Expression of CYP2B6 Enzyme in Human Liver Tissue of HIV and HCV Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Demographic and Clinical Data
2.2. Inclusion and Exclusion Criteria
2.3. Liver Biopsy
2.4. Tissue Homogenization and RNA Extraction from Liver Tissue
2.5. cDNA Synthesis
2.6. Gene Expression—Real-Time qRT-PCR and Data Analysis
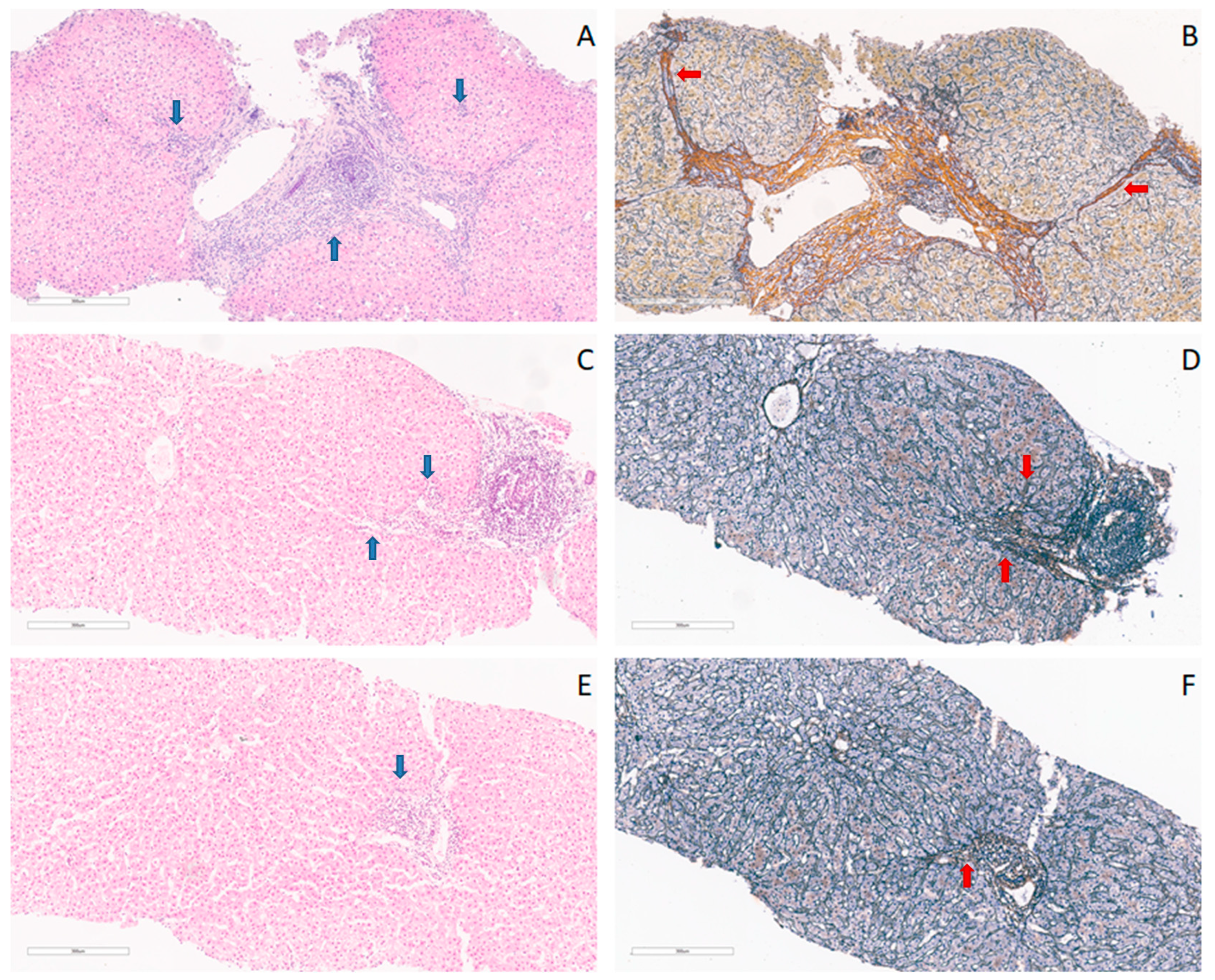
2.7. Histopathological Processing
2.8. Statistical Analysis
3. Results
3.1. Study Population
3.2. CYP2B6 Expression in the Study Population
3.2.1. Expression of CYP2B6 in Groups of HIV, HCV, and HIV/HCV Co-Infected Patients
3.2.2. CYP2B6 Gene Expression in the Groups of HCV-Infected Treated and Untreated Patients
3.2.3. Independent Correlations of CYP2B6
3.3. CYP3A4 Gene Expression in the Study Population
3.3.1. CYP3A4 Gene Expression in Groups of HIV, HCV and HIV/HCV Co-Infected Patients
3.3.2. Expression of CYP3A4 Gene in between Groups of HCV Treated and Untreated Patients
3.3.3. Independent Correlations of CYP3A4 Gene Expression
3.4. ABCB1 Expression in the Study Population
3.4.1. ABCB1 Expression in Groups of HIV, HCV and HIV/HCV Co-Infected Patients
3.4.2. ABCB1 Expression in the Group of HCV Treated and Untreated Patients
3.4.3. Independent Correlations of ABCB1 Expression
4. Discussion
4.1. HIV/HCV Co-Infection Correlated with Increased Expression of CYP2B6
4.2. Choice of Therapy of HCV Mono-Infected Patients Independently Correlates with Expression of CYP2B6
4.3. An Increase in CYP3A4 Expression Correlates with the Presence of HIV Infection
4.4. Lower ABCB1 Expression Correlates with the Use of Lamivudine
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, A.Y.; Chung, R.T. Coinfection with HIV-1 and HCV—A one-two punch. Gastroenterology 2009, 137, 795–814. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Mehraj, V.; Trottier, B.; Baril, J.G.; Leblanc, R.; Lebouche, B.; Cox, J.; Tremblay, C.; Lu, W.; Singer, J.; et al. Early Initiation Rather Than Prolonged Duration of Antiretroviral Therapy in HIV Infection Contributes to the Normalization of CD8 T-Cell Counts. Clin. Infect. Dis. 2016, 62, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Sarıgül Yıldırım, F.; Candevir, A.; Akhan, S.; Kaya, S.; Çabalak, M.; Ersöz, G.; İnan, D.; Ceren, N.; Karaoğlan, İ.; Damar Çakırca, T.; et al. Comparison of Immunological and Virological Recovery with Rapid, Early, and Late Start of Antiretroviral Treatment in Naive Plwh: Real-World Data. Int. J. Gen. Med. 2023, 16, 1867–1877. [Google Scholar] [CrossRef] [PubMed]
