What Is the Optimal Digoxin Level? Challenging Case of Fetal Atrial Flutter Treatment in a Monochorionic Diamniotic Twin
Abstract
1. Introduction
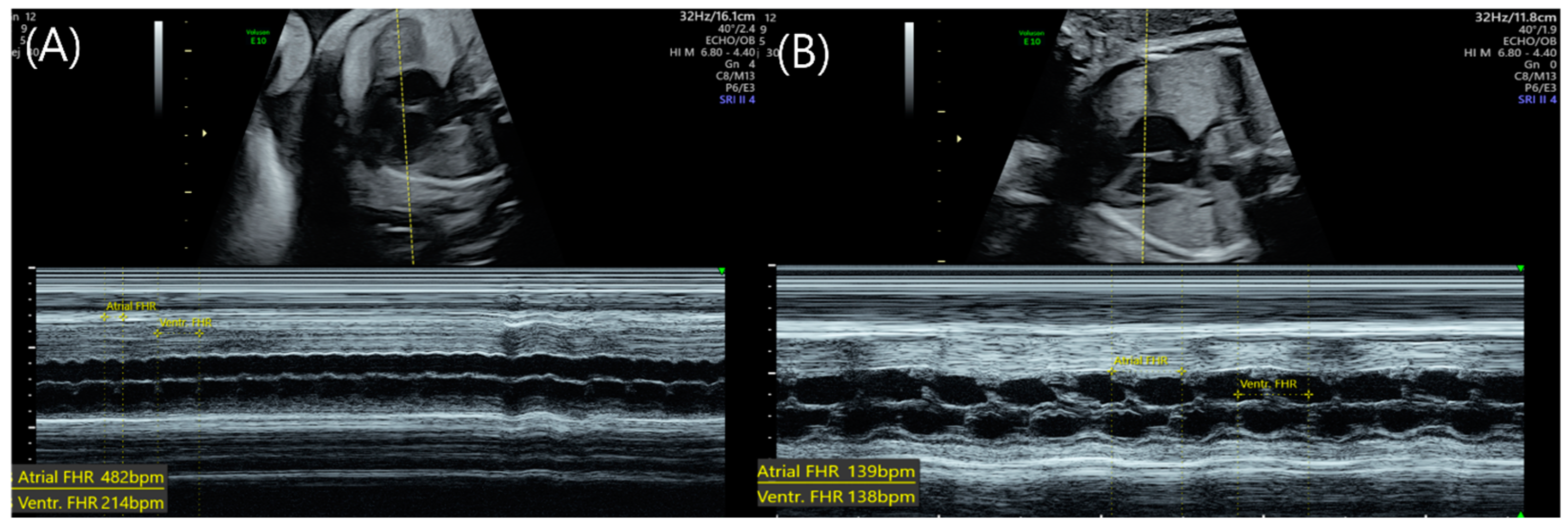
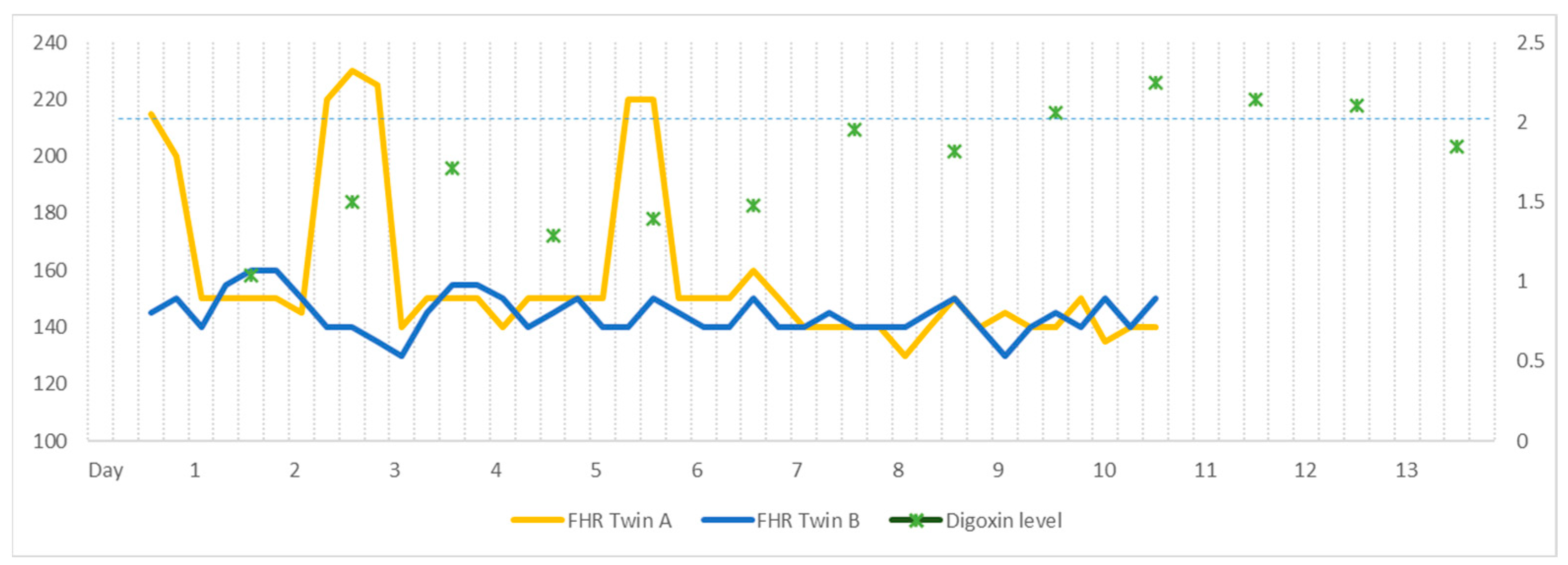
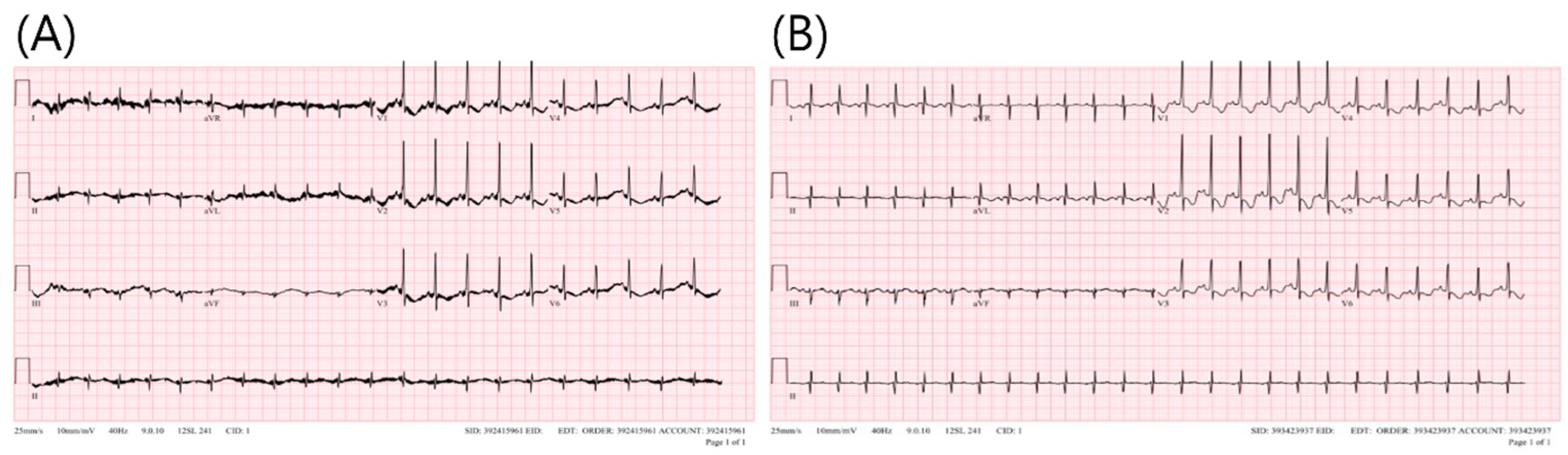
2. Case
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wacker-Gussmann, A.; Strasburger, J.F.; Srinivasan, S.; Cuneo, B.F.; Lutter, W.; Wakai, R.T. Fetal atrial flutter: Electrophysiology and associations with rhythms involving an accessory pathway. J. Am. Heart Assoc. 2016, 5, e003673. [Google Scholar] [CrossRef]
- Gozar, L.; Gabor-Miklosi, D.; Toganel, R.; Fagarasan, A.; Gozar, H.; Toma, D.; Cerghit-Paler, A. Fetal tachyarrhythmia management from digoxin to amiodarone—A review. J. Clin. Med. 2022, 11, 804. [Google Scholar] [CrossRef]
- Chimenea, Á.; García-Díaz, L.; Méndez, A.; Antiñolo, G. Maternal effects induced by oral digoxin during treatment of fetal tachyarrhythmia: Case series and literature review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 256, 354–357. [Google Scholar] [CrossRef]
- Zhou, K.; Hua, Y.; Zhu, Q.; Liu, H.; Yang, S.; Zhou, R.; Guo, N. Transplacental digoxin therapy for fetal tachyarrhythmia with multiple evaluation systems. J. Matern.-Fetal Neonatal Med. 2011, 24, 1378–1383. [Google Scholar] [CrossRef]
- Krapp, M.; Kohl, T.; Simpson, J.; Sharland, G.; Katalinic, A.; Gembruch, U. Review of diagnosis, treatment, and outcome of fetal atrial flutter compared with supraventricular tachycardia. Heart 2003, 89, 913–917. [Google Scholar] [CrossRef]
