sFLT1, PlGF, the sFLT1/PlGF Ratio and Their Association with Pre-Eclampsia in Twin Pregnancies—A Review of the Literature
Abstract
1. Introduction
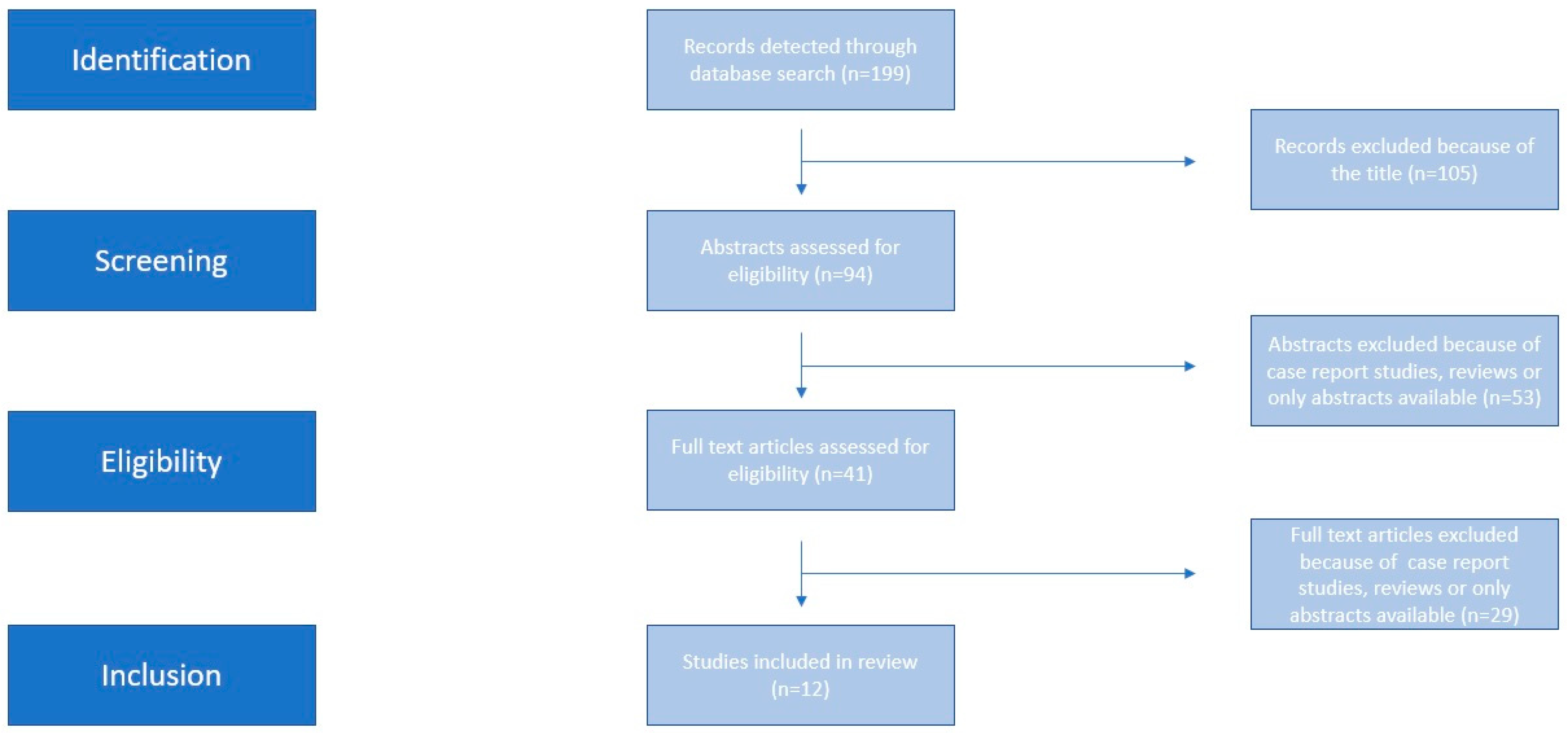
2. Materials and Methods
2.1. Eligibility Criteria, Information Sources, Search Strategy
2.2. Study Selection
2.3. Data Extraction
2.4. Assessment of Risk of Bias
3. Results
3.1. sFLT1, PlGF, sFLT1/PlGF Ratio Alterations in Twin Pregnancies
3.2. Mean Time until Delivery (MTUD)
3.3. Pre-Eclampsia and Adverse Outcomes
4. Discussion
4.1. Main Findings and General Discussion
4.2. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Say, L.; Chou, D.; Gemmill, A.; Tunçalp, Ö.; Moller, A.B.; Daniels, J.; Gülmezoglu, A.M.; Temmerman, M.; Alkema, L. Global causes of maternal death: A WHO systematic analysis. Lancet Glob. Health 2014, 2, e323–e333. [Google Scholar] [CrossRef]
- Oppong, S.A.; Bakari, A.; Bell, A.J.; Bockarie, Y.; Adu, J.A.; Turpin, C.A.; Obed, S.A.; Adanu, R.M.; Moyer, C.A. Incidence, causes and correlates of maternal near-miss morbidity: A multi-centre cross-sectional study. BJOG Int. J. Obs. Gynaecol. 2019, 126, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Weiner, E.; Feldstein, O.; Schreiber, L.; Grinstein, E.; Barber, E.; Dekalo, A.; Bar, J.; Kovo, M. Placental Component and Pregnancy Outcome in Singleton versus Twin Pregnancies Complicated by Pre-eclampsia. Fetal Diagn. Ther. 2018, 44, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Krotz, S.; Fajardo, J.; Ghandi, S.; Patel, A.; Keith, L.G. Hypertensive disease in twin pregnancies: A review. Twin Res. 2002, 5, 8–14. [Google Scholar] [CrossRef]
- Pourali, L.; Ayati, S.; Jelodar, S.; Zarifian, A.; Sheikh Andalibi, M.S. Obstetrics and perinatal outcomes of dichorionic twin pregnancy following ART compared with spontaneous pregnancy. Int. J. Reprod. Biomed. 2016, 14, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Verlohren, S.; Herraiz, I.; Lapaire, O.; Schlembach, D.; Moertl, M.; Zeisler, H.; Calda, P.; Holzgreve, W.; Galindo, A.; Engels, T.; et al. The sFLT1/PlGF ratio in different types of hypertensive pregnancy disorders and its prognostic potential in preeclamptic patients. Am. J. Obs. Gynecol. 2012, 206, 58.e1–58.e8. [Google Scholar] [CrossRef] [PubMed]
- Verlohren, S.; Herraiz, I.; Lapaire, O.; Schlembach, D.; Zeisler, H.; Calda, P.; Sabria, J.; Markfeld-Erol, F.; Galindo, A.; Schoofs, K.; et al. New gestational phase-specific cutoff values for the use of the soluble fms-like tyrosine kinase-1/placental growth factor ratio as a diagnostic test for pre-eclampsia. Hypertension 2014, 63, 346–352. [Google Scholar] [CrossRef]
- Perry, H.; Binder, J.; Kalafat, E.; Jones, S.; Thilaganathan, B.; Khalil, A. Angiogenic marker prognostic models in pregnant women with hypertension. Hypertension 2020, 75, 755–761. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. Hypertension in Pregnancy: Diagnosis and Management (Clinical Guideline NG133); National Institute for Health and Care Excellence (NICE): London, UK, 2019; Bookshelf ID: NBK546004. [Google Scholar]