- Guss, D.; Sherigar, J.; Rosen, P.; Mohanty, S.R. Diagnosis and Management of Hepatitis C Infection in Primary Care Settings. J. Gen. Intern. Med. 2018, 33, 551–557. [Google Scholar] [CrossRef]
- Blackard, J.T.; Sherman, K.E. HCV/HIV co-infection: Time to re-evaluate the role of HIV in the liver? J. Viral Hepat. 2008, 15, 323–330. [Google Scholar] [CrossRef]
- Gobran, S.T.; Ancuta, P.; Shoukry, N.H. A Tale of Two Viruses: Immunological Insights Into HCV/HIV Coinfection. Front. Immunol. 2021, 12, 726419. [Google Scholar] [CrossRef]
- Ward, B.A.; Gorski, J.C.; Jones, D.R.; Hall, S.D.; Flockhart, D.A.; Desta, Z. The cytochrome P450 2B6 (CYP2B6) is the main catalyst of efavirenz primary and secondary metabolism: Implication for HIV/AIDS therapy and utility of efavirenz as a substrate marker of CYP2B6 catalytic activity. J. Pharmacol. Exp. Ther. 2003, 306, 287–300. [Google Scholar] [CrossRef]
- Geddawy, A.; Ibrahim, Y.F.; Elbahie, N.M.; Ibrahim, M.A. Direct Acting Anti-hepatitis C Virus Drugs: Clinical Pharmacology and Future Direction. J. Transl. Int. Med. 2017, 5, 8–17. [Google Scholar] [CrossRef]
- Hariparsad, N.; Nallani, S.C.; Sane, R.S.; Buckley, D.J.; Buckley, A.R.; Desai, P.B. Induction of CYP3A4 by efavirenz in primary human hepatocytes: Comparison with rifampin and phenobarbital. J. Clin. Pharmacol. 2004, 44, 1273–1281. [Google Scholar] [CrossRef]
- Achenbach, C.J.; Darin, K.M.; Murphy, R.L.; Katlama, C. Atazanavir/ritonavir-based combination antiretroviral therapy for treatment of HIV-1 infection in adults. Future Virol. 2011, 6, 157–177. [Google Scholar] [CrossRef]
- Ahmed, H.; Abushouk, A.I.; Menshawy, A.; Attia, A.; Mohamed, A.; Negida, A.; Abdel-Daim, M.M. Meta-Analysis of Grazoprevir plus Elbasvir for Treatment of Hepatitis C Virus Genotype 1 Infection. Ann. Hepatol. 2018, 17, 18–32. [Google Scholar] [CrossRef]
- Klomp, F.; Wenzel, C.; Drozdzik, M.; Oswald, S. Drug-Drug Interactions Involving Intestinal and Hepatic CYP1A Enzymes. Pharmaceutics 2020, 12, 1201. [Google Scholar] [CrossRef]
- Lenoir, C.; Rollason, V.; Desmeules, J.; Samer, C. Influence of Inflammation on Cytochromes P450 Activity in Adults: A Systematic Review of the Literature. Front. Pharmacol. 2021, 12, 733935. [Google Scholar] [CrossRef]
- Gwak, H.; Yoo, Z.; Kim, H. Effects of Diabetes Mellitus on the Disposition of Tofacitinib, a Janus Kinase Inhibitor, in Rats. Biomol. Ther. 2020, 28, 361–369. [Google Scholar] [CrossRef]
