Multimodality Cardiac Imaging in COVID-19 Infection
Abstract
:1. Introduction
2. Pathophysiology of Cardiovascular Complications Secondary to COVID-19
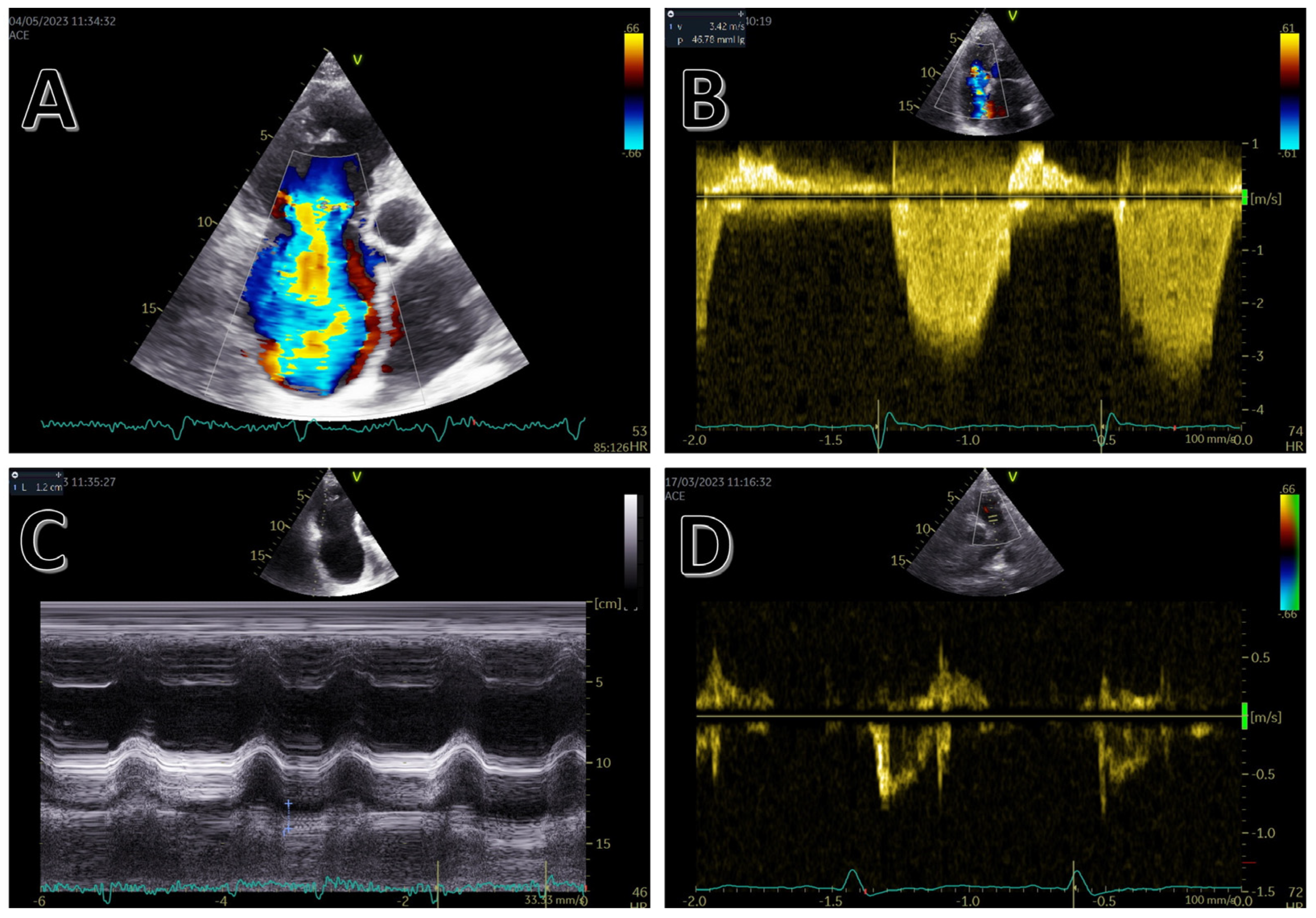
3. Echocardiography
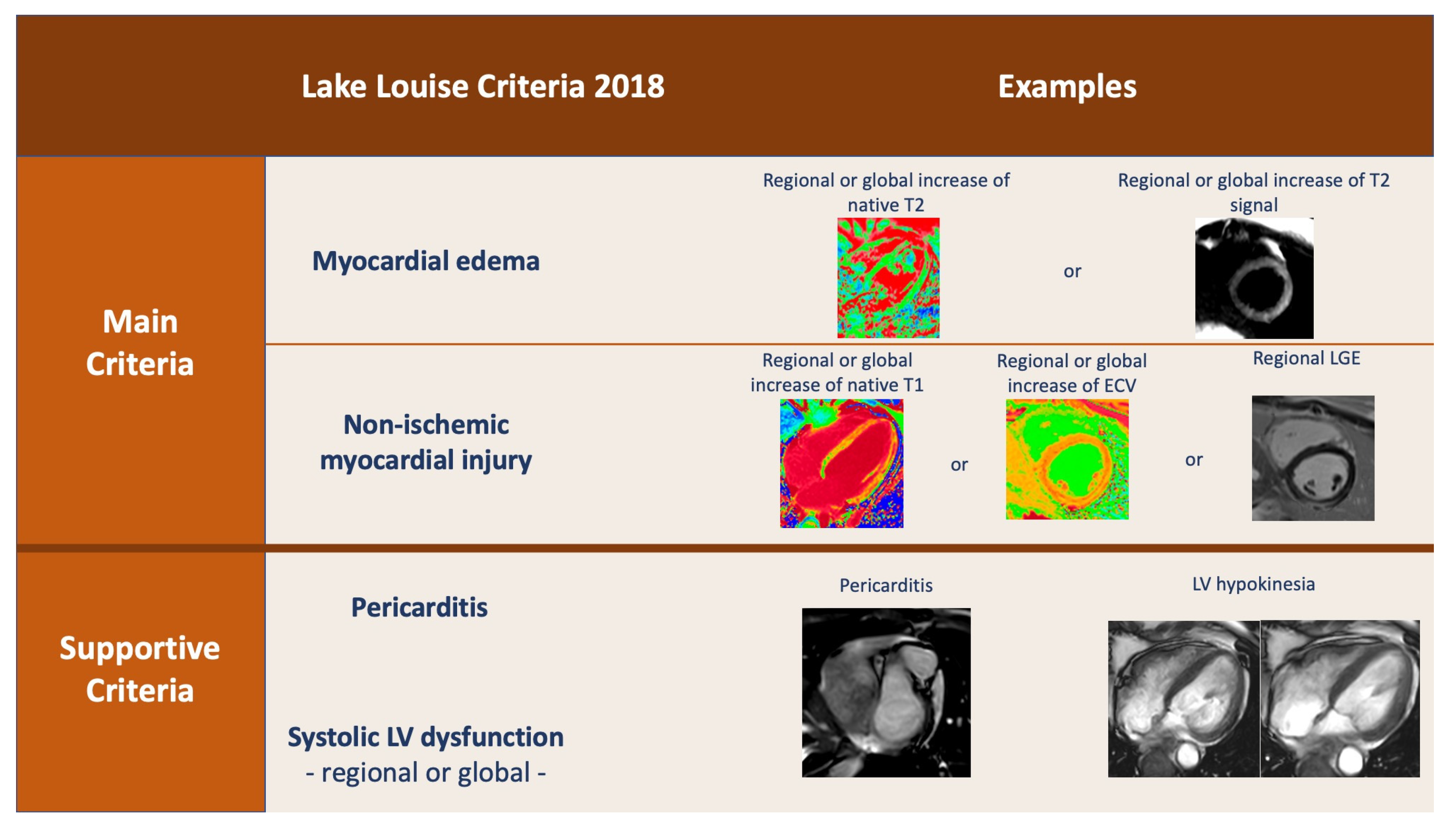
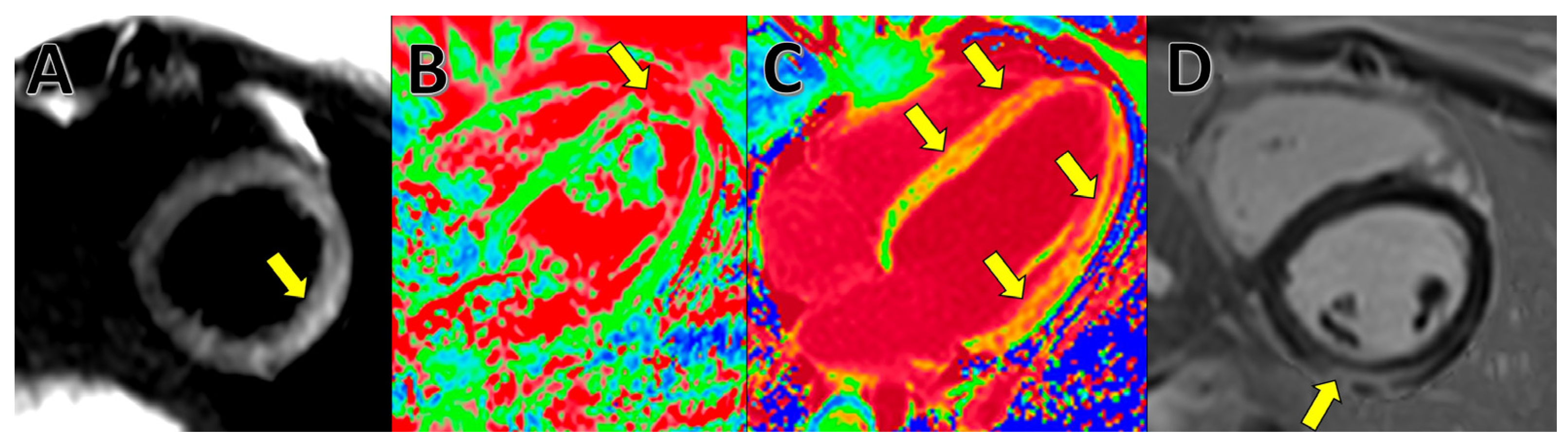
4. Cardiac Magnetic Resonance (CMR)
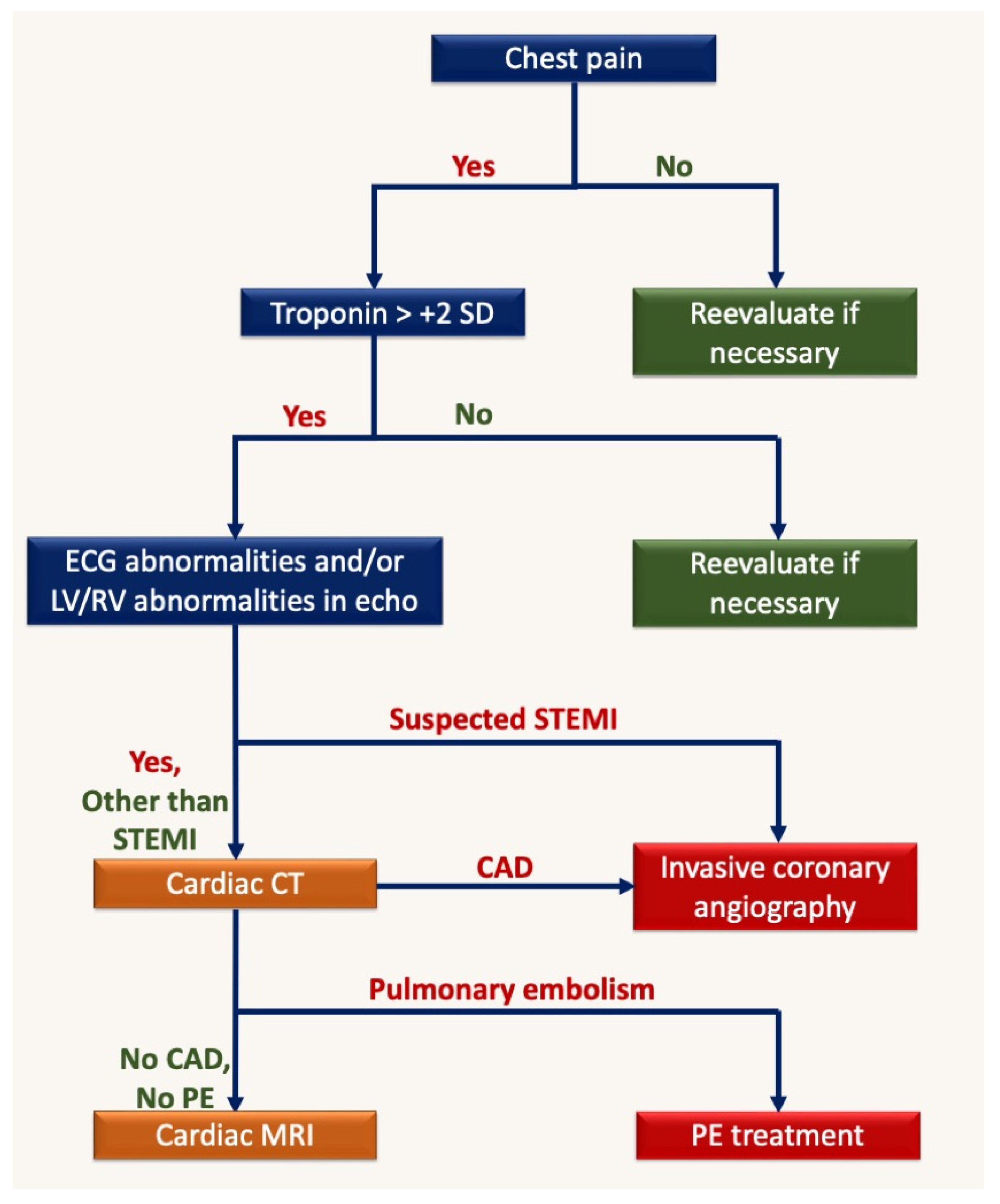
5. Computed Tomography (CT)
6. Discussion
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Esposito, A.; Palmisano, A.; Cao, R.; Rancoita, P.; Landoni, G.; Grippaldi, D.; Boccia, E.; Cosenza, M.; Messina, A.; La Marca, S.; et al. Quantitative assessment of lung involvement on chest CT at admission: Impact on hypoxia and outcome in COVID-19 patients. Clin. Imaging 2021, 77, 194. [Google Scholar] [CrossRef] [PubMed]
- Fried, J.A.; Ramasubbu, K.; Bhatt, R.; Topkara, V.K.; Clerkin, K.J.; Horn, E.; Rabbani, L.; Brodie, D.; Jain, S.S.; Kirtane, A.J.; et al. The Variety of Cardiovascular Presentations of COVID-19. Circulation 2020, 141, 1930–1936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopes, R.D.; Macedo, A.V.S.; de Barros E Silva, P.G.M.; Moll-Bernardes, R.J.; Dos Santos, T.M.; Mazza, L.; Feldman, A.; D’Andréa Saba Arruda, G.; de Albuquerque, D.C.; Camiletti, A.S.; et al. Effect of Discontinuing vs Continuing Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers on Days Alive and Out of the Hospital in Patients Admitted With COVID-19: A Randomized Clinical Trial. JAMA 2021, 325, 254. [Google Scholar] [CrossRef]
- Hendren, N.S.; Drazner, M.H.; Bozkurt, B.; Cooper, L.T. Description and Proposed Management of the Acute COVID-19 Cardiovascular Syndrome. Circulation 2020, 141, 1903–1914. [Google Scholar] [CrossRef] [Green Version]
- Palmisano, A.; Gambardella, M.; D’Angelo, T.; Vignale, D.; Ascione, R.; Gatti, M.; Peretto, G.; Federico, F.; Shah, A.; Esposito, A. Advanced cardiac imaging in the spectrum of COVID-19 related cardiovascular involvement. Clin. Imaging 2022, 90, 78. [Google Scholar] [CrossRef]
- Panigada, M.; Bottino, N.; Tagliabue, P.; Grasselli, G.; Novembrino, C.; Chantarangkul, V.; Pesenti, A.; Peyvandi, F.; Tripodi, A. Hypercoagulability of COVID-19 patients in intensive care unit: A report of thromboelastography findings and other parameters of hemostasis. J. Thromb. Haemost. 2020, 18, 1738–1742. [Google Scholar] [CrossRef] [PubMed]
