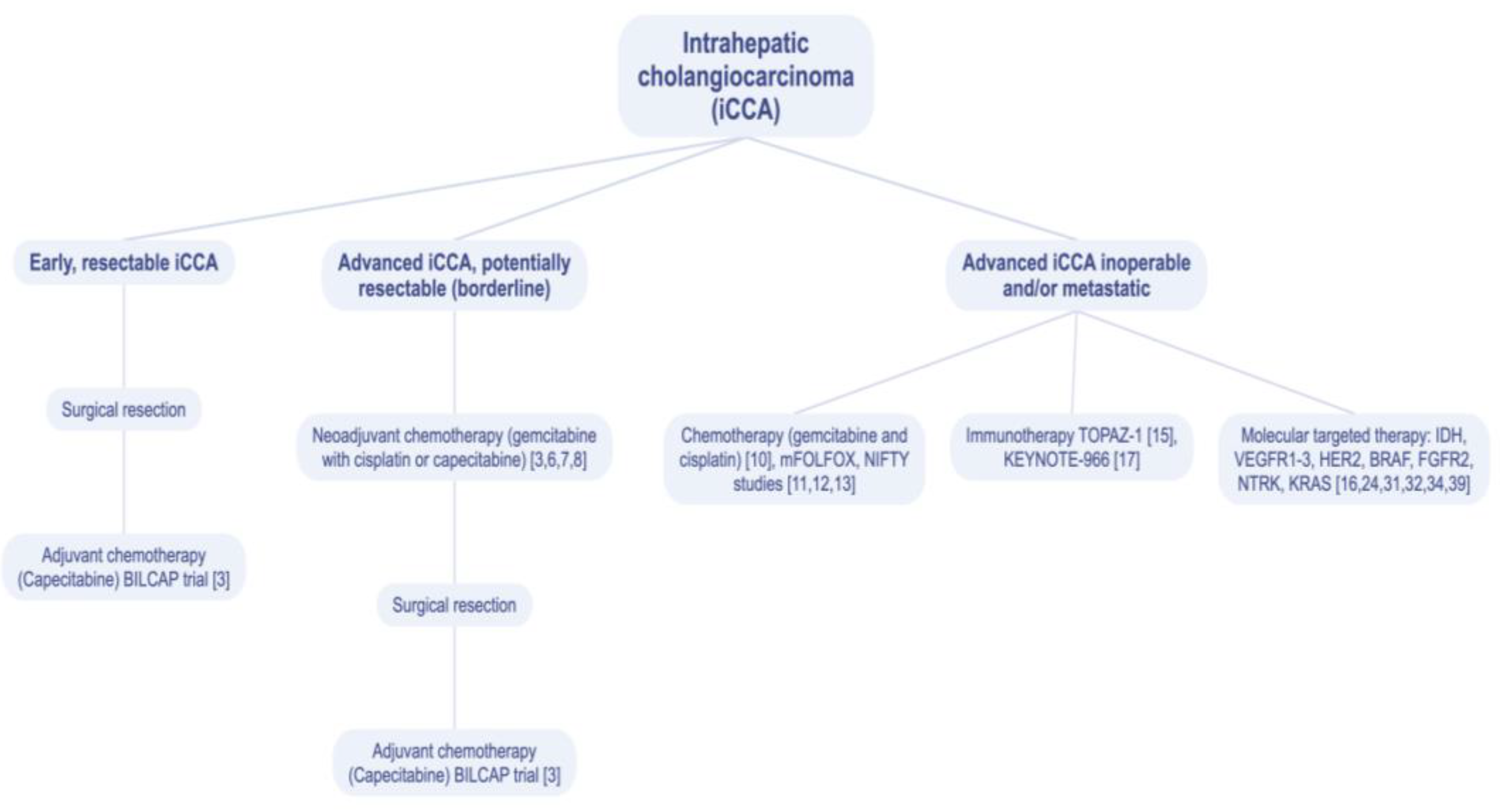
:1. Introduction
2. Chemotherapy
3. Immunotherapy
4. Targeted Treatment
| Molecularly Targeted Treatment iCCA | ||
|---|---|---|
| Molecular Target | Targeted Therapy | References |
| VEGFR1-3, PDGFRβ, FGFR1 | Regorafenib | [22] |
| IDH | Ivozydenib (ClarIDHy trial) | [24] |
| FGFR2 | Pemigatinib, infigratinib, futibatinib | [25,26,27] |
| HER-2-mutated | Trastuzumab deruxtecan (T-DXd) | [28] |
| HER-2-amplified | Trastuzumab, pertuzumab | [29] |
| BRAF V600E | Dabrafenib (ROAR trial) | [30] |
| BRCA1/2, PALB2 mutation | Niraparib (NCT 03207347 trial) | [31] |
| NTRK | Larotrectinib, entrectinib | [32,33] |
| KRAS G12C mutation | Adagrasib (KRISTAL-1 trial) | [34,35] |
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rizzo, A. Targeted Therapies in Advanced Cholangiocarcinoma: A Focus on FGFR Inhibitors. Medicina 2021, 57, 458. [Google Scholar] [CrossRef]
- Buettner, S.; Van Vugt, J.L.; IJzermans, J.N.; Koerkamp, B.G. Intrahepatic cholangiocarcinoma: Current perspectives. OncoTargets Ther. 2017, 10, 1131–1142. [Google Scholar] [CrossRef] [Green Version]
- Rizzo, A.; Brandi, G. Neoadjuvant therapy for cholangiocarcinoma: A comprehensive literature review. Cancer Treat. Res. Commun. 2021, 27, 100354. [Google Scholar] [CrossRef]
