Epithelial Ovarian Cancer: A Five Year Review
Abstract
:1. Introduction
2. Morphology and Insights
3. Current Data Based on Studies
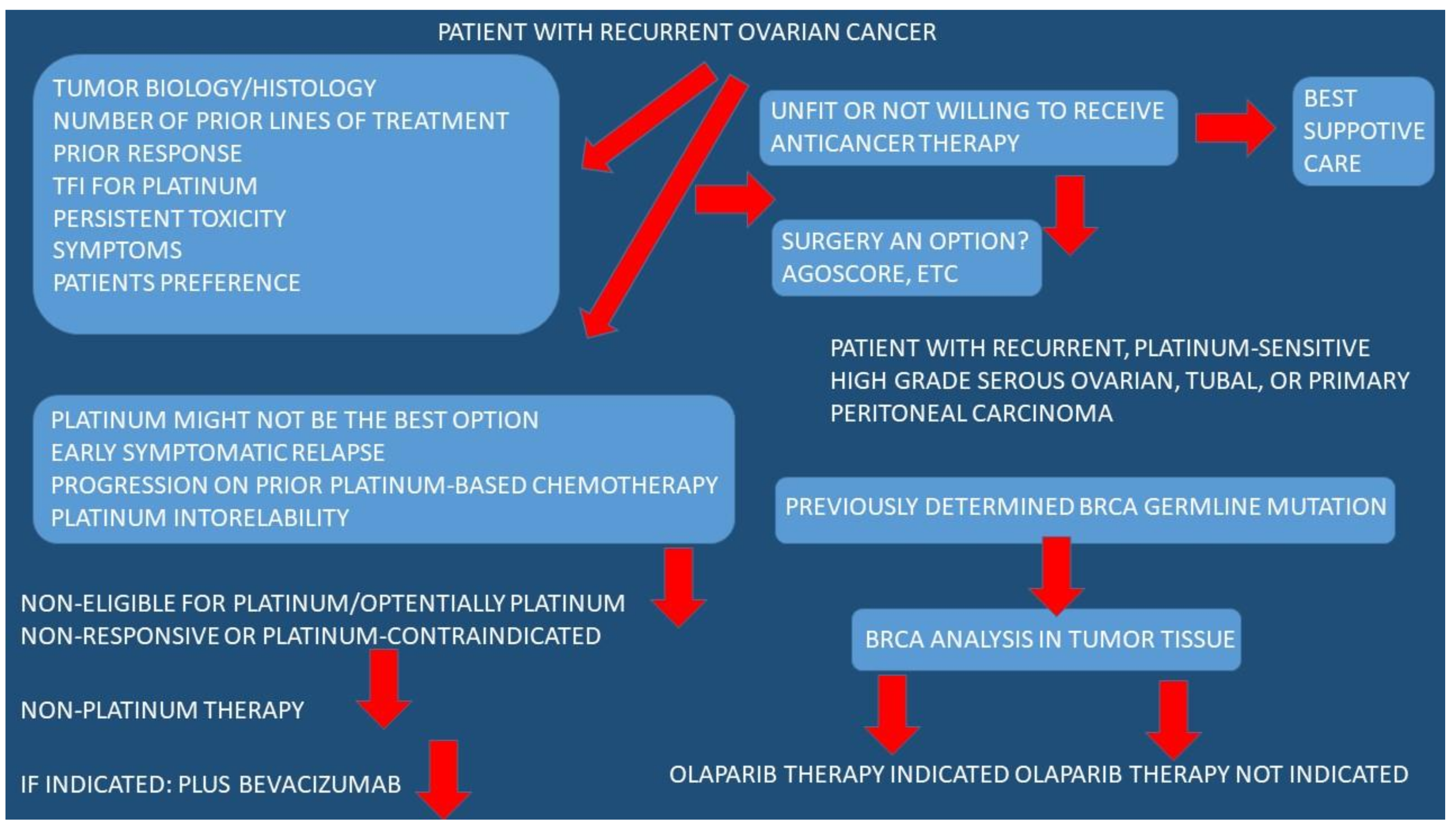
3.1. Conventional Treatment
3.2. Biological Treatment
3.3. Ovarian Cancer and Stem Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Doll, K.M.; Rademaker, A.; Sosa, J.A. Practical Guide to Surgical Data Sets: Surveillance, Epidemiology, and End Results (SEER) Database. JAMA Surg. 2018, 153, 588–589. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA A Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Jelovac, D.; Armstrong, D.K. Recent progress in the diagnosis and treatment of ovarian cancer. CA A Cancer J. Clin. 2011, 61, 183–203. [Google Scholar] [CrossRef]
- Elzakkers, J.C.J.; van der Aa, M.A.; van Altena, A.M.; de Hullu, J.A.; Harmsen, M.G. Further insights into the role of tumour characteristics in survival of young women with epithelial ovarian cancer. Gynecol. Oncol. 2019, 155, 213–219. [Google Scholar] [CrossRef]
- Cress, R.D.; Chen, Y.S.; Morris, C.R.; Petersen, M.; Leiserowitz, G.S. Characteristics of Long-Term Survivors of Epithelial Ovarian Cancer. Obstet. Gynecol. 2015, 126, 491–497. [Google Scholar] [CrossRef]
- Atallah, G.A.; Abd Aziz, N.H.; Teik, C.K.; Shafiee, M.N.; Kampan, N.C. New Predictive Biomarkers for Ovarian Cancer. Diagnostics 2021, 11, 465. [Google Scholar] [CrossRef]
- Cheng, B.; Lu, W.; Xiaoyun, W.; YaXia, C.; Xie, X. Extra-abdominal metastases from epithelial ovarian carcinoma: An analysis of 20 cases. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2009, 19, 611–614. [Google Scholar] [CrossRef]
- Peres, L.C.; Cushing-Haugen, K.L.; Kobel, M.; Harris, H.R.; Berchuck, A.; Rossing, M.A.; Schildkraut, J.M.; Doherty, J.A. Invasive Epithelial Ovarian Cancer Survival by Histotype and Disease Stage. J. Natl. Cancer Inst. 2019, 111, 60–68. [Google Scholar] [CrossRef] [Green Version]
- Guth, U.; Huang, D.J.; Bauer, G.; Stieger, M.; Wight, E.; Singer, G. Metastatic patterns at autopsy in patients with ovarian carcinoma. Cancer 2007, 110, 1272–1280. [Google Scholar] [CrossRef]
- Marchetti, C.; Ferrandina, G.; Cormio, G.; Gambino, A.; Cecere, S.; Lorusso, D.; De Giorgi, U.; Bogliolo, S.; Fagotti, A.; Mammoliti, S.; et al. Brain metastases in patients with EOC: Clinico-pathological and prognostic factors. A multicentric retrospective analysis from the MITO group (MITO 19). Gynecol. Oncol. 2016, 143, 532–538. [Google Scholar] [CrossRef]
- Gasparri, M.L.; Grandi, G.; Bolla, D.; Gloor, B.; Imboden, S.; Panici, P.B.; Mueller, M.D.; Papadia, A. Hepatic resection during cytoreductive surgery for primary or recurrent epithelial ovarian cancer. J. Cancer Res. Clin. Oncol. 2016, 142, 1509–1520. [Google Scholar] [CrossRef]
- Yu, Z.; Pestell, T.G.; Lisanti, M.P.; Pestell, R.G. Cancer stem cells. Int. J. Biochem. Cell Biol. 2012, 44, 2144–2151. [Google Scholar] [CrossRef] [Green Version]