- Stepan, H.; Hund, M.; Andraczek, T. Combining biomarkers to predict pregnancy complications and redefine pre-eclampsia: The angiogenic-placental syndrome. Hypertension 2020, 75, 918–926. [Google Scholar] [CrossRef]
- Shinohara, S.; Sunami, R.; Kasai, M.; Yasuda, G.; Uchida, Y. Predictive value of the sFLT1/PlGF ratio for pre-eclampsia in twin pregnancies: A retrospective study. Hypertens. Pregnancy 2021, 40, 330–335. [Google Scholar] [CrossRef]
- Martínez-Varea, A.; Martínez-Sáez, C.; Domenech, J.; Desco-Blay, J.; Monfort-Pitarch, S.; Hueso, M.; Diago-Almela, V. sFLT1/PlGF Ratio at 24 Weeks Gestation in Twin Pregnancies as a Predictor of Pre-eclampsia or Fetal Growth Restriction. Fetal Diagn. Ther. 2022, 49, 206–214. [Google Scholar] [CrossRef] [PubMed]
- De La Calle, M.; Delgado, J.L.; Verlohren, S.; Escudero, A.I.; Bartha, J.L.; Campillos, J.M.; Aguarón De La Cruz, A.; Chantraine, F.; García Hernández, J.Á.; Herraiz, I.; et al. Gestational Age-Specific Reference Ranges for the sFLT1/PlGF Immunoassay Ratio in Twin Pregnancies. Fetal Diagn. Ther. 2021, 48, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Karge, A.; Seiler, A.; Flechsenhar, S.; Haller, B.; Ortiz, J.U.; Lobmaier, S.M.; Axt-Fliedner, R.; Enzensberger, C.; Abel, K.; Kuschel, B.; et al. Prediction of adverse perinatal outcome and the mean time until delivery in twin pregnancies with suspected pre-eclampsia using sFLT1/PIGF ratio. Pregnancy Hypertens. 2021, 24, 37–43. [Google Scholar] [CrossRef]
- Binder, J.; Palmrich, P.; Pateisky, P.; Kalafat, E.; Kuessel, L.; Zeisler, H.; Munkhbaatar, M.; Windsperger, K.; Thilaganathan, B.; Khalil, A. The Prognostic Value of Angiogenic Markers in Twin Pregnancies to Predict Delivery due to Maternal Complications of Pre-eclampsia. Hypertension 2020, 76, 176–183. [Google Scholar] [CrossRef]
- Saleh, L.; Tahitu, S.I.M.; Danser, A.H.J.; van den Meiracker, A.H.; Visser, W. The predictive value of the sFLT1/PlGF ratio on short-term absence of pre-eclampsia and maternal and fetal or neonatal complications in twin pregnancies. Pregnancy Hypertens. 2018, 14, 222–227. [Google Scholar] [CrossRef]
- Dröge, L.; Herraìz, I.; Zeisler, H.; Schlembach, D.; Stepan, H.; Küssel, L.; Henrich, W.; Galindo, A.; Verlohren, S. Maternal serum sFLT1/PlGF ratio in twin pregnancies with and without pre-eclampsia in comparison with singleton pregnancies. Ultrasound Obs. Gynecol. 2015, 45, 286–293. [Google Scholar] [CrossRef]
- Boucoiran, I.; Thissier-Levy, S.; Wu, Y.; Wei, S.Q.; Luo, Z.C.; Delvin, E.; Fraser, W.D.; Audibert, F.; MIROS Study Group. Risks for pre-eclampsia and small for gestational age: Predictive values of placental growth factor, soluble fms-like tyrosine kinase-1, and inhibin A in singleton and multiple-gestation pregnancies. Am. J. Perinatol. 2013, 30, 607–612. [Google Scholar] [CrossRef]
