Innovative Strategies in Microvascular Head and Neck Reconstruction
Abstract
1. Introduction
2. Soft Tissue Reconstruction
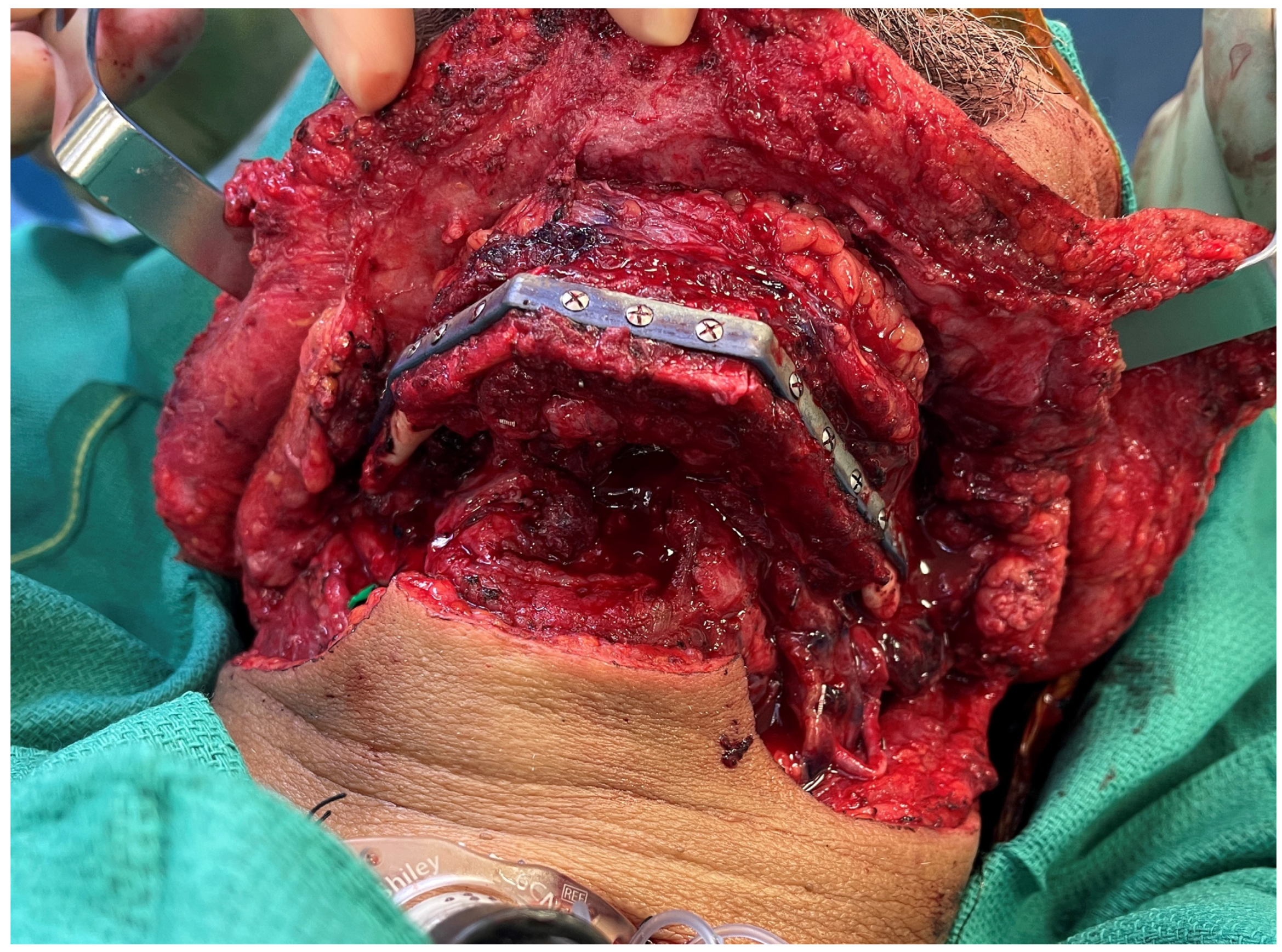
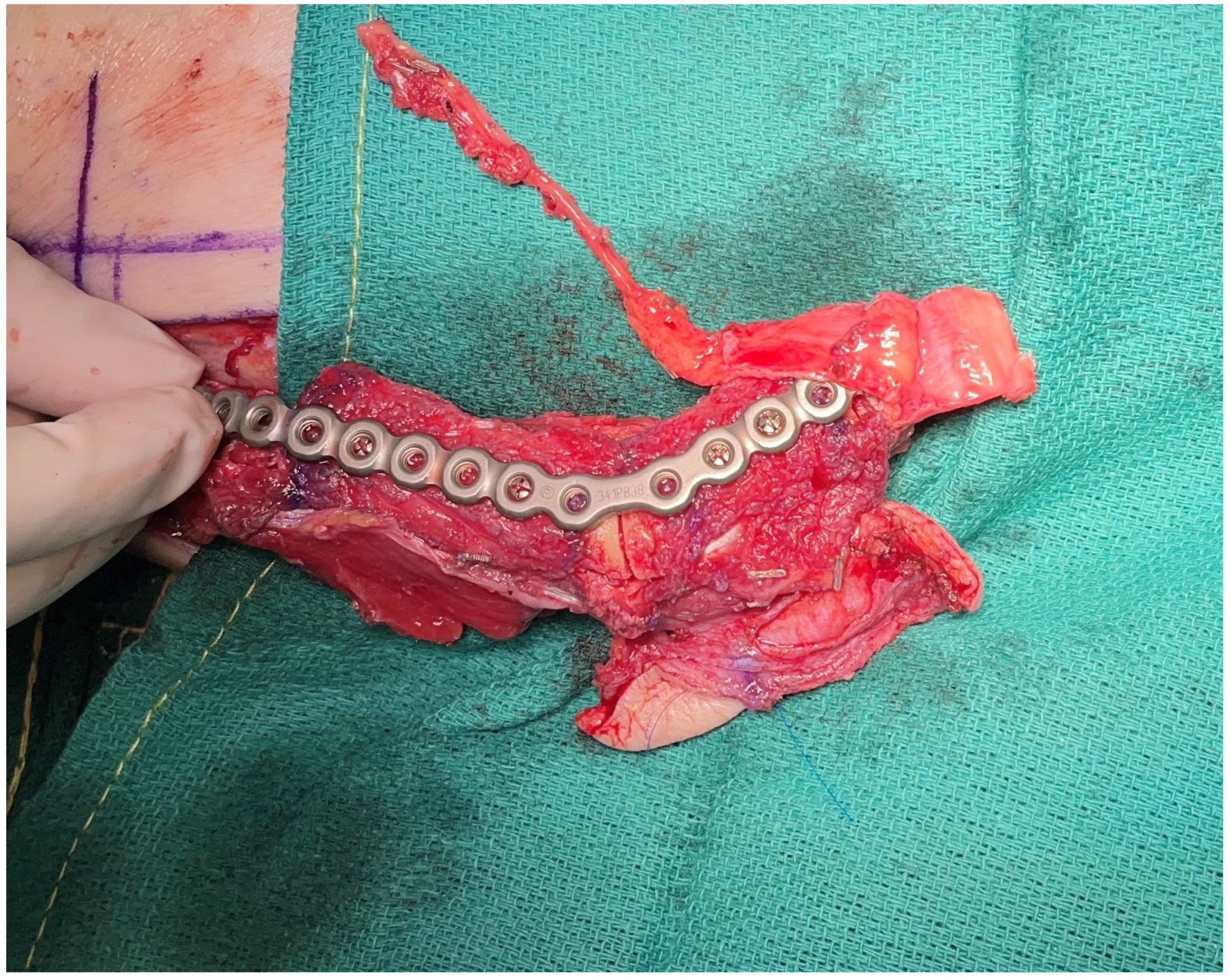
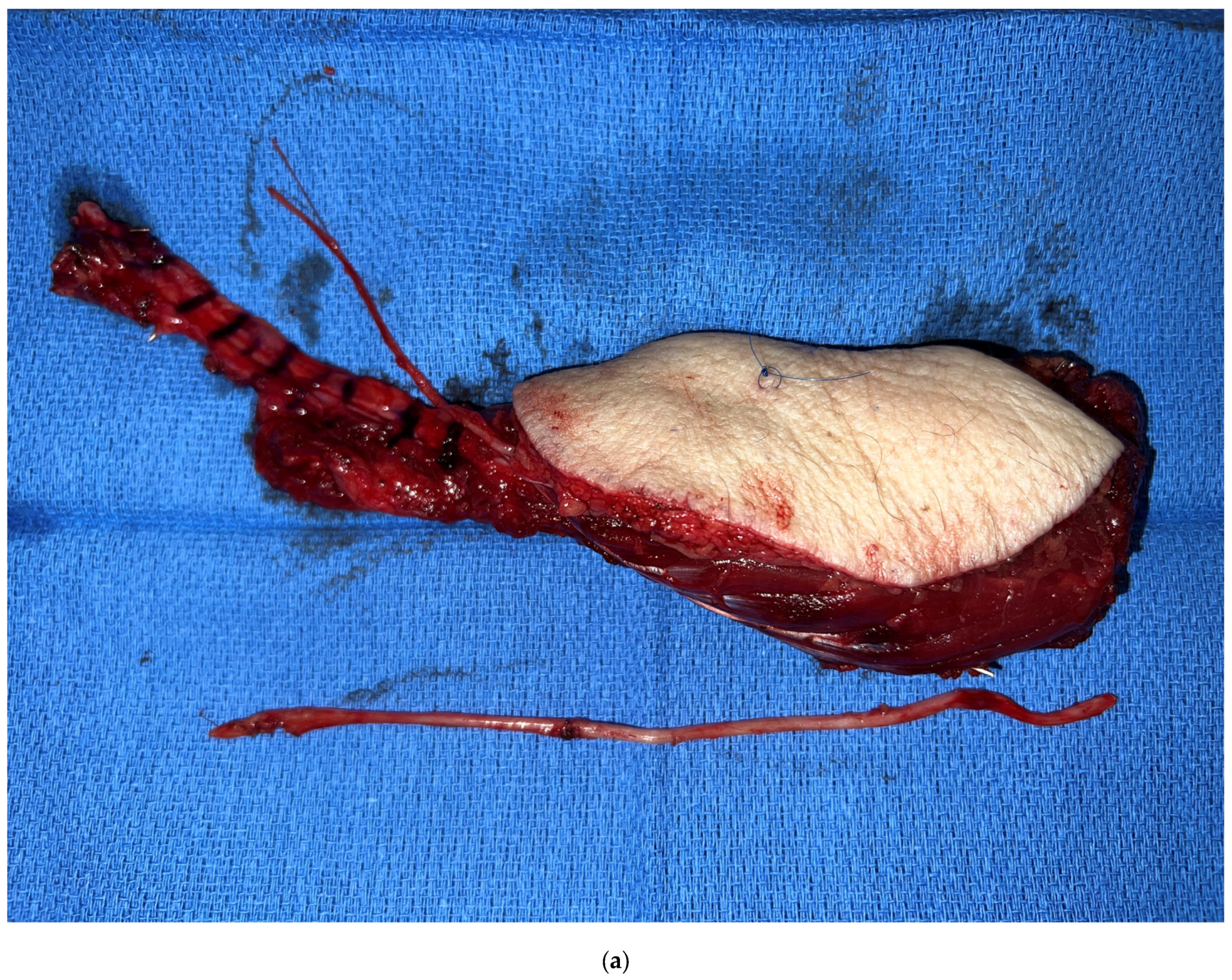
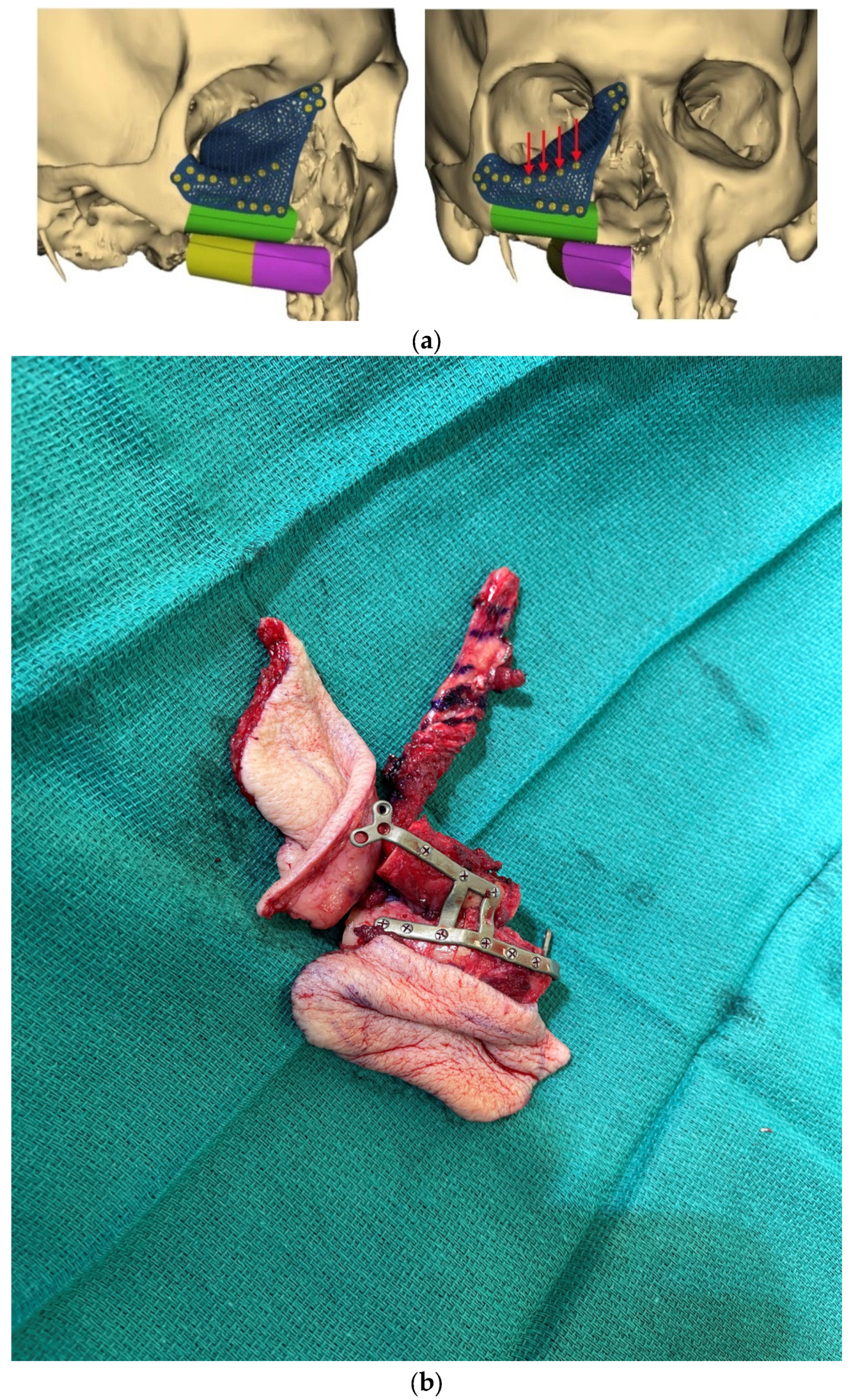
3. Virtual Surgical Planning and CAD-CAM
4. Dental Rehabilitation
5. Nerve Reconstruction and Reinnervation
6. Alternate Bone Flaps
7. Facial Reanimation
8. Future Directions
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carrel, A. Results of the transplantation of blood vessels, organs and limbs. JAMA 1983, 250, 944–953. [Google Scholar] [CrossRef]
- Converse, J.M. Alexis Carrel: The man, the unknown. Plast. Reconstr. Surg. 1981, 68, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Skladman, R.; Hanto, D.W.M.; Sacks, J.M.M. The Story of Charles Guthrie and Alexis Carrel. J. Am. Coll. Surg. 2022, 235, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Morris, D.J.; Pribaz, J.J. The interrupted-continuous microsurgical suture technique. Microsurgery 1992, 13, 103–105. [Google Scholar] [CrossRef]
- Haidar, Y.M.; Kuan, E.C.; Verma, S.P.; Goddard, J.A.; Armstrong, W.B.; Tjoa, T. Free Flap Versus Pedicled Flap Reconstruction of Laryngopharyngeal Defects: A 10-Year National Surgical Quality Improvement Program Analysis. Laryngoscope 2019, 129, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Gabrysz-Forget, F.; Tabet, P.; Rahal, A.; Bissada, E.; Christopoulos, A.; Ayad, T. Free versus pedicled flaps for reconstruction of head and neck cancer defects: A systematic review. J. Otolaryngol.—Head Neck Surg. 2019, 48, 13. [Google Scholar] [CrossRef]
- Nguyen, S.; Thuot, F. Functional outcomes of fasciocutaneous free flap and pectoralis major flap for salvage total laryngectomy. Head Neck 2017, 39, 1797–1805. [Google Scholar] [CrossRef]
- Chao, J.W.; Spector, J.A.; Taylor, E.M.; Otterburn, D.M.; Kutler, D.I.; Caruana, S.M.; Rohde, C.H. Pectoralis Major Myocutaneous Flap versus Free Fasciocutaneous Flap for Reconstruction of Partial Hypopharyngeal Defects: What Should We Be Doing? J. Reconstr. Microsurg. 2015, 31, 198–204. [Google Scholar] [CrossRef]
- Chang, E.I.; Zhang, H.; Liu, J.; Yu, P.; Skoracki, R.J.; Hanasono, M.M. Analysis of risk factors for flap loss and salvage in free flap head and neck reconstruction. Head Neck 2015, 38 (Suppl. S1), E771–E775. [Google Scholar] [CrossRef]
- Dermody, S.M.; Kahng, P.W.; Rao, V.M.; Casper, K.A.; Malloy, K.M.; Rosko, A.J.; Stucken, C.L.; Chinn, S.B.; Spector, M.E.; Feng, A.L. Color Match Following Free Flap Surgery in Head and Neck Reconstruction: A Colorimetric and Aesthetic Analysis. Plast. Reconstr. Surg. 2023; Online ahead of print. [Google Scholar] [CrossRef]