- Campbell, D.; MacGillivray, I. The importance of plasma volume expansion and nutrition in twin pregnancy. Acta Genet. Med. Gemellol. 1984, 33, 19–24. [Google Scholar] [CrossRef]
- Torres, X.; Bennasar, M.; García-Otero, L.; Martínez-Portilla, R.J.; Valenzuela-Alcaraz, B.; Crispi, F.; Goncé, A.; Gratacós, E.; Figueras, F.; Martínez, J.M. Uncomplicated monochorionic twins: Two normal hearts sharing one placenta. J. Clin. Med. 2020, 9, 3602. [Google Scholar] [CrossRef]
- Wójtowicz-Marzec, M.; Wysokińska, B.; Respondek-Liberska, M. Successful treatment of neonatal atrial flutter by synchronized cardioversion: Case report and literature review. BMC Pediatr. 2020, 20, 370. [Google Scholar] [CrossRef]
- Jaeggi, E.; Öhman, A. Fetal and neonatal arrhythmias. Clin. Perinatol. 2016, 43, 99–112. [Google Scholar] [CrossRef]
- Bravo-Valenzuela, N.J.; Rocha, L.A.; Nardozza, L.M.M.; Júnior, E.A. Fetal cardiac arrhythmias: Current evidence. Ann. Pediatr. Cardiol. 2018, 11, 148–163. [Google Scholar]
- Donofrio, M.T.; Moon-Grady, A.J.; Hornberger, L.K.; Copel, J.A.; Sklansky, M.S.; Abuhamad, A.; Cuneo, B.F.; Huhta, J.C.; Jonas, R.A.; Krishnan, A.; et al. Diagnosis and treatment of fetal cardiac disease: A scientific statement from the American Heart Association. Circulation 2014, 129, 2183–2242. [Google Scholar] [CrossRef]