- Kap, M.; Sieuwerts, A.; Kubista, M.; Oomen, M.; Arshad, S.; Riegman, P. The influence of tissue procurement procedures on RNA integrity, gene expression, and morphology in porcine and human liver tissue. Biopreserv. Biobank. 2015, 13, 200–206. [Google Scholar] [CrossRef]
- Hesse, L.M.; Venkatakrishnan, K.; Court, M.H.; von Moltke, L.L.; Duan, S.X.; Shader, R.I.; Greenblatt, D.J. CYP2B6 mediates the In vitro hydroxylation of bupropion: Potential drug interactions with other antidepressants. Drug Metab. Dispos. 2000, 28, 1176–1183. [Google Scholar]
- Faucette, S.R.; Hawke, R.L.; Lecluyse, E.L.; Shord, S.S.; Yan, B.; Laethem, R.M.; Lindley, C.M. Validation of bupropion hydroxylation as a selective marker of human cytochrome P450 2B6 catalytic activity. Drug Metab. Dispos. 2000, 28, 1222–1230. [Google Scholar]
- Hesse, L.M.; He, P.; Krishnaswamy, S.; Hao, Q.; Hogan, K.; von Moltke, L.L.; Greenblatt, D.J.; Court, M.H. Pharmacogenetic determinants of interindividual variability in bupropion hydroxylation by cytochrome P450 2B6 in human liver microsomes. Pharmacogenetics 2004, 14, 225–238. [Google Scholar] [CrossRef]
- Pfaffl, M. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Zanger, U.M.; Schwab, M. Cytochrome P450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol. Ther. 2013, 138, 103–141. [Google Scholar] [CrossRef]
- Wang, P.F.; Neiner, A.; Kharasch, E.D. Efavirenz Metabolism: Influence of Polymorphic CYP2B6 Variants and Stereochemistry. Drug Metab. Dispos. 2019, 47, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.A.; Caixas, U.; Branco, T.; Germano, I.; Lampreia, F.; Papoila, A.L.; Monteiro, E.C. Efavirenz concentrations in HIV-infected patients with and without viral hepatitis. Br. J. Clin. Pharmacol. 2008, 66, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Apostolova, N.; Funes, H.A.; Blas-Garcia, A.; Galindo, M.J.; Alvarez, A.; Esplugues, J.V. Efavirenz and the CNS: What we already know and questions that need to be answered. J. Antimicrob. Chemother. 2015, 70, 2693–2708. [Google Scholar] [CrossRef] [PubMed]
- Calza, L.; Danese, I.; Colangeli, V.; Manfredi, R.; Magistrelli, E.; Verucchi, G.; Conti, M.; Motta, R.; Viale, P. Plasma concentrations of efavirenz, darunavir/ritonavir and raltegravir in HIV-HCV-coinfected patients without liver cirrhosis in comparison with HIV-monoinfected patients. Infect. Dis. 2015, 47, 625–636. [Google Scholar] [CrossRef]