- Rana, S.; Hacker, M.R.; Modest, A.M.; Salahuddin, S.; Lim, K.H.; Verlohren, S.; Perschel, F.H.; Karumanchi, S.A. Circulating angiogenic factors and risk of adverse maternal and perinatal outcomes in twin pregnancies with suspected pre-eclampsia. Hypertension 2012, 60, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Powers, R.W.; Jeyabalan, A.; Clifton, R.G.; Van Dorsten, P.; Hauth, J.C.; Klebanoff, M.A.; Lindheimer, M.D.; Sibai, B.; Landon, M.; Miodovnik, M.; et al. Soluble fms-Like tyrosine kinase 1 (sFlt1), endoglin and placental growth factor (PlGF) in pre-eclampsia among high risk pregnancies. PLoS ONE 2010, 5, e13263. [Google Scholar] [CrossRef]
- Kozłowski, S.; Stelmaszczyk-Emmel, A.; Szymusik, I.; Saletra-Bielińska, A.; Brawura-Biskupski-Samaha, R.; Pietruski, P.; Osińska, A.; Kosińska-Kaczyńska, K. sFLT1, Not PlGF, Is Related to Twin Gestation Choronicity in the First and Third Trimesters of Pregnancy. Diagnostics 2021, 11, 1181. [Google Scholar] [CrossRef]
- Faupel-Badger, J.M.; McElrath, T.F.; Lauria, M.; Houghton, L.C.; Lim, K.H.; Parry, S.; Cantonwine, D.; Lai, G.; Karumanchi, S.A.; Hoover, R.N.; et al. Maternal circulating angiogenic factors in twin and singleton pregnancies. Am. J. Obs. Gynecol. 2015, 212, 636.e1–636.e8. [Google Scholar] [CrossRef]
- Sánchez, O.; Llurba, E.; Marsal, G.; Domínguez, C.; Aulesa, C.; Sánchez-Durán, M.A.; Goya, M.M.; Alijotas-Reig, J.; Carreras, E.; Cabero, L. First trimester serum angiogenic/anti-angiogenic status in twin pregnancies: Relationship with assisted reproduction technology. Hum. Reprod. 2012, 27, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M.; QUADAS-2 Group. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef] [PubMed]
- McElrath, T.F.; Lim, K.-H.; Pare, E.; Rich-Edwards, J.; Pucci, D.; Troisi, R.; Parry, S. Longitudinal evaluation of predictive value for pre-eclampsia of circulating angiogenic factors through pregnancy. Am. J. Obs. Gynecol. 2012, 207, 407.e1–407.e7. [Google Scholar] [CrossRef] [PubMed]
- Maynard, S.E.; Moore Simas, T.A.; Solitro, M.J.; Rajan, A.; Crawford, S.; Soderland, P.; Meyer, B.A. Circulating angiogenic factors in singleton vs multiple-gestation pregnancies. Am. J. Obs. Gynecol. 2008, 198, 200.e1–200.e7. [Google Scholar] [CrossRef]
- Hund, M.; Allegranza, D.; Schoedl, M.; Dilba, P.; Verhagen-Kamerbeek, W.; Stepan, H. Multicenter prospective clinical study to evaluate the prediction of short-term outcome in pregnant women with suspected pre-eclampsia (PROGNOSIS): Study protocol. BMC Pregnancy Childbirth 2014, 14, 324. [Google Scholar] [CrossRef]
- Perales, A.; Delgado, J.L.; De La Calle, M.; GarcíaHernández, J.A.; Escudero, A.I.; Campillos, J.M.; Sarabia, M.D.; Laíz, B.; Duque, M.; Navarro, M.; et al. STEPS investigators: sFLT1/PlGF for earlyonset pre-eclampsia prediction: STEPS (study of early pre-eclampsia in Spain). Ultrasound Obs. Gynecol. 2016, 50, 373–382. [Google Scholar] [CrossRef]