- Malhamé, I.; Gandhi, C.; Tarabulsi, G.; Esposito, M.; Lombardi, K.; Chu, A.; Chen, K.K. Maternal monitoring and safety considertions during antiarrhythmic treatment for fetal supraventricular tachycardia. Obstet. Med. 2019, 12, 66–75. [Google Scholar] [CrossRef]
- Jaeggi, E.T.; Carvalho, J.S.; De Groot, E.; Api, O.; Clur, S.-A.B.; Rammeloo, L.; McCrindle, B.W.; Ryan, G.; Manlhiot, C.; Blom, N.A. Comparison of transplacental treatment of fetal supraventricular tachyarrhythmias with digoxin, flecainide, and sotalol: Results of a nonrandomized multicenter study. Circulation 2011, 124, 1747–1754. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, S.; Sullivan, I.; Tomek, V.; Wolfenden, J.; Škovránek, J.; Yates, R.; Janoušek, J.; Dominguez, T.E.; Marek, J. Flecainide versus digoxin for fetal supraventricular tachycardia: Comparison of two drug treatment protocols. Heart Rhythm. 2016, 13, 1913–1919. [Google Scholar] [CrossRef]
- Miyoshi, T.; Maeno, Y.; Hamasaki, T.; Inamura, N.; Yasukochi, S.; Kawataki, M.; Horigome, H.; Yoda, H.; Taketazu, M.; Nii, M.; et al. Antenatal therapy for fetal supraventricular tachyarrhythmias: Multicenter trial. J. Am. Coll. Cardiol. 2019, 74, 874–885. [Google Scholar] [CrossRef]
- Rotar, I.C.; Zaharie, G.; Staicu, A.; Preda, A.; Mureşan, D. Fetal cardiovascular alterations in twin-to-twin transfusion syndrome. Med. Pharm. 2020, 93, 5–11. [Google Scholar] [CrossRef] [PubMed]
| Tachycardia | Rhythm | Atrial Rate (Beats per Minute) | Atrioventricular Conduction | Ventricular Rate (Beats per Minutes) |
|---|---|---|---|---|