- Rusu, E.L.E.N.A.; Sarbu, I.; Cristescu, C.; Coculescu, B.I.; Moldovan, H.; Petrut, S.; Muresan, A.; Vassu, T.; Pelinescu, D. Highlighting the Antimicrobial Activity of Organic Compounds Isolated from Some Strains of Lactic Acid Bacteria. Rev. De Chim. 2016, 67, 2417–2421. [Google Scholar]
- Giustino, G.; Croft, L.B.; Stefanini, G.G.; Bragato, R.; Silbiger, J.J.; Vicenzi, M.; Danilov, T.; Kukar, N.; Shaban, N.; Kini, A.; et al. Characterization of Myocardial Injury in Patients With COVID-19. J. Am. Coll. Cardiol. 2020, 76, 2043–2055. [Google Scholar] [CrossRef]
- Babapoor-Farrokhran, S.; Gill, D.; Walker, J.; Rasekhi, R.T.; Bozorgnia, B.; Amanullah, A. Myocardial injury and COVID-19: Possible mechanisms. Life Sci. 2020, 253, 117723. [Google Scholar] [CrossRef]
- Bangalore, S.; Sharma, A.; Slotwiner, A.; Yatskar, L.; Harari, R.; Shah, B.; Ibrahim, H.; Friedman, G.H.; Thompson, C.; Alviar, C.L.; et al. ST-Segment Elevation in Patients with Covid-19—A Case Series. N. Engl. J. Med. 2020, 382, 2478–2480. [Google Scholar] [CrossRef]
- Doyen, D.; Moceri, P.; Ducreux, D.; Dellamonica, J. Myocarditis in a patient with COVID-19: A cause of raised troponin and ECG changes. Lancet 2020, 395, 1516. [Google Scholar] [CrossRef] [PubMed]
- Moody, W.E.; Mahmoud-Elsayed, H.M.; Senior, J.; Gul, U.; Khan-Kheil, A.M.; Horne, S.; Banerjee, A.; Bradlow, W.M.; Huggett, R.; Hothi, S.S.; et al. Impact of Right Ventricular Dysfunction on Mortality in Patients Hospitalized With COVID-19, According to Race. CJC Open 2021, 3, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Fujiyoshi, K.; Ako, J.; Ishida, K.; Ishida, M.; Minami, Y.; Inomata, T. Tako-tsubo-like left ventricular dysfunction in a patient with COVID-19 demonstrated by non-invasive multi-modality imaging. J. Nucl. Cardiol. 2022, 29, 863–865. [Google Scholar] [CrossRef] [PubMed]
- Barman, H.A.; Atici, A.; Tekin, E.A.; Baycan, O.F.; Alici, G.; Meric, B.K.; Sit, O.; Genc, O.; Er, F.; Gungor, B.; et al. Echocardiographic features of patients with COVID-19 infection: A cross-sectional study. Int. J. Cardiovasc. Imaging 2021, 37, 825–834. [Google Scholar] [CrossRef]
- Lazzerini, P.E.; Boutjdir, M.; Capecchi, P.L. COVID-19, Arrhythmic Risk, and Inflammation: Mind the Gap! Circulation 2020, 142, 7–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.; Desai, R.; Gandhi, Z.; Fong, H.K.; Doreswamy, S.; Desai, V.; Chockalingam, A.; Mehta, P.K.; Sachdeva, R.; Kumar, G. Takotsubo Syndrome in Patients with COVID-19: A Systematic Review of Published Cases. SN Compr. Clin. Med. 2020, 2, 2102–2108. [Google Scholar] [CrossRef]