- Colak, S.; Medema, J.P. Cancer stem cells--important players in tumor therapy resistance. FEBS J. 2014, 281, 4779–4791. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Visvader, J.E.; Lindeman, G.J. Cancer stem cells in solid tumours: Accumulating evidence and unresolved questions. Nat. Rev. Cancer 2008, 8, 755–768. [Google Scholar] [CrossRef]
- Maugeri-Sacca, M.; Vigneri, P.; De Maria, R. Cancer stem cells and chemosensitivity. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2011, 17, 4942–4947. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.; Deleyrolle, L.; Vedam-Mai, V.; Azari, H.; Abd-El-Barr, M.; Reynolds, B.A. The cancer stem cell hypothesis: Failures and pitfalls. Neurosurgery 2011, 68, 531–545, discussion 545. [Google Scholar] [CrossRef] [Green Version]
- Keyvani, V.; Farshchian, M.; Esmaeili, S.A.; Yari, H.; Moghbeli, M.; Nezhad, S.K.; Abbaszadegan, M.R. Ovarian cancer stem cells and targeted therapy. J. Ovarian Res. 2019, 12, 120. [Google Scholar] [CrossRef] [Green Version]
- Bapat, S.A.; Mali, A.M.; Koppikar, C.B.; Kurrey, N.K. Stem and progenitor-like cells contribute to the aggressive behavior of human epithelial ovarian cancer. Cancer Res. 2005, 65, 3025–3029. [Google Scholar] [CrossRef] [Green Version]
- Zou, K.; Yuan, Z.; Yang, Z.; Luo, H.; Sun, K.; Zhou, L.; Xiang, J.; Shi, L.; Yu, Q.; Zhang, Y.; et al. Production of offspring from a germline stem cell line derived from neonatal ovaries. Nat. Cell Biol. 2009, 11, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Flesken-Nikitin, A.; Hwang, C.I.; Cheng, C.Y.; Michurina, T.V.; Enikolopov, G.; Nikitin, A.Y. Ovarian surface epithelium at the junction area contains a cancer-prone stem cell niche. Nature 2013, 495, 241–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szotek, P.P.; Pieretti-Vanmarcke, R.; Masiakos, P.T.; Dinulescu, D.M.; Connolly, D.; Foster, R.; Dombkowski, D.; Preffer, F.; Maclaughlin, D.T.; Donahoe, P.K. Ovarian cancer side population defines cells with stem cell-like characteristics and Mullerian Inhibiting Substance responsiveness. Proc. Natl. Acad. Sci. USA 2006, 103, 11154–11159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubeau, L.; Drapkin, R. Coming into focus: The nonovarian origins of ovarian cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2013, 24 (Suppl. S8), viii28–viii35. [Google Scholar] [CrossRef]
- Flesken-Nikitin, A.; Choi, K.C.; Eng, J.P.; Shmidt, E.N.; Nikitin, A.Y. Induction of carcinogenesis by concurrent inactivation of p53 and Rb1 in the mouse ovarian surface epithelium. Cancer Res. 2003, 63, 3459–3463. [Google Scholar]
- Lee, H.Y.; Hong, I.S. Double-edged sword of mesenchymal stem cells: Cancer-promoting versus therapeutic potential. Cancer Sci. 2017, 108, 1939–1946. [Google Scholar] [CrossRef] [Green Version]
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA A Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef] [Green Version]