- Al Omran, Y.; Evans, E.; Jordan, C.; Borg, T.-M.; AlOmran, S.; Sepehripour, S.; Akhavani, M.A. The Medial Sural Artery Perforator Flap versus Other Free Flaps in Head and Neck Reconstruction: A Systematic Review. Arch. Plast. Surg. 2023, 50, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Chim, H. Perforator mapping and clinical experience with the superthin profunda artery perforator flap for reconstruction in the upper and lower extremities. J. Plast. Reconstr. Aesthetic Surg. 2023, 81, 60–67. [Google Scholar] [CrossRef]
- Hurrell, M.; Clark, J.; Ch’ng, S.; Low, T.-H.; Nguyen, K.; Elliott, M.; Palme, C.; Wykes, J. Comparison between the radial forearm and superficial circumflex iliac artery perforator free flaps for oral soft tissue reconstruction. Int. J. Oral Maxillofac. Surg. 2023, 52, 181–187. [Google Scholar] [CrossRef]
- Patel, N.K.M.; Tipps, J.A.B.; Bartlett, S.P.; Kovach, S.J.I., 3rd; Levin, L.S.; Mendenhall, S.D. Expanding Indications of the Medial Femoral Condyle Free Flap: Systematic Review in Head and Neck Reconstruction. Plast. Reconstr. Surg.—Glob. Open 2023, 11, e4925. [Google Scholar] [CrossRef]
- Manzie, T.; MacDougall, H.; Cheng, K.; Venchiarutti, R.; Fox, R.; Sharman, A.; Charters, E.; Seyfi, D.; Dunn, M.; Mukherjee, P.; et al. Virtual reality digital surgical planning for jaw reconstruction: A usability study. ANZ J. Surg. 2023, 93, 1341–1347. [Google Scholar] [CrossRef]
- Al-Sabahi, M.E.; Jamali, O.M.; Shindy, M.I.; Moussa, B.G.; Amin, A.A.-W.; Zedan, M.H. Aesthetic Reconstruction of Onco-surgical Mandibular Defects Using Free Fibular Flap with and without CAD/CAM Customized Osteotomy Guide: A Randomized Controlled Clinical Trial. BMC Cancer 2022, 22, 1252. [Google Scholar] [CrossRef]
- Cho, M.-J.; Hanasono, M.M. Virtual Surgical Planning in Free Tissue Transfer for Orbito-Maxillary Reconstruction. Semin. Plast. Surg. 2022, 36, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, K.S.; Morris, J.M.; Alexander, A.E.; Nathan, J.M.; Arce, K. Accuracy and Precision of the Computed Tomographic Angiography Perforator Localization Technique for Virtual Surgical Planning of Composite Osteocutaneous Fibular Free Flaps in Head and Neck Reconstruction. J. Oral Maxillofac. Surg. 2022, 80, 1434–1444. [Google Scholar] [CrossRef]
- Roy, M.; Corkum, J.P.; Shah, P.S.; Borschel, G.H.; Ho, E.S.; Zuker, R.M.; Davidge, K.M. Effectiveness and safety of the use of gracilis muscle for dynamic smile restoration in facial paralysis: A systematic review and meta-analysis. J. Plast. Reconstr. Aesthetic Surg. 2019, 72, 1254–1264. [Google Scholar] [CrossRef] [PubMed]
- Formeister, E.J.; Baum, R.; Knott, P.D.; Seth, R.; Ha, P.; Ryan, W.; El-Sayed, I.; George, J.; Larson, A.; Plonowska, K.; et al. Machine Learning for Predicting Complications in Head and Neck Microvascular Free Tissue Transfer. Laryngoscope 2020, 130, E843–E849. [Google Scholar] [CrossRef]
- Tighe, D.; McMahon, J.; Schilling, C.; Ho, M.; Provost, S.; Freitas, A. Machine learning methods applied to risk adjustment of cumulative sum chart methodology to audit free flap outcomes after head and neck surgery. Br. J. Oral Maxillofac. Surg. 2022, 60, 1353–1361. [Google Scholar] [CrossRef]