- Tajbakhsh, A.; Gheibi Hayat, S.M.; Taghizadeh, H.; Akbari, A.; Inabadi, M.; Savardashtaki, A.; Johnston, T.P.; Sahebkar, A. COVID-19 and cardiac injury: Clinical manifestations, biomarkers, mechanisms, diagnosis, treatment, and follow up. Expert Rev. Anti. Infect. Ther. 2021, 19, 345–357. [Google Scholar] [CrossRef]
- Lang, J.P.; Wang, X.; Moura, F.A.; Siddiqi, H.K.; Morrow, D.A.; Bohula, E.A. A current review of COVID-19 for the cardiovascular specialist. Am. Heart J. 2020, 226, 29–44. [Google Scholar] [CrossRef]
- Kirkpatrick, J.N.; Mitchell, C.; Taub, C.; Kort, S.; Hung, J.; Swaminathan, M. ASE Statement on Protection of Patients and Echocardiography Service Providers During the 2019 Novel Coronavirus Outbreak: Endorsed by the American College of Cardiology. J. Am. Soc. Echocardiogr. 2020, 33, 648. [Google Scholar] [CrossRef]
- Huang, G.; Vengerovsky, A.; Morris, A.; Town, J.; Carlbom, D.; Kwon, Y. Development of a COVID-19 Point-of-Care Ultrasound Protocol. J. Am. Soc. Echocardiogr. 2020, 33, 903. [Google Scholar] [CrossRef]
- Bonnemain, J.; Ltaief, Z.; Liaudet, L. The Right Ventricle in COVID-19. J. Clin. Med. 2021, 10, 2535. [Google Scholar] [CrossRef] [PubMed]
- Szekely, Y.; Lichter, Y.; Taieb, P.; Banai, A.; Hochstadt, A.; Merdler, I.; Oz, A.G.; Rothschild, E.; Baruch, G.; Peri, Y.; et al. Spectrum of Cardiac Manifestations in COVID-19: A Systematic Echocardiographic Study. Circulation 2020, 142, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, H.; Zhu, S.; Xie, Y.; Wang, B.; He, L.; Zhang, D.; Zhang, Y.; Yuan, H.; Wu, C.; et al. Prognostic Value of Right Ventricular Longitudinal Strain in Patients With COVID-19. JACC Cardiovasc. Imaging 2020, 13, 2287–2299. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, E.; Baruch, G.; Szekely, Y.; Lichter, Y.; Kaplan, A.; Taieb, P.; Laufer-Perl, M.; Beer, G.; Kapusta, L.; Topilsky, Y. The Predictive Role of Left and Right Ventricular Speckle-Tracking Echocardiography in COVID-19. JACC Cardiovasc. Imaging 2020, 13, 2471–2474. [Google Scholar] [CrossRef]
- McErlane, J.; McCall, P.; Willder, J.; Berry, C.; Ben Shelley, B.; Reece, A.; Kitchen, C.; Gillies, M.; Dabek, V.; Irvine, V.; et al. Right ventricular free wall longitudinal strain is independently associated with mortality in mechanically ventilated patients with COVID-19. Ann. Intensive Care 2022, 12, 104. [Google Scholar] [CrossRef]