- Shaked, Y. The pro-tumorigenic host response to cancer therapies. Nat. Rev. Cancer 2019, 19, 667–685. [Google Scholar] [CrossRef]
- Eble, J.A.; Niland, S. The extracellular matrix in tumor progression and metastasis. Clin. Exp. Metastasis 2019, 36, 171–198. [Google Scholar] [CrossRef]
- Ozbek, S.; Balasubramanian, P.G.; Chiquet-Ehrismann, R.; Tucker, R.P.; Adams, J.C. The evolution of extracellular matrix. Mol. Biol. Cell 2010, 21, 4300–4305. [Google Scholar] [CrossRef] [Green Version]
- Naba, A.; Clauser, K.R.; Ding, H.; Whittaker, C.A.; Carr, S.A.; Hynes, R.O. The extracellular matrix: Tools and insights for the “omics” era. Matrix Biol. J. Int. Soc. Matrix Biol. 2016, 49, 10–24. [Google Scholar] [CrossRef]
- Winkler, J.; Abisoye-Ogunniyan, A.; Metcalf, K.J.; Werb, Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat. Commun. 2020, 11, 5120. [Google Scholar] [CrossRef]
- Hastings, J.F.; Skhinas, J.N.; Fey, D.; Croucher, D.R.; Cox, T.R. The extracellular matrix as a key regulator of intracellular signalling networks. Br. J. Pharmacol. 2019, 176, 82–92. [Google Scholar] [CrossRef] [Green Version]
- Kechagia, J.Z.; Ivaska, J.; Roca-Cusachs, P. Integrins as biomechanical sensors of the microenvironment. Nat. Rev. Mol. Cell Biol. 2019, 20, 457–473. [Google Scholar] [CrossRef]
- Case, L.B.; Waterman, C.M. Integration of actin dynamics and cell adhesion by a three-dimensional, mechanosensitive molecular clutch. Nat. Cell Biol. 2015, 17, 955–963. [Google Scholar] [CrossRef]
- Sun, Z.; Guo, S.S.; Fassler, R. Integrin-mediated mechanotransduction. J. Cell Biol. 2016, 215, 445–456. [Google Scholar] [CrossRef] [Green Version]
- Alexander, V.M.; Gordon, A.N.; Howard, D.H.; Khanna, N. Outcomes and Cost Analysis of Surveillance Strategies After Initial Treatment for Women with Recurrent Ovarian Cancer. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2017, 27, 1333–1342. [Google Scholar] [CrossRef]
- Aigner, K.R.; Gailhofer, S.; Aigner, K. Hypoxic isolated abdominal perfusion breaks through chemoresistance in recurrent FIGO stage IIIC and IV ovarian cancer. Mol. Clin. Oncol. 2021, 14, 129. [Google Scholar] [CrossRef]
- Banik, B.; Ashokan, A.; Choi, J.H.; Surnar, B.; Dhar, S. Platin-C containing nanoparticles: A recipe for the delivery of curcumin-cisplatin combination chemotherapeutics to mitochondria. Dalton Trans. 2023, 52, 3575–3585. [Google Scholar] [CrossRef]
- Yee, S.S.; Risinger, A.L. Efficacy of a Covalent Microtubule Stabilizer in Taxane-Resistant Ovarian Cancer Models. Molecules 2021, 26, 4077. [Google Scholar] [CrossRef]
- Lee, H.R.; Kim, D.W.; Jones, V.O.; Choi, Y.; Ferry, V.E.; Geller, M.A.; Azarin, S.M. Sonosensitizer-Functionalized Graphene Nanoribbons for Adhesion Blocking and Sonodynamic Ablation of Ovarian Cancer Spheroids. Adv. Healthc. Mater. 2021, 10, e2001368. [Google Scholar] [CrossRef] [PubMed]