- Chang, E.I.; Yu, P.; Skoracki, R.J.; Liu, J.; Hanasono, M.M. Comprehensive Analysis of Functional Outcomes and Survival After Microvascular Reconstruction of Glossectomy Defects. Ann. Surg. Oncol. 2015, 22, 3061–3069. [Google Scholar] [CrossRef] [PubMed]
- Selber, J.C.; Xue, A.; Liu, J.; Hanasono, M.M.; Skoracki, R.J.; Chang, E.I.; Yu, P.; Selber, J.C. Pharyngoesophageal Reconstruction Outcomes Following 349 Cases. J. Reconstr. Microsurg. 2014, 30, 641–654. [Google Scholar] [CrossRef] [PubMed]
- Lee, Z.-H.; Hanasono, M.M. Pharyngeaoesophageal Reconstruction. Otolaryngol. Clin. N. Am. 2023; Online ahead of print. [Google Scholar] [CrossRef]
- Chao, A.H.; Yu, P.; Skoracki, R.J.; DeMonte, F.; Hanasono, M.M. Microsurgical reconstruction of composite scalp and calvarial defects in patients with cancer: A 10-year experience. Head Neck 2012, 34, 1759–1764. [Google Scholar] [CrossRef]
- Chang, E.I. Alternate Soft Tissue Free Flaps for Head and Neck Reconstruction: The Next Generation of Workhorse Flaps. Plast. Reconstr. Surg. 2023; Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Largo, R.D.M.; Chu, C.K.M.; Chang, E.I.M.; Liu, J.; Abu-Ghname, A.M.; Wang, H.M.; Schaverien, M.V.M.; Mericli, A.F.M.; Hanasono, M.M.M.; Yu, P.M. Perforator Mapping of the Profunda Artery Perforator Flap: Anatomy and Clinical Experience. Plast. Reconstr. Surg. 2020, 146, 1135–1145. [Google Scholar] [CrossRef]
- Largo, R.D.; Bhadkamkar, M.A.; Asaad, M.; Chu, C.K.; Garvey, P.B.; Butler, C.E.; Yu, P.; Hanasono, M.M.; Chang, E.I. The Profunda Artery Perforator Flap: A Versatile Option for Head and Neck Reconstruction. Plast. Reconstr. Surg. 2021, 147, 1401–1412. [Google Scholar] [CrossRef]
- Yu, P.; Chang, E.I.; Selber, J.C.; Hanasono, M.M. Perforator Patterns of the Ulnar Artery Perforator Flap. Plast. Reconstr. Surg. 2012, 129, 213–220. [Google Scholar] [CrossRef]
- Chang, E.I.; Liu, J. Prospective Comparison of Donor-Site Morbidity following Radial Forearm and Ulnar Artery Perforator Flap Harvest. Plast. Reconstr. Surg. 2020, 145, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Shuck, J.M.; Chang, E.I.M.; Mericli, A.F.M.; Gross, N.F.M.; Hanasono, M.M.M.; Garvey, P.B.M.; Yu, P.M.; Largo, R.D.M. Free Lateral Forearm Flap in Head and Neck Reconstruction: An Attractive Alternative to the Radial Forearm Flap. Plast. Reconstr. Surg. 2020, 146, 446e–450e. [Google Scholar] [CrossRef]
- Chang, E.I.; Ibrahim, A.; Papazian, N.; Jurgus, A.; Nguyen, A.T.; Suami, H.; Yu, P. Perforator Mapping and Optimizing Design of the Lateral Arm Flap: Anatomy Revisited and Clinical Experience. Plast. Reconstr. Surg. 2016, 138, 300e–306e. [Google Scholar] [CrossRef] [PubMed]
- Taghizadeh, F.; Troob, S.H.; Wax, M.K. The Role of Fluorescent Angiography in Free Flap Reconstruction of the Head and Neck. Laryngoscope 2023, 133, 1388–1393. [Google Scholar] [CrossRef]