- Ngayo, M.O.; Oluka, M.; Kwena, Z.A.; Bulimo, W.D.; Okalebo, F.A. Effects of cytochrome P450 2B6 and constitutive androstane receptor genetic variation on Efavirenz plasma concentrations among HIV patients in Kenya. PLoS ONE 2022, 17, e0260872. [Google Scholar] [CrossRef]
- Gufford, B.T.; Metzger, I.F.; Bamfo, N.O.; Benson, E.A.; Masters, A.R.; Lu, J.B.L.; Desta, Z. Influence of CYP2B6 Pharmacogenetics on Stereoselective Inhibition and Induction of Bupropion Metabolism by Efavirenz in Healthy Volunteers. J. Pharmacol. Exp. Ther. 2022, 382, 313–326. [Google Scholar] [CrossRef]
- Hammond, T.G.; Birdsall, H.H. Hepatocyte CYP2B6 Can Be Expressed in Cell Culture Systems by Exerting Physiological Levels of Shear: Implications for ADME Testing. J. Toxicol. 2017, 2017, 1907952. [Google Scholar] [CrossRef]
- Drozdzik, M.; Lapczuk-Romanska, J.; Wenzel, C.; Skalski, L.; Szeląg-Pieniek, S.; Post, M.; Parus, A.; Syczewska, M.; Kurzawski, M.; Oswald, S. Protein Abundance of Drug Metabolizing Enzymes in Human Hepatitis C Livers. Int. J. Mol. Sci. 2023, 24, 4543. [Google Scholar] [CrossRef]
- Chen, X.; Pan, L.Q.; Naranmandura, H.; Zeng, S.; Chen, S.Q. Influence of various polymorphic variants of cytochrome P450 oxidoreductase (POR) on drug metabolic activity of CYP3A4 and CYP2B6. PLoS ONE 2012, 7, e38495. [Google Scholar] [CrossRef]
- Vo, T.; Varghese Gupta, S. Role of cytochrome P450 2B6 pharmacogenomics in determining efavirenz-mediated central nervous system toxicity, treatment outcomes, and dosage adjustments in patients with human immunodeficiency virus infection. Pharmacotherapy 2016, 36, 1245–1254. [Google Scholar] [CrossRef]
- Hanada, K.; Nakai, K.; Tanaka, H.; Suzuki, F.; Kumada, H.; Ohno, Y.; Ozawa, S.; Ogata, H. Effect of nuclear receptor downregulation on hepatic expression of cytochrome P450 and transporters in chronic hepatitis C in association with fibrosis development. Drug Metab. Pharmacokinet. 2012, 27, 301–306. [Google Scholar] [CrossRef]
- Jones, A.E.; Brown, K.C.; Werner, R.E.; Gotzkowsky, K.; Gaedigk, A.; Blake, M. Variability in drug metabolizing enzyme activity in HIV-infected patients. Eur. J. Clin. Pharm. 2010, 66, 475–485. [Google Scholar] [CrossRef]
- Jetter, A.; Fätkenheuer, G.; Frank, D.; Klaassen, T.; Seeringer, A.; Doroshyenko, O. Do activities of cytochrome P450 (CYP)3A, CYP2D6 and P-glycoprotein differ between healthy volunteers and HIV-infected patients? Antivir. Ther. 2010, 15, 975–983. [Google Scholar] [CrossRef]