- Huang, S.; Vignon, P.; Mekontso-Dessap, A.; Tran, S.; Prat, G.; Chew, M.; Balik, M.; Sanfilippo, F.; Banauch, G.; Clau-Terre, F.; et al. Echocardiography findings in COVID-19 patients admitted to intensive care units: A multi-national observational study (the ECHO-COVID study). Intensive Care Med. 2022, 48, 667. [Google Scholar] [CrossRef]
- Li, Y.; Fang, L.; Zhu, S.; Xie, Y.; Wang, B.; He, L.; Zhang, D.; Zhang, Y.; Yuan, H.; Wu, C.; et al. Echocardiographic Characteristics and Outcome in Patients With COVID-19 Infection and Underlying Cardiovascular Disease. Front. Cardiovasc. Med. 2021, 8, 642973. [Google Scholar] [CrossRef]
- Hothi, S.S.; Jiang, J.; Steeds, R.P.; Moody, W.E. Utility of Non-invasive Cardiac Imaging Assessment in Coronavirus Disease 2019. Front. Cardiovasc. Med. 2021, 8, 663864. [Google Scholar] [CrossRef]
- Goerlich, E.; Gilotra, N.A.; Minhas, A.S.; Bavaro, N.; Hays, A.G.; Cingolani, O.H. Prominent Longitudinal Strain Reduction of Basal Left Ventricular Segments in Patients With Coronavirus Disease-19. J. Card. Fail. 2021, 27, 100. [Google Scholar] [CrossRef]
- Friedrich, M.G.; Marcotte, F. Cardiac magnetic resonance assessment of myocarditis. Circ. Cardiovasc. Imaging 2013, 6, 833–839. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, V.M.; Schulz-Menger, J.; Holmvang, G.; Kramer, C.M.; Carbone, I.; Sechtem, U.; Kindermann, I.; Gutberlet, M.; Cooper, L.T.; Liu, P.; et al. Cardiovascular Magnetic Resonance in Nonischemic Myocardial Inflammation: Expert Recommendations. J. Am. Coll. Cardiol. 2018, 72, 3158–3176. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.K.; Hong, Y.J.; Im, D.J.; Suh, Y.J.; Park, C.H.; Kim, J.Y.; Chang, S.; Lee, H.-J.; Hur, J.; Kim, Y.J.; et al. Myocardial T1 and T2 Mapping: Techniques and Clinical Applications. Korean J. Radiol. 2017, 18, 113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eitel, I.; Friedrich, M.G. T2-weighted cardiovascular magnetic resonance in acute cardiac disease. J. Cardiovasc. Magn. Reson. 2011, 13, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Brien, A.T.; Gil, K.E.; Varghese, J.; Simonetti, O.P.; Zareba, K.M. T2 mapping in myocardial disease: A comprehensive review. J. Cardiovasc. Magn. Reson. 2022, 24, 33. [Google Scholar] [CrossRef] [PubMed]
- Kravchenko, D.; Isaak, A.; Mesropyan, N.; Bischoff, L.M.; Pieper, C.C.; Attenberger, U.; Kuetting, D.; Zimmer, S.; Hart, C.; Luetkens, J.A. Cardiac magnetic resonance follow-up of COVID-19 vaccine associated acute myocarditis. Front. Cardiovasc. Med. 2022, 9, 1049256. [Google Scholar] [CrossRef]