- Ejaz, A.; Cloyd, J.M.; Pawlik, T.M. Advances in the Diagnosis and Treatment of Patient with Intrahepatic Cholangiocarcinoma. Ann. Surg. Oncol. 2020, 27, 552–560. [Google Scholar] [CrossRef]
- Amini, N.; Ejaz, A.; Spolverato, G.; Kim, Y.; Herman, J.M.; Pawlik, T.M. Temporal trends in liver-directed therapy of patients with intrahepatic cholangiocarcinoma in the United States: A population-based analysis. J. Surg. Oncol. 2014, 110, 163–170. [Google Scholar] [CrossRef]
- Lunsford, K.E.; Javle, M.; Heyne, K.; Shroff, R.T.; Abdel-Wahab, R.; Gupta, N.; Mobley, C.M.; Saharia, A.; Victor, D.W.; Nguyen, D.T.; et al. Methodist-MD Anderson Joint Cholangiocarcinoma Collaborative Committee (NMAJCCC). Liver transplantation for locally advanced intrahepatic cholangiocarcinoma treated with neoadjuvant therapy: A prospective case-series. Lancet Gastroenterol. Hepatol. 2018, 3, 337–348. [Google Scholar] [CrossRef]
- Gemcitabine, Cisplatin, and Nab-Paclitaxel Before Surgery in Patients with High-Risk Liver Bile Duct Cancer (Trial No. NCT03579771). Available online: https://clinicaltrials.gov/ct2/show/NCT03579771?id=NCT03579771&draw=2&rank=1 (accessed on 7 June 2023).
- PD1 Antibody (Toripalimab), GEMOX and Lenvatinib Neoadjuvant Treatment for Resectable Intrahepatic Cholangiocarcinoma with High-Risk Recurrence Factors (Trial No. NCT04506281). Available online: https://clinicaltrials.gov/ct2/show/NCT04506281?id=NCT04506281&draw=2&rank=1 (accessed on 7 June 2023).