- Schöpper, S.; Smeets, R.; Gosau, M.; Hanken, H. Intraoperative ICG-based fluorescence-angiography in head and neck reconstruction: Predictive value for impaired perfusion of free flaps. J. Cranio-Maxillofacial Surg. 2022, 50, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Hanasono, M.M.; Skoracki, R.J. Computer-assisted design and rapid prototype modeling in microvascular mandible reconstruction. Laryngoscope 2013, 123, 597–604. [Google Scholar] [CrossRef]
- Hanasono, M.M.; Jacob, R.F.; Bidaut, L.; Robb, G.L.; Skoracki, R.J. Midfacial Reconstruction Using Virtual Planning, Rapid Prototype Modeling, and Stereotactic Navigation. Plast. Reconstr. Surg. 2010, 126, 2002–2006. [Google Scholar] [CrossRef]
- Levine, J.P.; Bae, J.S.; Soares, M.; Brecht, L.E.; Saadeh, P.B.; Ceradini, D.J.; Hirsch, D.L. Jaw in a day: Total maxillofacial reconstruction using digital technology. Plast Reconstr Surg. 2013, 131, 1386–1391. [Google Scholar] [CrossRef]
- Padilla, P.L.; Mericli, A.F.; Largo, R.D.; Garvey, P.B. Computer-Aided Design and Manufacturing versus Conventional Surgical Planning for Head and Neck Reconstruction: A Systematic Review and Meta-Analysis. Plast. Reconstr. Surg. 2021, 148, 183–192. [Google Scholar] [CrossRef]
- Garvey, P.B.; Chang, E.I.; Selber, J.C.; Skoracki, R.J.; Madewell, J.E.; Liu, J.; Yu, P.; Hanasono, M.M. A Prospective Study of Preoperative Computed Tomographic Angiographic Mapping of Free Fibula Osteocutaneous Flaps for Head and Neck Reconstruction. Plast. Reconstr. Surg. 2012, 130, 541e–549e. [Google Scholar] [CrossRef] [PubMed]
- Lee, Z.-H.; Alfonso, A.R.B.; Ramly, E.P.; Kantar, R.S.; Yu, J.W.D.; Daar, D.M.; Hirsch, D.L.M.; Jacobson, A.; Levine, J.P. The Latest Evolution in Virtual Surgical Planning: Customized Reconstruction Plates in Free Fibula Flap Mandibular Reconstruction. Plast. Reconstr. Surg. 2020, 146, 872–879. [Google Scholar] [CrossRef]
- Vranckx, J.J.; Desmet, O.; Bila, M.M.; Wittesaele, W.; Wilssens, N.; Poorten, V.V. Maxillo-mandibular reconstruction with vascularized bone flaps using insourced virtual surgical planning and home-made CAD-CAM: A 5-year single-center evolution in 75 patients. Plast. Reconstr. Surg. 2023; Online ahead of print. [Google Scholar] [CrossRef]
- Nelson, J.A.; Allen, R.J.; Rosen, E.B.; Matros, E. Cost-Effectiveness and Virtual Surgical Planning in Head and Neck Reconstruction: Measuring What Matters Most. Plast. Reconstr. Surg. 2021, 147, 1091e–1092e. [Google Scholar] [CrossRef] [PubMed]
- Kurlander, D.E.; Garvey, P.B.; Largo, R.D.; Yu, P.; Chang, E.I.; Hanasono, M.M.; Mericli, A.F. The Cost Utility of Virtual Surgical Planning and Computer-Assisted Design/Computer-Assisted Manufacturing in Mandible Reconstruction Using the Free Fibula Osteocutaneous Flap. J. Reconstr. Microsurg. 2023, 39, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Knitschke, M.; Sonnabend, S.; Roller, F.C.; Pons-Kühnemann, J.; Schmermund, D.; Attia, S.; Streckbein, P.; Howaldt, H.-P.; Böttger, S. Osseous Union after Mandible Reconstruction with Fibula Free Flap Using Manually Bent Plates vs. Patient-Specific Implants: A Retrospective Analysis of 89 Patients. Curr. Oncol. 2022, 29, 274. [Google Scholar] [CrossRef]