- Georgiopoulos, G.; Figliozzi, S.; Sanguineti, F.; Aquaro, G.D.; Di Bella, G.; Stamatelopoulos, K.; Chiribiri, A.; Garot, J.; Masci, P.G.; Ismail, T.F. Prognostic Impact of Late Gadolinium Enhancement by Cardiovascular Magnetic Resonance in Myocarditis: A Systematic Review and Meta-Analysis. Circ. Cardiovasc. Imaging 2021, 14, E011492. [Google Scholar] [CrossRef]
- Haaf, P.; Garg, P.; Messroghli, D.R.; Broadbent, D.A.; Greenwood, J.P.; Plein, S. Cardiac T1 Mapping and Extracellular Volume (ECV) in clinical practice: A comprehensive review. J. Cardiovasc. Magn. Reson. 2017, 18, 89. [Google Scholar] [CrossRef] [Green Version]
- Kato, S.; Azuma, M.; Fukui, K.; Kodama, S.; Nakayama, N.; Kitamura, H.; Hagiwara, E.; Ogura, T.; Horita, N.; Namkoong, H.; et al. Cardiac involvement in coronavirus disease 2019 assessed by cardiac magnetic resonance imaging: A meta-analysis. Heart Vessels 2022, 37, 1570. [Google Scholar] [CrossRef]
- Puntmann, V.O.; Carerj, M.L.; Wieters, I.; Fahim, M.; Arendt, C.; Hoffmann, J.; Shchendrygina, A.; Escher, F.; Vasa-Nicotera, M.; Zeiher, A.M.; et al. Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered From Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 1265. [Google Scholar] [CrossRef]
- Shah, R.M.; Shah, M.; Shah, S.; Li, A.; Jauhar, S. Takotsubo Syndrome and COVID-19: Associations and Implications. Curr. Probl. Cardiol. 2021, 46, 100763. [Google Scholar] [CrossRef]
- Shirani, F.; Shayganfar, A.; Hajiahmadi, S. COVID-19 pneumonia: A pictorial review of CT findings and differential diagnosis. Egypt. J. Radiol. Nucl. Med. 2021, 52, 38. [Google Scholar] [CrossRef]
- Cosyns, B.; Lochy, S.; Luchian, M.L.; Gimelli, A.; Pontone, G.; Allard, S.D.; De Mey, J.; Rosseel, P.; Dweck, M.; E Petersen, S.; et al. The role of cardiovascular imaging for myocardial injury in hospitalized COVID-19 patients. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Artico, J.; Shiwani, H.; Moon, J.C.; Gorecka, M.; McCann, G.P.; Roditi, G.; Morrow, A.; Mangion, K.; Lukaschuk, E.; Shanmuganathan, M.; et al. Myocardial Involvement after Hospitalization for COVID-19 Complicated by Troponin Elevation: A Prospective, Multicenter, Observational Study. Circulation 2023, 147, 364–374. [Google Scholar] [CrossRef] [PubMed]