- Verlohren, S.; Galindo, A.; Schlembach, D.; Zeisler, H.; Herraiz, I.; Moertl, M.G.; Pape, J.; Dudenhausen, J.W.; Barbara, D.; Holger, S.; et al. An automated method for the determination of the sFLT1/PIGF ratio in the assessment of pre-eclampsia. Am. J. Obs. Gynecol. 2010, 202, 161.e1–161.e11. [Google Scholar] [CrossRef]
- Herraiz, I.; Llurba, E.; Verlohren, S.; Galindo, A.; Spanish Group for the Study of Angiogenic Markers in Pre-eclampsia. Update on the diagnosis and prognosis of pre-eclampsia with the aid of the sFLT1/PlGF ratio in singleton pregnancies. Fetal Diagn. Ther. 2018, 43, 81–89. [Google Scholar] [CrossRef]
- Bdolah, Y.; Lam, C.; Rajakumar, A.; Shivalingappa, V.; Mutter, W.; Sachs, B.P.; Lim, K.H.; Bdolah-Abram, T.; Epstein, F.H.; Karumanchi, S.A. Twin pregnancy and the risk of pre-eclampsia: Bigger placenta or relative ischemia? Am. J. Obs. Gynecol. 2008, 198, 428.e1–428.e6. [Google Scholar] [CrossRef]
- Kametas, N.A.; McAuliffe, F.; Krampl, E.; Chambers, J.; Nicolaides, K.H. Maternal cardiac function in twin pregnancy. Obs. Gynecol. 2003, 102, 806–815. [Google Scholar] [PubMed]
- Lane-Cordova, A.D.; Khan, S.S.; Grobman, W.A.; Greenland, P.; Shah, S.J. Long-Term Cardiovascular Risks Associated with Adverse Pregnancy Outcomes: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019, 73, 2106–2116. [Google Scholar] [CrossRef] [PubMed]
| Authors | Type of Study | Mean GA at Blood Sampling in Weeks of Gestation (Range) | Inclusion | N (Twins) | Control | Outcome | Pregnancy Duration from Presentation to Delivery | sFLT1/PlGF Ratio | sFLT1 (pg/mL) | PIGF (pg/mL) |
|---|---|---|---|---|---|---|---|---|---|---|
| Powers et al., 2010 [20] | Secondary analysis of a multicentre randomised controlled trial | Visit 1: 7–26 Visit 2: 24–28 Visit 3: 34–38 | Multifetal gestations | 39 with PET | 195 without PET | Prediction of PET in high-risk pregnancies | N/A | N/A | With PET 7330 ± 5420 Without PET 5950 ± 2470 | With PET 386.1 ± 318.17 Without PET 554.73 ± 388 |
| Rana et al., 2012 [19] | Prospective Cohort | 33.9 (31.9–36.0) | Twins with suspected PET | 52 with adverse outcome 27 without adverse outcome | N/A | (1) Adverse outcomes (2) Time until delivery | With adverse 3.5 days Without adverse 14.5 days | With adverse 74.2 Without adverse 36.2 | With adverse: 11,461.5 Without adverse adverse: 7495.0 | With adverse: 162.5 Without adverse adverse: 224.0 |
| Boucoiran et al., 2013 [18] | Prospective Cohort | Visit 1 12–18 Visit 2 24–26 | Twins and singletons | 69 | 703 | Predictive accuracy of PlGF, sFLT1, and inhibin A plasma concentrations in multiple compared to singleton pregnancies | N/A | Visit 1 With PET 2.53 (0.96–3.45) MoM Without PET 1.09 (0.55–1.71) MoM Visit 2 With PET 21.23 (0.55–34.31) MoM Without PET 0.73 (0.42–1.74) MoM | Visit 1 With PET 0.72 (0.70–0.87) MoM Without PET 1.06 (0.73–1.56) MoM Visit 2 With PET 2.21 (0.99–3.87) MoM Without PET 1.03 (0.55–1.65) MoM | Visit 1 With PET 0.34 (0.31–0.49) MoM Without PET 1.08 (0.74–1.47) MoM Visit 2 With PET 0.22 (0.10–1.03) MoM Without PET 1.08 (0.54–1.78) MoM |