- Olesen, K.D.; Larsen, A.T.R.; Jensen, L.H.; Steffensen, K.D.; Sondergaard, S.R. Treosulfan in platinum-resistant ovarian cancer. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2021, 31, 1045–1051. [Google Scholar] [CrossRef] [PubMed]
- Swisher, E.M.; Kwan, T.T.; Oza, A.M.; Tinker, A.V.; Ray-Coquard, I.; Oaknin, A.; Coleman, R.L.; Aghajanian, C.; Konecny, G.E.; O’Malley, D.M.; et al. Molecular and clinical determinants of response and resistance to rucaparib for recurrent ovarian cancer treatment in ARIEL2 (Parts 1 and 2). Nat. Commun. 2021, 12, 2487. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Wang, J.; Li, H.; Yu, Y.; Wang, X.; Lu, L.; Lv, C.; Chang, B.; Jin, W.; Guo, W.; et al. Extracellular matrix protein-1 secretory isoform promotes ovarian cancer through increasing alternative mRNA splicing and stemness. Nat. Commun. 2021, 12, 4230. [Google Scholar] [CrossRef]
- Bhojnagarwala, P.S.; Perales-Puchalt, A.; Cooch, N.; Sardesai, N.Y.; Weiner, D.B. A synDNA vaccine delivering neoAg collections controls heterogenous, multifocal murine lung and ovarian tumors via robust T cell generation. Mol. Ther. Oncolytics 2021, 21, 278–287. [Google Scholar] [CrossRef]
- Thouvenin, L.; Charrier, M.; Clement, S.; Christinat, Y.; Tille, J.C.; Frigeri, M.; Homicsko, K.; Michielin, O.; Bodmer, A.; Chappuis, P.O.; et al. Ovarian cancer with high-level focal ERBB2 amplification responds to trastuzumab and pertuzumab. Gynecol. Oncol. Rep. 2021, 37, 100787. [Google Scholar] [CrossRef]
- Larroque, M.; Mounicou, S.; Sgarbura, O.; Arnaudguilhem, C.; Rebel, L.; Leaha, C.; Faye, P.A.; Enjalbal, C.; Quenet, F.; Bouyssiere, B.; et al. Study of oxaliplatin penetration into ovaries of patients treated with hyperthermic intraperitoneal chemotherapy (HIPEC) for peritoneal metastases of colorectal and appendiceal origin using mass spectrometry imaging. Pleura Peritoneum 2021, 6, 67–74. [Google Scholar] [CrossRef]
- Valenciaga, A.; Iwenofu, O.H.; Tinoco, G. Larotrectinib in a Patient With Advanced Pleomorphic Liposarcoma of the Uterus. J. Natl. Compr. Cancer Netw. JNCCN 2021, 19, 775–779. [Google Scholar] [CrossRef]
- Kang, Y.; Flores, L.; Ngai, H.W.; Cornejo, Y.R.; Haber, T.; McDonald, M.; Moreira, D.F.; Gonzaga, J.M.; Abidi, W.; Zhang, Y.; et al. Large, Anionic Liposomes Enable Targeted Intraperitoneal Delivery of a TLR 7/8 Agonist To Repolarize Ovarian Tumors’ Microenvironment. Bioconjug. Chem. 2021, 32, 1581–1592. [Google Scholar] [CrossRef]
- Wang, C.; Wang, J.; Han, X.; Liu, J.; Ma, M.; Tian, S.; Zhang, L.; Tang, J. Ultra-small lipid carriers with adjustable release profiles for synergistic treatment of drug-resistant ovarian cancer. Nanotechnology 2022, 33, 355102. [Google Scholar] [CrossRef]