- Kreutzer, K.; Steffen, C.; Nahles, S.; Koerdt, S.; Heiland, M.; Rendenbach, C.; Beck-Broichsitter, B. Removal of patient-specific reconstruction plates after mandible reconstruction with a fibula free flap: Is the plate the problem? Int. J. Oral Maxillofac. Surg. 2022, 51, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Rendenbach, C.; Steffen, C.; Hanken, H.; Schluermann, K.; Henningsen, A.; Beck-Broichsitter, B.; Kreutzer, K.; Heiland, M.; Precht, C. Complication rates and clinical outcomes of osseous free flaps: A retrospective comparison of CAD/CAM versus conventional fixation in 128 patients. Int. J. Oral Maxillofac. Surg. 2019, 48, 1156–1162. [Google Scholar] [CrossRef]
- Ch’Ng, S.; Skoracki, R.J.; Selber, J.C.; Yu, P.; Martin, J.W.; Dds, T.M.H.; Chambers, M.S.; Liu, J.; Hanasono, M.M. Osseointegrated implant-based dental rehabilitation in head and neck reconstruction patients. Head Neck 2016, 38 (Suppl. S1), E321–E327. [Google Scholar] [CrossRef]
- Panchal, H.; Shamsunder, M.G.; Petrovic, I.; Rosen, E.B.; Allen, R.J., Jr.; Hernandez, M.; Ganly, I.; Boyle, J.O.; Matros, E.; Nelson, J.A. Dental Implant Survival in Vascularized Bone Flaps: A Systematic Review and Meta-Analysis. Plast. Reconstr. Surg. 2020, 146, 637–648. [Google Scholar] [CrossRef]
- Rosen, E.B.D.; Gazdeck, R.D.K.; Goldman, D.A.M.; Panchal, H.M.; Jones, E.B.; Nguyen, J.P.D.; Allen, R.J., Jr.; Nelson, J.M.; Huryn, J.M.D.; Matros, E.M. An Anatomic Analysis of Fibula Flap Mandible Reconstructions: Implications for Endosseous Implant Placement. Plast. Reconstr. Surg. 2022, 149, 1419–1428. [Google Scholar] [CrossRef]
- Allen, R.J.; Nelson, J.A.; Polanco, T.O.; Shamsunder, M.G.; Ganly, I.; Boyle, J.; Rosen, E.; Matros, E. Short-Term Outcomes following Virtual Surgery–Assisted Immediate Dental Implant Placement in Free Fibula Flaps for Oncologic Mandibular Reconstruction. Plast. Reconstr. Surg. 2020, 146, 768e–776e. [Google Scholar] [CrossRef]
- Ong, A.; Williams, F.; Tokarz, E.; Shokri, T.; Hammer, D.; Ducic, Y. Jaw in a Day: Immediate Dental Rehabilitation during Fibula Reconstruction of the Mandible. Facial Plast. Surg. 2021, 37, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Salinero, L.; Boczar, D.; Barrow, B.; Berman, Z.P.; Diep, G.K.; Trilles, J.; Howard, R.; Chaya, B.F.; Colon, R.R.; Rodriguez, E.D. Patient-centred outcomes and dental implant placement in computer-aided free flap mandibular reconstruction: A systematic review and meta-analysis. Br. J. Oral Maxillofac. Surg. 2022, 60, 1283–1291. [Google Scholar] [CrossRef]