| Sinus tachycardia | Regular | 180–200 | 1:01 | 180–200 |
| Atrial flutter | Regular | 350–500 | 1:1, 2:1, 3:1, 4:1 | Variable depending on AV conduction |
| Supra-ventricular tachycardia | Regular | 220–300 | 1:1 ventricular contraction followed by atrial contraction | 220–300 |
| Atrial ectopic tachycardia | Irregular | 160–250 | 1:01 | 160–250 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.J.; Jeon, H.D.; Shim, S.-Y.; Kim, Y.-S.; Park, M.-H.; Lee, K.A. What Is the Optimal Digoxin Level? Challenging Case of Fetal Atrial Flutter Treatment in a Monochorionic Diamniotic Twin. Medicina 2023, 59, 1198. https://doi.org/10.3390/medicina59071198
Kim SJ, Jeon HD, Shim S-Y, Kim Y-S, Park M-H, Lee KA. What Is the Optimal Digoxin Level? Challenging Case of Fetal Atrial Flutter Treatment in a Monochorionic Diamniotic Twin. Medicina. 2023; 59(7):1198. https://doi.org/10.3390/medicina59071198
Chicago/Turabian StyleKim, Soo Jung, Hee Do Jeon, So-Yeon Shim, Yi-Seul Kim, Mi-Hye Park, and Kyung A. Lee. 2023. "What Is the Optimal Digoxin Level? Challenging Case of Fetal Atrial Flutter Treatment in a Monochorionic Diamniotic Twin" Medicina 59, no. 7: 1198. https://doi.org/10.3390/medicina59071198
APA StyleKim, S. J., Jeon, H. D., Shim, S.-Y., Kim, Y.-S., Park, M.-H., & Lee, K. A. (2023). What Is the Optimal Digoxin Level? Challenging Case of Fetal Atrial Flutter Treatment in a Monochorionic Diamniotic Twin. Medicina, 59(7), 1198. https://doi.org/10.3390/medicina59071198