| Droge et al., 2015 [17] | Multicenter case–control study | No PE twins: 30.5 PE twins: 32.9 | Twins and singletons | 18 with PET 31 without PET | 54 singletons with PET 238 singletons without PET | PET | N/A | With PET 164.22 Without PET 13.29 | With PET 20,011.50 pg/mL Without PET 4503.00 pg/mL | With PET 138.80 pg/mL Without PET 403.00 pg/mL |
| Faupel-Badger et al., 2015 [22] | Data analysis from two studies | BIRTH: 4 visits 9.7 (8.4–11.6), 17.8 (16.8–18.7), 25.9 (24.8–28.1), and 35.1 (34.6–35.9) Geisel School of Medicine: 31–39 weeks and after admission for labor | Twins and signletons without PET | BIRTH 91 Geisel School of Medicine 41 | BIRTH 2193 Geisel School of Medicine 62 | Comparison of angiogenic factors between twins and singletons | N/A | BIRTH (Twins vs. Singletons) 9.7 weeks: 294.6 vs. 202.4 17.8 weeks: 58.7 vs. 44.3 25.9 weeks: 19.4 vs. 13.2 35.1 weeks: 168.4 vs. 29 Geisel School of Medicine(Twins vs. Singletons) 3rd trimester:15.9 vs. 4.51 Delivery: 107.8 vs. 47.8 | BIRTH (Twins vs. Singletons) 9.7 weeks: 7037 vs. 4485 17.8 weeks: 12,543 vs. 6131 25.9 weeks: 12,968 vs. 5898 35.1 weeks: 36,916 vs. 10,151 Geisel School of Medicine(Twins vs. Singletons) 3rd trimester: 6129 vs. 2108 Delivery: 15,899 vs. 7278 | BIRTH (Twins vs. Singletons) 9.7 weeks: 24.9 vs. 22.8 17.8 weeks: 213.5 vs. 138.4 25.9 weeks: 668 vs. 445.9 35.1 weeks: 219.2 vs. 350.2 Geisel School of Medicine (Twins vs. Singletons) 3rd trimester: 386.2 vs. 467.3 Delivery: 147.5 vs. 152.4 |
| Saleh et al., 2018 [16] | Secondary analysis of a prospective multicenter cohort study | 29 (23–34) 30 (24–34) | Twins with suspected PET | 21 | 21 singletons | (1) PET (2) Adverse pregnancy outcomes | N/A | Confirmed PET twins: 49 Suspected PET twins: 26 | Confirmed PET twins: 9134 Suspected PET twins: 6377 | Confirmed PET twins: 185 Suspected PET twins: 228 |
| Binder et al., 2020 [15] | Retrospective analysis | 33.6 (30.0–35.2) | Twins with suspected PET | 164 | N/A | Delivery because of PET within 1 or 2 weeks of blood sampling | N/A | ≤1 wk due to PET: 98.9 ≤2 wk due to PET: 84.2 Delivery >2 wk due to PET or at Any Time Reasons Other Than PET: 23.5 | ≤1 wk due to PET: 15,034 ≤2 wk due to PET: 14,620 Delivery > 2 wk due to PET or at Any Time Reasons Other Than PET: 6954 | ≤1 wk due to PET: 150.2 ≤2 wk due to PET: 62.1 Delivery > 2 wk due to PET or at Any Time Reasons Other Than PET: 344.8 |
| Calle et al., 2021 [13] | Reference range analysis | PROGNOSIS: 26–37 STEPS: Visit 1: 19 or 20, Visit 2: 23–24, Visit 3: 27–28 | Twins | 269 | N/A | (1) Reference ranges for sFLT1/PlGF ratio in twin pregnancies (2) Predictive performance short-term PE | N/A | Cut-off of 38 NPV of 91.9 and 83.8% to rule out PE within 1 and 4 weeks | N/A | N/A |