- Venuto, C.S.; Lim, J.; Messing, S.; Hunt, P.W.; McComsey, G.A.; Morse, G.D. Inflammation Investigated as a Source of Pharmacokinetic Variability of Atazanavir in AIDS Clinical Trials Group Protocol A5224s. Antivir. Ther. 2018, 23, 345–351. [Google Scholar] [CrossRef]
- Li, F.; Lu, J.; Ma, X. CYP3A4-mediated lopinavir bioactivation and its inhibition by ritonavir. Drug Metab. Dispos. 2012, 40, 18–24. [Google Scholar] [CrossRef]
- Shah, S.; McRae, A.F.; Marioni, R.E.; Harris, S.E.; Gibson, J.; Henders, A.K.; Redmond, P.; Cox, S.R.; Pattie, A.; Corley, J.; et al. Genetic and environmental exposures constrain epigenetic drift over the human life course. Genome Res. 2014, 24, 1725–1733. [Google Scholar] [CrossRef]
- Lee, L.S.; Soon, G.H.; Shen, P.; Yong, E.L.; Flexner, C.; Pham, P. Darunavir/ritonavir and efavirenz exert differential effects on MRP1 transporter expression and function in healthy volunteers. Antivir. Ther. 2010, 15, 275–279. [Google Scholar] [CrossRef]
- Lucia, M.B.; Rutella, S.; Leone, G.; Larocca, L.M.; Vella, S.; Cauda, R. In vitro and in vivo modulation of MDR1/P-glycoprotein in HIV-infected patients administered highly active antiretroviral therapy and liposomal doxorubicin. J. Acquir. Immune Defic. Syndr. 2002, 30, 369–378. [Google Scholar] [CrossRef]
- Meaden, E.R.; Hoggard, P.G.; Maher, B.; Khoo, S.H.; Back, D.J. Expression of P-glycoprotein and multidrug resistance-associated protein in healthy volunteers and HIV-infected patients. AIDS Res. Hum. Retrovir. 2001, 17, 1329–1332. [Google Scholar] [CrossRef]
- Whyte-Allman, S.K.; Kaul, R.; Bendayan, R. Regulation of ABC Drug Efflux Transporters in Human T-Cells Exposed to an HIV Pseudotype. Front. Pharmacol. 2021, 12, 711999. [Google Scholar] [CrossRef]
- Ebert, C.; Perner, F.; Wolleschak, D.; Schnöder, T.M.; Fischer, T.; Heidel, F.H. Expression and function of ABC-transporter protein ABCB1 correlates with inhibitory capacity of Ruxolitinib in vitro and in vivo. Haematologica 2016, 101, e81–e85. [Google Scholar] [CrossRef] [PubMed]
- Swart, M.; Ren, Y.; Smith, P.; Dandara, C. ABCB1 4036A>G and 1236C>T Polymorphisms Affect Plasma Efavirenz Levels in South African HIV/AIDS Patients. Front. Genet. 2012, 3, 236. [Google Scholar] [CrossRef] [PubMed]
| Groups | CYP2B6 Expression (Mean ± SD) | p Value | Posthoc | |
|---|---|---|---|---|