- Gonzalez-Junca, A.; Liu, F.D.; Nagaraja, A.S.; Mullenix, A.; Lee, C.T.; Gordley, R.M.; Frimannsson, D.O.; Maller, O.; Garrison, B.S.; Iyer, D.; et al. SENTI-101, a Preparation of Mesenchymal Stromal Cells Engineered to Express IL12 and IL21, Induces Localized and Durable Antitumor Immunity in Preclinical Models of Peritoneal Solid Tumors. Mol. Cancer Ther. 2021, 20, 1508–1520. [Google Scholar] [CrossRef]
- Chen, Y.; Li, F.; Li, D.; Liu, W.; Zhang, L. Atezolizumab and blockade of LncRNA PVT1 attenuate cisplatin resistant ovarian cancer cells progression synergistically via JAK2/STAT3/PD-L1 pathway. Clin. Immunol. 2021, 227, 108728. [Google Scholar] [CrossRef]
- Vaughan, S.; Coward, J.I.; Bast, R.C., Jr.; Berchuck, A.; Berek, J.S.; Brenton, J.D.; Coukos, G.; Crum, C.C.; Drapkin, R.; Etemadmoghadam, D.; et al. Rethinking ovarian cancer: Recommendations for improving outcomes. Nat. Rev. Cancer 2011, 11, 719–725. [Google Scholar] [CrossRef] [Green Version]
- Kartal-Yandim, M.; Adan-Gokbulut, A.; Baran, Y. Molecular mechanisms of drug resistance and its reversal in cancer. Crit. Rev. Biotechnol. 2016, 36, 716–726. [Google Scholar] [CrossRef] [Green Version]
- Yousefi, B.; Zarghami, N.; Samadi, N.; Majidinia, M. Peroxisome Proliferator-Activated Receptors and their Ligands in Cancer Drug- Resistance: Opportunity or Challenge. Anti-Cancer Agents Med. Chem. 2016, 16, 1541–1548. [Google Scholar] [CrossRef]
- Lengyel, E. Ovarian cancer development and metastasis. Am. J. Pathol. 2010, 177, 1053–1064. [Google Scholar] [CrossRef]
- Colombo, N.; Lorusso, D.; Scollo, P. Impact of Recurrence of Ovarian Cancer on Quality of Life and Outlook for the Future. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2017, 27, 1134–1140. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Ramena, G.; Elble, R.C. The role of cancer stem cells in relapse of solid tumors. Front. Biosci. 2012, 4, 1528–1541. [Google Scholar] [CrossRef]
- Mihanfar, A.; Aghazadeh Attari, J.; Mohebbi, I.; Majidinia, M.; Kaviani, M.; Yousefi, M.; Yousefi, B. Ovarian cancer stem cell: A potential therapeutic target for overcoming multidrug resistance. J. Cell. Physiol. 2019, 234, 3238–3253. [Google Scholar] [CrossRef]
- Paik, D.Y.; Janzen, D.M.; Schafenacker, A.M.; Velasco, V.S.; Shung, M.S.; Cheng, D.; Huang, J.; Witte, O.N.; Memarzadeh, S. Stem-like epithelial cells are concentrated in the distal end of the fallopian tube: A site for injury and serous cancer initiation. Stem Cells 2012, 30, 2487–2497. [Google Scholar] [CrossRef] [Green Version]
- Mansoori, M.; Roudi, R.; Abbasi, A.; Abolhasani, M.; Abdi Rad, I.; Shariftabrizi, A.; Madjd, Z. High GD2 expression defines breast cancer cells with enhanced invasiveness. Exp. Mol. Pathol. 2019, 109, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.K.; Pujade-Lauraine, E.; Aoki, D.; Mirza, M.R.; Lorusso, D.; Oza, A.M.; du Bois, A.; Vergote, I.; Reuss, A.; Bacon, M.; et al. Fifth Ovarian Cancer Consensus Conference of the Gynecologic Cancer InterGroup: Recurrent disease. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017, 28, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Pignata, S.; Lorusso, D.; Joly, F.; Gallo, C.; Colombo, N.; Sessa, C.; Bamias, A.; Salutari, V.; Selle, F.; Frezzini, S.; et al. Carboplatin-based doublet plus bevacizumab beyond progression versus carboplatin-based doublet alone in patients with platinum-sensitive ovarian cancer: A randomised, phase 3 trial. Lancet Oncol. 2021, 22, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Luvero, D.; Milani, A.; Ledermann, J.A. Treatment options in recurrent ovarian cancer: Latest evidence and clinical potential. Ther. Adv. Med. Oncol. 2014, 6, 229–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomao, F.; D’Incalci, M.; Biagioli, E.; Peccatori, F.A.; Colombo, N. Restoring platinum sensitivity in recurrent ovarian cancer by extending the platinum-free interval: Myth or reality? Cancer 2017, 123, 3450–3459. [Google Scholar] [CrossRef] [Green Version]
- Wagner, U.; Marth, C.; Largillier, R.; Kaern, J.; Brown, C.; Heywood, M.; Bonaventura, T.; Vergote, I.; Piccirillo, M.C.; Fossati, R.; et al. Final overall survival results of phase III GCIG CALYPSO trial of pegylated liposomal doxorubicin and carboplatin vs paclitaxel and carboplatin in platinum-sensitive ovarian cancer patients. Br. J. Cancer 2012, 107, 588–591. [Google Scholar] [CrossRef] [Green Version]
- Pujade-Lauraine, E.; Wagner, U.; Aavall-Lundqvist, E.; Gebski, V.; Heywood, M.; Vasey, P.A.; Volgger, B.; Vergote, I.; Pignata, S.; Ferrero, A.; et al. Pegylated liposomal Doxorubicin and Carboplatin compared with Paclitaxel and Carboplatin for patients with platinum-sensitive ovarian cancer in late relapse. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010, 28, 3323–3329. [Google Scholar] [CrossRef]
- Pignata, S.; Cecere, S.C.; Du Bois, A.; Harter, P.; Heitz, F. Treatment of recurrent ovarian cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017, 28, viii51–viii56. [Google Scholar] [CrossRef]
- Pignata, S.; Scambia, G.; Bologna, A.; Signoriello, S.; Vergote, I.B.; Wagner, U.; Lorusso, D.; Murgia, V.; Sorio, R.; Ferrandina, G.; et al. Randomized Controlled Trial Testing the Efficacy of Platinum-Free Interval Prolongation in Advanced Ovarian Cancer: The MITO-8, MaNGO, BGOG-Ov1, AGO-Ovar2.16, ENGOT-Ov1, GCIG Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017, 35, 3347–3353. [Google Scholar] [CrossRef]
- Loizzi, V.; Chan, J.K.; Osann, K.; Cappuccini, F.; DiSaia, P.J.; Berman, M.L. Survival outcomes in patients with recurrent ovarian cancer who were treated with chemoresistance assay-guided chemotherapy. Am. J. Obstet. Gynecol. 2003, 189, 1301–1307. [Google Scholar] [CrossRef]
- Lawrie, T.A.; Bryant, A.; Cameron, A.; Gray, E.; Morrison, J. Pegylated liposomal doxorubicin for relapsed epithelial ovarian cancer. Cochrane Database Syst. Rev. 2013, 2013, CD006910. [Google Scholar] [CrossRef]