- Primrose, J.N.; Fox, R.P.; Palmer, D.H.; Malik, H.Z.; Prasad, R.; Mirza, D.; Anthony, A.; Corrie, P.; Falk, S.; Finch-Jones, M.; et al. BILCAP study group. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): A randomized, controlled, multicentre phase 3 study. Lancet Oncol. 2019, 20, 663–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valle, J.; Wasan, H.S.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. ABC-02 Trial Investigators. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamarca, A.; Palmer, D.H.; Wasan, H.S.; Ross, P.J.; Ma, Y.T.; Arora, A.; Falk, S.; Gillmore, R.; Wadsley, J.; Patel, K.; et al. Advanced Biliary Cancer Working Group. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): A phase 3, open-label, randomised, controlled trial. Lancet Oncol. 2021, 22, 690–701. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.; Brandi, G. First-line Chemotherapy in Advanced Biliary Tract Cancer Ten Years After the ABC-02 Trial: “And Yet It Moves!”. Cancer Treat. Res. Commun. 2021, 27, 100335. [Google Scholar] [CrossRef] [PubMed]
- Yoo, C.; Kim, K.P.; Jeong, J.H.; Kim, I.; Kang, M.J.; Cheon, J.; Kang, B.W.; Ryu, H.; Lee, J.S.; Kim, K.W.; et al. Liposomal irinotecan plus fluorouracil and leucovorin versus fluorouracil and leucovorin for metastatic biliary tract cancer after progression on gemcitabine plus cisplatin (NIFTY): A multicentre, open-label, randomised, phase 2b study. Lancet Oncol. 2021, 22, 1560–1572. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Sprengers, D.; Mancham, S.; Erkens, R.; Boor, P.P.; Van Beek, A.A.; Doukas, M.; Noordam, L.; Campos Carrascosa, L.; De Ruiter, V.; et al. Reduction of immunosuppressive tumor microenvironment in cholangiocarcinoma by ex vivo targeting immune checkpoint molecules. J. Hepatol. 2019, 71, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Sabbatino, F.; Villani, V.; Yearley, J.H.; Deshpande, V.; Cai, L.; Konstantinidis, I.T.; Moon, C.; Nota, S.; Wang, Y.; Al-Sukaini, A.; et al. PD-L1 and HLA class I antigen expression and clinical course of the disease in intrahepatic cholangiocarcinoma. Clin. Cancer Res. 2016, 22, 470–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, D.Y.; He, A.R.; Qin, S.; Chen, L.T.; Okusaka, T.; Vogel, A.; Kim, J.W.; Suksombooncharoen, T.; Lee, M.A.; Kitano, M.; et al. The TOPAZ-1 Investigators. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer. NEJM Evid. 2022, 1, 1–11. [Google Scholar] [CrossRef]
- Kelly, R.K.; Ueno, M.; Yoo, C.; Finn, R.S.; Furuse, J.; Ren, Z.; Yau, T.; Klümpen, H.-J.; Chan, S.L.; Ozaka, M.; et al. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): A randomized, double-blind, placebo-controlled, phase 3 trial. Lancet 2023, 401, 1853–1865. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch repair deficiency predict response of solid tumors to PD-1 blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef] [Green Version]
- Maio, M.; Ascierto, P.A.; Manzyuk, L.; Motola-Kuba, D.; Penel, N.; Cassier, P.A.; Bariani, G.M.; De Jesus Acosta, A.; Doi, T.; Longo, F.; et al. Pembrolizumab in microsatellite instability high or mismatch repair deficient cancers: Updated analysis from the phase II KEYNOTE-158 study. Ann. Oncol. 2022, 33, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Marcus, L.; Fashoyin-Aje, L.A.; Donoghue, M.; Yuan, M.; Rodriguez, L.; Gallagher, P.S.; Philip, R.; Ghosh, S.; Theoret, M.R.; Beaver, J.A.; et al. FDA Approval Summary: Pembrolizumab for the treatment of Tumor Mutational Burden-High Solid Tumors. Clin. Cancer Res. 2021, 27, 4685–4689. [Google Scholar] [CrossRef]
- Schenker, M.; Burotto, M.; Richardet, M.; Ciuleanu, T.; Goncalves, A.; Steeghs, N.; Schöffski, P.; Ascierto, P.A.; Maio, M.; Lugowska, I.; et al. Abstract CT022: CheckMate 848: A randomized, open label, phase 2 study of nivolumab in combination with ipilimumab or nivolumab monotherapy in patients with advanced or metastatic solid tumors of high tumor mutational burden. Cancer Res. 2022, 82 (Suppl. S12), CT022. [Google Scholar] [CrossRef]
- Sun, W.; Patel, A.; Normolle, D.; Patel, K.; Ohr, J.; Lee, J.J.; Bahary, N.; Chu, E.; Streeter, N.; Drummond, S. A phase 2 trial of regorafenib as a single agent in patients with chemotherapy-refractory, advanced, and metastatic biliary tract adenocarcinoma. Cancer 2019, 125, 902–909. [Google Scholar] [CrossRef]