| HCV+ without HCV therapy | 1.711 ± 0.635 | 0.017 | 1* vs. 3* p = 0.007 2* vs. 3* p = 0.030 | |
| HIV+ | 1.813 ± 0.866 | |||
| HIV+/HCV+ without HCV therapy | 3.673 ± 2.704 | |||
| HCV+ without HCV therapy | 1.711 ± 0.635 | 0.252 | 1* vs. 4* EG IFN p = 0.021 1* vs. 4* DAA p > 0.05 | |
| HCV+ with HCV therapy | Total | 2.117 ± 1.081 | ||
| PEG IFN | 2.727 ± 1.144 | 0.028 | ||
| DAA | 1.601 ± 0.781 | |||
| Groups | CYP3A4 Expression (Mean ± SD) | p Value | Posthoc | |
|---|---|---|---|---|
| HCV+ without HCV therapy | 2.377 ± 1.227 | 0.359 | 1* vs. 3* p > 0.05 1* vs. 2* p > 0.05 2* vs. 3* p > 0.05 | |
| HIV+ | 3.730 ± 2.696 | |||
| HIV+/HCV+ without HCV therapy | 4.245 ± 4.799 | |||
| HCV+ without HCV therapy | 2.377 ± 1.227 | 0.149 | 1* vs. 4* PEG IFN p > 0.05 1* vs. 4* DAA p > 0.05 | |
| HCV+ with HCV therapy | Total | 1.637 ± 1.252 | ||
| PEG IFN | 1.174 ± 0.613 | 0.300 | ||
| DAA | 1.968 ± 1.521 | |||
| Groups | ABCB1 Expression (Mean ± SD) | p Value | Posthoc | |
|---|---|---|---|---|
| HCV+ without HCV therapy | 0.934 ± 0.379 | 0.116 | 1* vs. 3* p > 0.05 1* vs. 2* p > 0.05 2* vs. 3* p > 0.05 | |
| HIV+ | 0.903 ± 0.339 | |||
| HIV+/HCV+ without HCV therapy | 3.652 ± 5.325 | |||
| HCV+ without HCV therapy | 0.934 ± 0.379 | 0.466 | 1* vs. 4* PEG IFN p > 0.05 1* vs. 4* DAA p > 0.05 | |
| HCV+ with HCV therapy | Total | 1.060 ± 0.398 | ||
| PEG IFN | 0.986 ± 0.588 | 0.657 | ||
| DAA | 1.110 ± 0.267 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obradovic, B.; Roberts, O.; Owen, A.; Milosevic, I.; Milic, N.; Ranin, J.; Dragovic, G. Expression of CYP2B6 Enzyme in Human Liver Tissue of HIV and HCV Patients. Medicina 2023, 59, 1207. https://doi.org/10.3390/medicina59071207
Obradovic B, Roberts O, Owen A, Milosevic I, Milic N, Ranin J, Dragovic G. Expression of CYP2B6 Enzyme in Human Liver Tissue of HIV and HCV Patients. Medicina. 2023; 59(7):1207. https://doi.org/10.3390/medicina59071207
Chicago/Turabian StyleObradovic, Bozana, Owain Roberts, Andrew Owen, Ivana Milosevic, Natasa Milic, Jovan Ranin, and Gordana Dragovic. 2023. "Expression of CYP2B6 Enzyme in Human Liver Tissue of HIV and HCV Patients" Medicina 59, no. 7: 1207. https://doi.org/10.3390/medicina59071207
APA StyleObradovic, B., Roberts, O., Owen, A., Milosevic, I., Milic, N., Ranin, J., & Dragovic, G. (2023). Expression of CYP2B6 Enzyme in Human Liver Tissue of HIV and HCV Patients. Medicina, 59(7), 1207. https://doi.org/10.3390/medicina59071207