- Rivankar, S. An overview of doxorubicin formulations in cancer therapy. J. Cancer Res. Ther. 2014, 10, 853–858. [Google Scholar] [CrossRef]
- Hsiao, S.M.; Chen, C.A.; Lin, H.H.; Hsieh, C.Y.; Wei, L.H. Phase II trial of carboplatin and distearoylphosphatidylcholine pegylated liposomal doxorubicin (Lipo-Dox) in recurrent platinum-sensitive ovarian cancer following front-line therapy with paclitaxel and platinum. Gynecol. Oncol. 2009, 112, 35–39. [Google Scholar] [CrossRef]
- Katsumata, N.; Yasuda, M.; Isonishi, S.; Takahashi, F.; Michimae, H.; Kimura, E.; Aoki, D.; Jobo, T.; Kodama, S.; Terauchi, F.; et al. Long-term results of dose-dense paclitaxel and carboplatin versus conventional paclitaxel and carboplatin for treatment of advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer (JGOG 3016): A randomised, controlled, open-label trial. Lancet Oncol. 2013, 14, 1020–1026. [Google Scholar] [CrossRef]
- Chan, J.K.; Brady, M.F.; Penson, R.T.; Huang, H.; Birrer, M.J.; Walker, J.L.; DiSilvestro, P.A.; Rubin, S.C.; Martin, L.P.; Davidson, S.A.; et al. Weekly vs. Every-3-Week Paclitaxel and Carboplatin for Ovarian Cancer. N. Engl. J. Med. 2016, 374, 738–748. [Google Scholar] [CrossRef] [Green Version]
- Gladieff, L.; Ferrero, A.; De Rauglaudre, G.; Brown, C.; Vasey, P.; Reinthaller, A.; Pujade-Lauraine, E.; Reed, N.; Lorusso, D.; Siena, S.; et al. Carboplatin and pegylated liposomal doxorubicin versus carboplatin and paclitaxel in partially platinum-sensitive ovarian cancer patients: Results from a subset analysis of the CALYPSO phase III trial. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2012, 23, 1185–1189. [Google Scholar] [CrossRef]
- Mangili, G.; Sigismondi, C.; Lorusso, D.; Cormio, G.; Candiani, M.; Scarfone, G.; Mascilini, F.; Gadducci, A.; Mosconi, A.M.; Scollo, P.; et al. The role of staging and adjuvant chemotherapy in stage I malignant ovarian germ cell tumors (MOGTs): The MITO-9 study. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017, 28, 333–338. [Google Scholar] [CrossRef]
- Pfisterer, J.; Shannon, C.M.; Baumann, K.; Rau, J.; Harter, P.; Joly, F.; Sehouli, J.; Canzler, U.; Schmalfeldt, B.; Dean, A.P.; et al. Bevacizumab and platinum-based combinations for recurrent ovarian cancer: A randomised, open-label, phase 3 trial. Lancet Oncol. 2020, 21, 699–709. [Google Scholar] [CrossRef]
- Ruscito, I.; Bellati, F.; Ray-Coquard, I.; Mirza, M.R.; du Bois, A.; Gasparri, M.L.; Costanzi, F.; De Marco, M.P.; Nuti, M.; Caserta, D.; et al. Incorporating Parp-inhibitors in Primary and Recurrent Ovarian Cancer: A Meta-analysis of 12 phase II/III randomized controlled trials. Cancer Treat. Rev. 2020, 87, 102040. [Google Scholar] [CrossRef]
- Madariaga, A.; Bowering, V.; Ahrari, S.; Oza, A.M.; Lheureux, S. Manage wisely: Poly (ADP-ribose) polymerase inhibitor (PARPi) treatment and adverse events. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2020, 30, 903–915. [Google Scholar] [CrossRef] [Green Version]