- Hanasono, M.M.; Zevallos, J.P.; Skoracki, R.J.; Yu, P. A Prospective Analysis of Bony versus Soft-Tissue Reconstruction for Posterior Mandibular Defects. Plast. Reconstr. Surg. 2010, 125, 1413–1421. [Google Scholar] [CrossRef] [PubMed]
- Mosahebi, A.; Chaudhry, A.; McCarthy, C.M.; Disa, J.J.; Mehrara, B.J.; Pusic, A.L.; Hu, Q.; Cordeiro, P.G. Reconstruction of Extensive Composite Posterolateral Mandibular Defects Using Nonosseous Free Tissue Transfer. Plast. Reconstr. Surg. 2009, 124, 1571–1577. [Google Scholar] [CrossRef]
- Chang, E.I.; Boukovalas, S.; Liu, J.; Largo, R.D.; Hanasono, M.M.; Garvey, P.B. Reconstruction of Posterior Mandibulectomy Defects in the Modern Era of Virtual Planning and Three-Dimensional Modeling. Plast. Reconstr. Surg. 2019, 144, 453e–462e. [Google Scholar] [CrossRef]
- Lee, Z.-H.; Avraham, T.; Monaco, C.; Patel, A.A.; Hirsch, D.L.; Levine, J.P. Optimizing Functional Outcomes in Mandibular Condyle Reconstruction with the Free Fibula Flap Using Computer-Aided Design and Manufacturing Technology. J. Oral Maxillofac. Surg. 2018, 76, 1098–1106. [Google Scholar] [CrossRef]
- Shuck, J.W.; Andejani, D.F.; Garvey, P.B.; Chang, E.I. Vascularized Condyle Reconstruction with Free Medial Femoral Trochlea and Fibular Flow-through Flaps. Plast. Reconstr. Surg.—Glob. Open 2023, 11, e4738. [Google Scholar] [CrossRef] [PubMed]
- Pogrel, M.A. Recovery of Sensation Over the Distribution of the Inferior Alveolar Nerve Following Mandibular Resection without Nerve Reconstruction. J. Oral Maxillofac. Surg. 2021, 79, 2143–2146. [Google Scholar] [CrossRef]
- Kaplan, J.; Lee, Z.-H.; Grome, L.; Yao, C.M.; Mericli, A.F.; Roubaud, M.S.; Largo, R.D.; Garvey, P.B. Lower Lip Sensory Outcomes of Allograft Inferior Alveolar Nerve Reconstruction Following Free Fibula Mandible Reconstruction in Cancer Patients1. Plast. Reconstr. Surg. 2023; Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.I.; Hanasono, M.M. State-of-the-art reconstruction of midface and facial deformities. J. Surg. Oncol. 2016, 113, 962–970. [Google Scholar] [CrossRef]
- Ismail, T.; Kurlander, D.E.; Lee, Z.-H.; Lunger, A.; Shuck, J.W.; Largo, R.D.; Chang, E.I. The Medial Femoral Condyle Flap: A Novel Versatile Tool for Complex Microvascular Maxillofacial Reconstruction. Plast. Reconstr. Surg. 2023, 151, 115e–119e. [Google Scholar] [CrossRef]
- Hanasono, M.M.; Skoracki, R.J.; Ba, A.K.S.; Yu, P. Adipofascial perforator flaps for “aesthetic” head and neck reconstruction. Head Neck 2011, 33, 1513–1519. [Google Scholar] [CrossRef]
- Gidley, P.W.; Herrera, S.J.; Hanasono, M.M.; Yu, P.; Skoracki, R.; Roberts, D.B.; Weber, R.S. The impact of radiotherapy on facial nerve repair. Laryngoscope 2010, 120, 1985–1989. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.B.W.; Spuerck, F.; Kollar, B.; Eisenhardt, S.U. Age-related outcome of facial reanimation surgery using cross face nerve graft and gracilis free functional muscle transfer—A retrospective cohort study. Microsurgery 2022, 42, 557–567. [Google Scholar] [CrossRef]