- de Guadiana-Romualdo, L.G.; Morell-García, D.; Rodríguez-Fraga, O.; Morales-Indiano, C.; Jiménez, A.M.L.P.; Revilla, J.I.G.; Urrechaga, E.; Álamo, J.M.; Holgado, A.M.H.; Lorenzo-Lozano, M.D.C.; et al. Cardiac troponin and COVID-19 severity: Results from BIOCOVID study. Eur. J. Clin. Investig. 2021, 51, e13532. [Google Scholar] [CrossRef] [PubMed]
- Piccioni, A.; Brigida, M.; Loria, V.; Zanza, C.; Longhitano, Y.; Zaccaria, R.; Racco, S.; Gasbarrini, A.; Ojetti, V.; Franceschi, F.; et al. Role of troponin in COVID-19 pandemic: A review of literature. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 10293–10300. [Google Scholar] [PubMed]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromesThe Task Force for the diagnosis and management of chronic coronary syndromes of the European Society of Cardiology (ESC). Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cereda, A.; Toselli, M.; Palmisano, A.; Vignale, D.; Khokhar, A.; Campo, G.; Bertini, M.; Loffi, M.; Andreini, D.; Pontone, G.; et al. Coronary calcium score as a predictor of outcomes in the hypertensive Covid-19 population: Results from the Italian (S) Core-Covid-19 Registry. Hypertens. Res. 2021, 45, 333–343. [Google Scholar] [CrossRef]
- World Health Organization. Weekly Epidemiological Update on COVID-19—13 April 2023; World Health Organization: Geneva, Switzerland, 2023.
- Ojha, V.; Verma, M.; Pandey, N.N.; Mani, A.; Malhi, A.S.; Kumar, S.; Jagia, P.; Roy, A.; Sharma, S. Cardiac Magnetic Resonance Imaging in Coronavirus Disease 2019 (COVID-19): A Systematic Review of Cardiac Magnetic Resonance Imaging Findings in 199 Patients. J. Thorac. Imaging 2021, 36, 73–83. [Google Scholar] [CrossRef]
- Esposito, A.; Palmisano, A.; Natale, L.; Ligabue, G.; Peretto, G.; Lovato, L.; Vignale, D.; Fiocchi, F.; Marano, R.; Russo, V. Cardiac Magnetic Resonance Characterization of Myocarditis-Like Acute Cardiac Syndrome in COVID-19. JACC. Cardiovasc. Imaging 2020, 13, 2462. [Google Scholar] [CrossRef]
- D’Angelo, T.; Cattafi, A.; Carerj, M.L.; Booz, C.; Ascenti, G.; Cicero, G.; Blandino, A.; Mazziotti, S. Myocarditis After SARS-CoV-2 Vaccination: A Vaccine-Induced Reaction? Can. J. Cardiol. 2021, 37, 1665–1667. [Google Scholar] [CrossRef]
- Liga, R.; Gimelli, A. Cardiac Imaging on COVID-19 Pandemic Era: The Stand, The Lost, and Found. Curr. Cardiovasc. Imaging Rep. 2022, 15, 23. [Google Scholar] [CrossRef] [PubMed]