| Karge et al., 2021 [14] | Retrospective cohort study | N/A | Twins with suspected PET and/or HELLP syndrome | 49 | N/A | (1) Adverse perinatal outcome. (2) Mean time until delivery | < 34 weeks sFLT1/PIGF ratio ≤ 53: 905.23 h ± 643.08 sFLT1/PIGF ratio > 53: 220.90 h ± 217.65 sFLT1/PIGF ratio ≤ 85: 741.48 h ± 624.94 sFLT1/PIGF ratio > 85: 109.00 h ± 119.03 ≥34 weeks sFLT1/PIGF ratio ≤ 53: 113.70 h ± 157.03 sFLT1/PIGF ratio > 53: 123.03 h ±157.03 sFLT1/PIGF ratio ≤ 110: 127.42 h ± 144.88 sFLT1/PIGF ratio > 110: 74.67 h ± 82.16 | With suspected PE 69.80 With PE 49.50 With adverse 89.45 Without adverse 62.00 | N/A | N/A |
| Kozłowski et al., 2021 [21] | Prospective observational study | Visit 1: 11–14 Visit 2: 32–34 | Twins | 79 | N/A | Expression of angiogenic biomarkers in dichorionic and monochorionic twins | N/A | N/A | N/A | N/A |
| Shinohara et al., 2021 [11] | Retrospective observational cohort | 29 (28–30) | Twins | 10 with PET within 4 wks | 68 without PET within 4 weeks | Development of PET within 4 weeks | With PET 4.7 weeks Without PET 7.3 weeks | With PET: 46.2 (22.2–64.6) Without PET: 4.0 (0.5–70.7) | N/A | N/A |
| Martínez-Varea et al., 2022 [12] | Prospective study | 24 | Twins | 14 with PET/FGR | 94 without PET/FGR | Prediction of PET/FGR | N/A | With PET/FGR 20.286 (22.317) Without PET/FGR 4.309 (7.008) | N/A | N/A |
| Demographics | Powers et al., 2010 [20] | Rana et al., 2012 [19] | Boucoiran et al., 2013 [18] | Droge et al., 2015 [17] | Faupel-Badger et al., 2015 [22] | Saleh et al., 2018 [16] | Binder et al., 2020 [15] | Calle et al., 2021 [13] | Karge et al., 2021 [14] | Kozłowski et al., 2021 [21] | Shinohara et al., 2021 [11] | Martínez-Varea et al., 2022 [12] | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N (total) | 234 multifetal gestations | 79 twins with suspected PET | 772 twins and singletons | 341 twins and singletons | BIRTH 2284 twins and signletons without PET | Geisel School of Medicine 103 twins and singletons without PET | 42 twins with suspected PET | 164 twins with suspected PET | 269 twins | 49 twins with suspected PET and/or HELLP syndrome | 79 twins | 78 twins | 108 twins | ||
| n | 39 with PET | 52 twins with adverse outcome | 69 | 18 twins with PET | 91 twins | 41 twins | 13 twins with confirmed PET | Delivery ≤ 1 wk due to PET 29 | Delivery ≤ 2 wk due to PET 42 | PROGNOSIS 22 STEPS 222 Case-control study of the Elecsys 25 | Early onset PET/HELLP 18 | Late onset PET/HELLP 31 | 43 monochorionic pregnancies 36 dichorionic pregnancies | 10 twins with PET within 4 wks | 14 twins with PET and/or FGR |
| Group of control | 195 without PET | 27 twins without adverse outcome | N/A | 31 twins without PET 54 singletons with PET 238 singleton without PET | 2193 singletons | 62 singletons | 8 twins with suspected PET 6 singletons with suspected PET 15 singletons with confirmed PET | Delivery > 2 wk due to PET or at Any Time Reasons Other Than PET 122 | N/A | N/A | N/A | N/A | 68 twins without PET within 4 wk | 94 twins without PE or FGR | |
| Maternal age (years) | 26 ± 7 | 34.0 (32.0–38.0) * | N/A | 33.56 ± 5.35 ** | 35.1 (5.8) ** | 33.4 (5.9) * | 36 (31–44) | 37.0 (33.0–39.0) * | 36.0 (31.2–38.0) | 34 (mean) | 34.61 ± 4.67 | 33.74 ± 5.59 | 31.5 ± 4.3 | 34 (27–43) | 36.000 (4.930) |