- Vithayathil, M.; Bridegwater, J.; Khan, S.A. Medical therapies for intra-hepatic cholangiocarcinoma. J. Hepatol. 2021, 75, 981–983. [Google Scholar] [CrossRef] [PubMed]
- Abou-Alfa, G.K.; Macarulla, T.; Javle, M.M.; Kelley, R.K.; Lubner, S.J.; Adeva, J.; Cleary, J.M.; Catenacci, D.V.; Borad, M.J.; Bridgewater, J.; et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): A multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020, 21, 796–807. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Sahai, V.; Hollebecque, A.; Vaccaro, G.; Melisi, D.; Al-Rajabi, R.; Paulson, A.S.; Borad, M.J.; Gallinson, D.; Murphy, A.G.; et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: A multicentre, open-label, phase 2 study. Lancet Oncol. 2020, 21, 671–684. [Google Scholar] [CrossRef] [PubMed]
- Meric-Bernstam, F.; Bahleda, R.; Hierro, C.; Sanson, M.; Bridgewater, J.; Arkenau, H.T.; Tran, B.; Kelley, R.K.; Park, J.O.; Javle, M.; et al. Futibatinib, an irreversible FGFR1-4 inhibitor, in patients with advanced solid tumors harboring FGF/FGFR aberrations: A phase I dose-expansion study. Cancer Discov. 2022, 12, 402–415. [Google Scholar] [CrossRef] [PubMed]
- Goyal, L.; Meric-Bernstam, F.; Hollebecque, A.; Valle, J.W.; Morizane, C.; Karasic, T.B.; Abrams, T.A.; Furuse, J.; Kelley, R.K.; Cassier, P.A.; et al. FOENIX-CCA2 Study Investigators. Futibatinib for FGFR2-Rearranged Intrahepatic Cholangiocarcinoma. N. Engl. J. Med. 2023, 388, 228–239. [Google Scholar] [CrossRef]
- A Phase 2 Study of T-DXd in Patients with Selected HER2 Expressing Tumors (Trial No. NCT04482309). Available online: https://clinicaltrials.gov/ct2/show/NCT04482309?id=NCT04482309&draw=2&rank=1 (accessed on 7 June 2023).
- Javle, M.; Borad, M.J.; Azad, N.S.; Kurzrock, R.; Abou-Alfa, G.K.; George, B.; Hainsworth, J.; Meric-Bernstam, F.; Swanton, C.; Sweeney, C.J.; et al. Pertuzumab and trastuzumab for HER2-positive, metastatic biliary tract cancer (MyPathway): A multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 2021, 22, 1290–1300. [Google Scholar] [CrossRef]
- Subbiah, V.; Lassen, U.; Élez, E.; Italiano, A.; Curigliano, G.; Javle, M.; De Braud, F.; Prager, G.W.; Greil, R.; Stein, A.; et al. Dabrafenib plus trametinib in patients with BRAFV600E—Mutated biliary tract cancer (ROAR): A phase 2, open-label, single-arm, multicentre basket trial. Lancet Oncol. 2020, 21, 1234–1243. [Google Scholar] [CrossRef]
- A Trial of Niraparib in BAP1 and Other DNA Damage Response (DDR) Deficient Neoplasms (UF-STO-ETI-001) (Trial No. NCT03207347). Available online: https://clinicaltrials.gov/ct2/show/NCT03207347?id=NCT03207347&draw=2&rank=1 (accessed on 7 June 2023).
- Drilon, A.; Laetsch, T.W.; Kummar, S.; DuBois, S.G.; Lassen, U.N.; Demetri, G.D.; Nathenson, M.; Doebele, R.C.; Farago, A.F.; Pappo, A.S.; et al. Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N. Engl. J. Med. 2018, 378, 731–739. [Google Scholar] [CrossRef]
- Doebele, R.C.; Drilon, A.; Paz-Ares, L.; Siena, S.; Shaw, A.T.; Farago, A.F.; Blakely, C.M.; Seto, T.; Cho, B.C.; Tosi, D.; et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: Integrated analysis of three phase 1–2 trials. Lancet Oncol. 2020, 21, 271–282. [Google Scholar] [CrossRef]
- Loong, H.H.F.; Du, N.; Cheng, C.; Lin, H.; Guo, J.; Lin, G.; Li, M.; Jiang, T.; Shi, Z.; Cui, Y.; et al. KRAS G12C mutations in Asia: A landscape analysis of 11,951 Chinese tumor samples. Transl. Lung Cancer Res. 2020, 9, 1759–1796. [Google Scholar] [CrossRef]
- Bekaii-Saab, T.S.; Spira, A.I.; Yaeger, R.; Buchschacher, G.L.; McRee, A.J.; Sabari, J.K.; Johnson, M.L.; Barve, M.A.; Hafez, N.; Velastegui, K.; et al. KRYSTAL-1: Updated activity and safety of adagrasib (MRTX849) in patients (Pts) with unresectable or metastatic pancreatic cancer (PDAC) and other gastrointestinal (GI) tumors harboring a KRAS g12C mutation. J. Clin. Oncol. 2022, 40 (Suppl. S4), 519. [Google Scholar] [CrossRef]
- Futibatinib Versus Gemcitabine-Cisplatin Chemotherapy as First-Line Treatment of Patients with Advanced Cholangiocarcinoma Harboring FGFR2 Gene Rearrangements (Trial No. NCT04093362). Available online: https://clinicaltrials.gov/ct2/show/NCT04093362?id=NCT04093362&draw=2&rank=1 (accessed on 7 June 2023).