- Gyawali, B.; Iddawela, M. Bevacizumab in Advanced Cervical Cancer: Issues and Challenges for Low- and Middle-Income Countries. J. Glob. Oncol. 2017, 3, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Johns, E.A.; Stanley, J.A.; Toboni, M.D.; Schwarz, J.K.; Zhang, F.; Hagemann, A.R.; Fuh, K.C.; Thaker, P.H.; McCourt, C.K.; Mutch, D.G.; et al. Radiation therapy for vaginal and perirectal lesions in recurrent ovarian cancer. Gynecol. Oncol. Rep. 2021, 37, 100808. [Google Scholar] [CrossRef] [PubMed]
| Study | Therapeutic Target | Condition | Trial Type | Phase | Status | Identifier |
|---|---|---|---|---|---|---|
| JGOG-3017 | - | Stage I–IV | Randomized | 3 | Completed | - |
| GOG-268 | mTOR | Stage III–IV | Single Group | 2 | Completed | NCT01196429 |
| GOG-254 | VEGFR, PDGFR | Persistant–Recurrent | Single Group | 2 | Completed | NCT00979992 |
| ENMD-2076 | Aurora A, VEGFR, FGFR | Persistant–Recurrent | Single Group | 2 | Completed | NCT01104675 |
| NiCCC | VEGFR, PDGFR, FGFR | Persistant–Recurrent | Randomized | 2 | Ongoing | NCT02866370 |
| NRG-GY001 | MET, RET, VEGFR2 | Persistant–Recurrent | Single Group | 2 | Completed | NCT02315430 |
| GOG-283 | ABL, Src, c-kit | Persistant–Recurrent | Single Group | 2 | Completed | NCT02059265 |
| MOCCA | PD-L1 | Persistant–Recurrent | Randomized | 2 | Ongoing | NCT03405454 |
| BrUOG-354 | PD-1, CTLA-4 | Persistant–Recurrent | Randomized | 2 | Ongoing | NCT03355976 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arnaoutoglou, C.; Dampala, K.; Anthoulakis, C.; Papanikolaou, E.G.; Tentas, I.; Dragoutsos, G.; Machairiotis, N.; Zarogoulidis, P.; Ioannidis, A.; Matthaios, D.; et al. Epithelial Ovarian Cancer: A Five Year Review. Medicina 2023, 59, 1183. https://doi.org/10.3390/medicina59071183
Arnaoutoglou C, Dampala K, Anthoulakis C, Papanikolaou EG, Tentas I, Dragoutsos G, Machairiotis N, Zarogoulidis P, Ioannidis A, Matthaios D, et al. Epithelial Ovarian Cancer: A Five Year Review. Medicina. 2023; 59(7):1183. https://doi.org/10.3390/medicina59071183
Chicago/Turabian StyleArnaoutoglou, Christos, Kalliopi Dampala, Christos Anthoulakis, Evangelos G. Papanikolaou, Ioannis Tentas, Georgios Dragoutsos, Nikolaos Machairiotis, Paul Zarogoulidis, Aristeidis Ioannidis, Dimitris Matthaios, and et al. 2023. "Epithelial Ovarian Cancer: A Five Year Review" Medicina 59, no. 7: 1183. https://doi.org/10.3390/medicina59071183
APA StyleArnaoutoglou, C., Dampala, K., Anthoulakis, C., Papanikolaou, E. G., Tentas, I., Dragoutsos, G., Machairiotis, N., Zarogoulidis, P., Ioannidis, A., Matthaios, D., Perdikouri, E. I., Giannakidis, D., Sardeli, C., Petousis, S., Oikonomou, P., Nikolaou, C., Charalampidis, C., & Sapalidis, K. (2023). Epithelial Ovarian Cancer: A Five Year Review. Medicina, 59(7), 1183. https://doi.org/10.3390/medicina59071183