- Finkenzeller, T.; Lenhart, S.; Reinwald, M.; Lüth, S.; Dendl, L.M.; Paetzel, C.; Szczypien, N.; Klawonn, F.; Von Meyer, A.; Schreyer, A.G. Risk to Radiology Staff for Occupational COVID-19 Infection in a High-Risk and a Low-Risk Region in Germany: Lessons from the “First Wave. ” Rofo 2021, 193, 537–543. [Google Scholar] [CrossRef]
- Özdemir, H.I.; Savas, R.; Özbek, S.S. COVID-19 radiology CT personnel management. Diagnostic Interv. Radiol. 2021, 27, 302. [Google Scholar] [CrossRef] [PubMed]
- Sabetian, G.; Moghadami, M.; Haghighi, L.H.F.; Shahriarirad, R.; Fallahi, M.J.; Asmarian, N.; Moeini, Y.S. COVID-19 infection among healthcare workers: A cross-sectional study in southwest Iran. Virol. J. 2021, 18, 58. [Google Scholar] [CrossRef] [PubMed]
- Skulstad, H.; Cosyns, B.; A Popescu, B.; Galderisi, M.; Di Salvo, G.; Donal, E.; Petersen, S.; Gimelli, A.; Haugaa, K.H.; Muraru, D.; et al. COVID-19 pandemic and cardiac imaging: EACVI recommendations on precautions, indications, prioritization, and protection for patients and healthcare personnel. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 592–598. [Google Scholar] [CrossRef]
- Skali, H.; Murthy, V.L.; Al-Mallah, M.H.; Bateman, T.M.; Beanlands, R.; Better, N.; Calnon, D.A.; Dilsizian, V.; Gimelli, A.; Pagnanelli, R.; et al. Guidance and best practices for nuclear cardiology laboratories during the coronavirus disease 2019 (COVID-19) pandemic: An Information Statement from ASNC and SNMMI. J. Nucl. Cardiol. 2020, 27, 1022–1029. [Google Scholar] [CrossRef] [PubMed]

| COVID-19 Phase | Cardiac Manifestations |
|---|---|
| Acute phase From symptom onset to symptom resolution | RV dysfunction: pulmonary hypertension |
| Type I myocadiac infarction | |
| Type II myocardial infarction | |
| Myocarditis, Pericarditis | |
| Takotsubo cardiomyopathy | |
| Arrhythmias | |
| Post-acute phase 3–4 weeks after onset | Myocarditis, Pericardits |
| Vaccination | Microvascular ischemia and myocardial infarction |
| Myocardits, pericarditis |
| Sequence | Parameters |
|---|---|
| Survey | Low-resolution 3-axis standard survey of the thorax |
| Axial survey | 20–25 slice axial single-shot white blood/black blood survey of the whole thorax |
| False 2-chamber | 1 slice, 8 mm thickness SSFP cine |
| Short-axis SSFP cine | 12–15 slices, 8 mm thickness, no slice gap |
| T1 mapping | 3 slices, 8 mm slice thickness, 10–15 mm slice gap |
| T2 mapping | 3 slices, 8 mm slice thickness, 10–15 mm slice gap |
| T2-weighted short-axis | TSE or STIR: 8 slices, 8 mm slice thickness, 0–5 mm slice gap |