- David, A.P.; Seth, R.; Knott, P.D. Facial Reanimation and Reconstruction of the Radical Parotidectomy. Facial Plast. Surg. Clin. North Am. 2021, 29, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.M.K.; Jozaghi, Y.; Danker, S.; Karami, R.; Asaad, M.; Lai, S.Y.; Hanna, E.Y.; Esmaeli, B.; Gidley, P.W.; Chang, E.I. The combined profunda artery perforator-gracilis flap for immediate facial reanimation and resurfacing of the radical parotidectomy defect. Microsurgery 2023, 43, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Cristóbal, L.; Linder, S.; Lopez, B.; Mani, M.; Rodríguez-Lorenzo, A. Free anterolateral thigh flap and masseter nerve transfer for reconstruction of extensive periauricular defects: Surgical technique and clinical outcomes. Microsurgery 2017, 37, 479–486. [Google Scholar] [CrossRef]
- Asaad, M.; Lu, S.-C.; Hassan, A.M.; Kambhampati, P.; Mitchell, D.; Chang, E.I.; Yu, P.; Hanasono, M.M.; Sidey-Gibbons, C. The Use of Machine Learning for Predicting Complications of Free-Flap Head and Neck Reconstruction. Ann. Surg. Oncol. 2023, 30, 2343–2352. [Google Scholar] [CrossRef] [PubMed]
- Costantino, A.; Festa, B.M.; Spriano, G.; De Virgilio, A. The Use of Machine Learning for Predicting Complications of Free Flap Head and Neck Reconstruction: Caution Needed. Ann. Surg. Oncol. 2023, 30, 4232–4233. [Google Scholar] [CrossRef]
- Lantieri, L.; Cholley, B.; Lemogne, C.; Guillemain, R.; Ortonne, N.; Grimbert, P.; Thervet, E.; Lellouch, A.G. First human facial retransplantation: 30-month follow-up. Lancet 2020, 396, 1758–1765. [Google Scholar] [CrossRef]
- Tasigiorgos, S.; Kollar, B.; Turk, M.; Perry, B.; Alhefzi, M.; Kiwanuka, H.; Nizzi, M.-C.; Marty, F.M.; Chandraker, A.; Tullius, S.G.; et al. Five-Year Follow-up after Face Transplantation. New Engl. J. Med. 2019, 380, 2579–2581. [Google Scholar] [CrossRef]
- Venchiarutti, R.L.; Templeton, S.; Mathers, L.; Charters, E.; Clark, J.R. Treatment approaches and outcomes of a head and neck lymphedema service at an Australian comprehensive cancer center. Head Neck 2023, 45, 1539–1548. [Google Scholar] [CrossRef]
- Starmer, H.; Cherry, M.G.; Patterson, J.; Young, B.; Fleming, J. Assessment of Measures of Head and Neck Lymphedema Following Head and Neck Cancer Treatment: A Systematic Review. Lymphat. Res. Biol. 2023, 21, 42–51. [Google Scholar] [CrossRef]
- Beederman, M.; Garza, R.M.; Agarwal, S.; Chang, D.W. Outcomes for Physiologic Microsurgical Treatment of Secondary Lymphedema Involving the Extremity. Ann. Surg. 2022, 276, e255–e263. [Google Scholar] [CrossRef] [PubMed]
- Inatomi, Y.; Yoshida, S.; Kamizono, K.; Hanada, M.; Yasumatsu, R.; Kadota, H. Successful treatment of severe facial lymphedema by lymphovenous anastomosis. Head Neck 2018, 40, E73–E76. [Google Scholar] [CrossRef] [PubMed]