- Vogel, A.; Bridgewater, J.; Edeline, J.; Kelley, R.K.; Klümpen, H.J.; Malka, D.; Primrose, J.N.; Rimassa, L.; Stenzinger, A.; Valle, J.W.; et al. ESMO Guidelines Committee. Biliary tract cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 127–140. [Google Scholar] [CrossRef]
- Domagała, P.; Kowalik, A. Examination of molecular markers used in the treatment of colon cancer. Pol. J. Pathol. 2014, 65 (Suppl. S1), S59–S77. [Google Scholar] [PubMed]
- Grossmann, A.H.; Samowitz, W.S. Epidermal growth factor receptor pathway mutations and colorectal cancer therapy. Arch. Pathol. Lab. Med. 2011, 135, 1278–1282. [Google Scholar] [CrossRef]
- Salem, M.; El-Refai, S.; Sha, W.; Grothey, A.; Puccini, A.; George, T.; Hwang, J.; Kadakia, K.; Musselwhite, L.; Van Cutsem, E.; et al. O-3 Characterization of KRAS mutation variants and prevalence of KRAS-G12C in gastrointestinal malignancies. In Proceedings of the ESMO World Congress on Gastrointestinal Cancer, Virtual, 30 June–3 July 2021. [Google Scholar]
- Skoulidis, F.; Li, B.T.; Dy, G.K.; Price, T.J.; Falchook, G.S.; Wolf, J.; Italiano, A.; Schuler, M.; Borghaei, H.; Barlesi, F.; et al. Sotorasib for Lung Cancers with KRAS p.G12C Mutation. N. Engl. J. Med. 2021, 384, 2371–2381. [Google Scholar] [CrossRef] [PubMed]
- Bridgewater, J.; Galle, P.R.; Khan, S.A.; Llovet, J.M.; Park, J.W.; Patel, T.; Pawlik, T.M.; Gores, G.J. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J. Hepatol. 2014, 60, 1268–1289. [Google Scholar] [CrossRef] [Green Version]
- Suda, R.; Saki, N.; Matsushita, K.; Ishige, T.; Kawasaki, Y.; Shiko, Y.; Furukawa, K.; Mishima, T.; Nakadai, E.; Ohtsuka, M. Prediction of mismatch repair deficient biliary tract cancer: Role of morphological features and host immune response detected by routine hematoxylin- eosin staining. J. Hepatobiliary Pancreat. Sci. 2021, 28, 680–691. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Becht, R.; Wasilewicz, M.P. New Options for Systemic Therapies in Intrahepatic Cholangiocarcinoma (iCCA). Medicina 2023, 59, 1174. https://doi.org/10.3390/medicina59061174
Becht R, Wasilewicz MP. New Options for Systemic Therapies in Intrahepatic Cholangiocarcinoma (iCCA). Medicina. 2023; 59(6):1174. https://doi.org/10.3390/medicina59061174
Chicago/Turabian StyleBecht, Rafał, and Michał P. Wasilewicz. 2023. "New Options for Systemic Therapies in Intrahepatic Cholangiocarcinoma (iCCA)" Medicina 59, no. 6: 1174. https://doi.org/10.3390/medicina59061174
APA StyleBecht, R., & Wasilewicz, M. P. (2023). New Options for Systemic Therapies in Intrahepatic Cholangiocarcinoma (iCCA). Medicina, 59(6), 1174. https://doi.org/10.3390/medicina59061174