| T2-weighted long-axis | TSE or STIR: 1 slice, 8 |
| Contrast injection | 0.15–0.2 mmol/kg, 3–4 mL/s |
| 4-chamber SSFP cine | 1 slice, 8 mm thickness |
| 3-chamber SSFP cine | 1 slice, 8 mm thickness |
| 2-chamber SSFP cine | 1 slice, 8 mm thickness |
| Right ventricle 2-chamber SSFP cine | 1 slice, 8 mm thickness |
| LookLocker (MOLLI) | 1 slice, 8 mm thickness |
| Short axis PSIR | 10 slices, 8 mm slice thickness, 0–2 mm slice gap |
| 4-chamber PSIR | 1 slice, 8 mm thickness |
| 3-chamber PSIR | 1 slice, 8 mm thickness |
| 2-chamber PSIR | 1 slice, 8 mm thickness |
| Enhanced T1-mapping | 3 slices, 8 mm slice thickness, 10–15 mm slice gap |
| Phase | Details |
|---|---|
| Preparation | Administer B-blockers until heart rate is <70 bpm (not appliable for the newest machines with 256+ slices) |
| Use venous canula of at least 20 G | |
| Coronary Calcium Score | ECG-gated, breathhold |
| Acquisition from tracheal carina until 2 cm under cardiac shadow on survey scans | |
| Calcium score calculation using dedicated software | |
| CT Angiography | Injection protocol |
| Use contrast agent with concentration of 300–400 mg of iodine/mL Two phase injection: 1. 80–120 mL of contrast media, 5–6 mL/s 2. 30–40 mL of saline, 5–6 mL/s | |
| ECG-gated, breathhold | |
| Acquisition from just over the aortic arch until 2 cm under the heart | |
| Acquisition window 30–80% Contrast monitor plane on the tracheal carina | |
| Manual breathhold and acquisition start: 1. Breathhold command when pulmonary artery has maximum opacification 2. Start 3 s delay acquisition when aorta starts opacifying |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Militaru, S.; Mihu, A.; Genunche-Dumitrescu, A.V.; Neagoe, C.D.; Avramescu, T.E.; Istratoaie, O.; Gheonea, I.-A.; Militaru, C. Multimodality Cardiac Imaging in COVID-19 Infection. Medicina 2023, 59, 1223. https://doi.org/10.3390/medicina59071223
Militaru S, Mihu A, Genunche-Dumitrescu AV, Neagoe CD, Avramescu TE, Istratoaie O, Gheonea I-A, Militaru C. Multimodality Cardiac Imaging in COVID-19 Infection. Medicina. 2023; 59(7):1223. https://doi.org/10.3390/medicina59071223
Chicago/Turabian StyleMilitaru, Sebastian, Anca Mihu, Amelia Valentina Genunche-Dumitrescu, Carmen Daniela Neagoe, Taina Elena Avramescu, Octavian Istratoaie, Ioana-Andreea Gheonea, and Cristian Militaru. 2023. "Multimodality Cardiac Imaging in COVID-19 Infection" Medicina 59, no. 7: 1223. https://doi.org/10.3390/medicina59071223