- Ayestaray, B.; Bekara, F.; Andreoletti, J.-B. π-Shaped lymphaticovenular anastomosis for head and neck lymphoedema: A preliminary study. J. Plast. Reconstr. Aesthetic Surg. 2013, 66, 201–206. [Google Scholar] [CrossRef]
- Ranganath, K.; Miller, L.E.; Goss, D.; Lin, D.T.; Faden, D.L.; Deschler, D.G.; Emerick, K.S.; Richmon, J.D.; Varvares, M.A.; Feng, A.L. Comparison of patient-reported upper extremity disability following free flaps in head and neck reconstruction: A systematic review and meta-analysis. Head Neck 2023, 45, 1832–1840. [Google Scholar] [CrossRef]
- Bruin, L.L.; Hundepool, C.A.; Duraku, L.S.; Luijsterburg, A.J.; De Jong, T.; Willems, W.F.; Mureau, M.A.; Zuidam, M. Neuropathic donor-site pain following radial forearm free flap harvest: A multicenter study on incidence, prognostic factors, and quality of life. J. Reconstr. Microsurg. 2023, 39, 320–326. [Google Scholar] [CrossRef]
- Emanuelli, E.; Egan, K.G.; Bins, G.B.; Nazir, N.; Bur, A.M.; Kakarala, K.; Przylecki, W.; Endress, R. Preoperative Examination Is Not Associated with Postoperative Function following Radial Forearm Free Flap Harvest. Plast. Reconstr. Surg. 2023, 151, 828e–837e. [Google Scholar] [CrossRef] [PubMed]
- Papanikolas, M.J.; Hurrell, M.J.L.; Clark, J.R.; Low, T.; Ch’Ng, S.; Elliott, M.S.; Palme, C.E.; Wykes, J. Anterolateral thigh, radial forearm and superficial circumflex iliac perforator flaps in oral reconstruction: A comparative analysis. ANZ J. Surg. 2023, 93, 1335–1340. [Google Scholar] [CrossRef]
- Russell, J.; Volker, G.; McGarvey, D.; Sharpe, C.; Breik, O.; Borgna, S.C.; Pateman, K.; Batstone, M. An objective analysis of composite free flap donor site morbidity in head and neck surgery: Prospective series. Head Neck 2023, 45, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Nyirjesy, S.C.; Heller, M.; von Windheim, N.; Gingras, A.; Kang, S.Y.; Ozer, E.; Agrawal, A.; Old, M.O.; Seim, N.B.; Carrau, R.L.; et al. The role of computer aided design/computer assisted manufacturing (CAD/CAM) and 3-dimensional printing in head and neck oncologic surgery: A review and future directions. Oral Oncol. 2022, 132, 105976. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Z.-H.; Ismail, T.; Shuck, J.W.; Chang, E.I. Innovative Strategies in Microvascular Head and Neck Reconstruction. Medicina 2023, 59, 1194. https://doi.org/10.3390/medicina59071194
Lee Z-H, Ismail T, Shuck JW, Chang EI. Innovative Strategies in Microvascular Head and Neck Reconstruction. Medicina. 2023; 59(7):1194. https://doi.org/10.3390/medicina59071194
Chicago/Turabian StyleLee, Z-Hye, Tarek Ismail, John W. Shuck, and Edward I. Chang. 2023. "Innovative Strategies in Microvascular Head and Neck Reconstruction" Medicina 59, no. 7: 1194. https://doi.org/10.3390/medicina59071194
APA StyleLee, Z.-H., Ismail, T., Shuck, J. W., & Chang, E. I. (2023). Innovative Strategies in Microvascular Head and Neck Reconstruction. Medicina, 59(7), 1194. https://doi.org/10.3390/medicina59071194