| Nulliparous | 19 * | 43/52 * | N/A | N/A | 27/91 | 12/41 | N/A | 16 /29 | 25/42 | N/A | 15/18 | 21/31 | 39/79 | 6/10 | 11 (78.6) |
| BMI (kg/m2) | 29 ± 8 | 21.9 (27.8–35.2) | N/A | 24.09 ± 4.97 | N/A | N/A | N/A | 24.5 (21.8–26.0) | 25.1 (21.9–29.1) | 25 (mean) | 24.80 ± 5.11 | 23.94 ± 6.57 | 23.8 ± 4.6 | 19.7 (16.9–28.2) | 24.192 (3.241) |
| Medical History | N/A | ||||||||||||||
| Previous PE | N/A | 1/52 * | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 0/14 |
| Chronic Hypertension | N/A | 7/52 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 14/18 | 1/31 | N/A | N/A | 0/14 |
| Renal disease | N/A | 1/52 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Diabetes | N/A | 1/52 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 1/18 (GDM) | 4/31 (GDM) | 21/79 (GDM) | 3/10 * (GDM) | 0/14 |
| Antihypertensive medication | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 8/29 | 13/42 * | N/A | 11/18 ** | 4/31 ** | N/A | N/A | N/A |
| Aspirin during pregnancy | 14 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 5/18 | 4/31 | N/A | N/A | N/A |
| Chorionicity/ Method of conception | N/A | ||||||||||||||
| Dichorionic | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 23/29 | 33/42 | N/A | 11/18 * | 28/31 * | N/A | N/A | 9/14 |
| Monochorionic | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 6/29 | 9/42 | N/A | 7/18 | 3/31 | N/A | N/A | 5/14 |
| ART | N/A | N/A | N/A | N/A | 64/91 ** | 16/41 * | 10/13 | 13/29 | 18/42 | N/A | 11/18 | 17/31 | 17/79 | 3/10 | 7/14 |
| Race | N/A | ||||||||||||||
| White/Caucasian | N/A | 47/52 | N/A | 14/18 * | 64/91 | 40/41 | 13/13 | N/A | N/A | N/A | 17/18 | 28/31 | N/A | N/A | N/A |
| Black/African American | N/A | 1/52 | N/A | 1/18 | 9/91 | 0/41 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Asian | N/A | 4/52 | N/A | 0/18 | 9/91 | 0/41 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Other/Unknown | N/A | 0/52 | N/A | 3/18 | 2/91 | 1/41 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Family history of PE | N/A | ||||||||||||||
| Yes | N/A | N/A | N/A | 0/18 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 3/10 | N/A |
| No | N/A | N/A | N/A | 14/18 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Unknown | N/A | N/A | N/A | 4/18 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Authors | Type of Study | Conclusions/Observations |
|---|---|---|
| Powers et al., 2010 [20] | Secondary analysis of a multicentre randomized controlled trial | sFlt1 and PlGF are significantly higher among women with multifetal gestations compared with other high-risk groups Observed that sFlt1 and PlGF modest significant differences of at least one of these factors during the third trimester in women who develop preeclampsia in all high-risk groups including multifetal gestations |
| Rana et al., 2012 [19] | Prospective Cohort | sFlt1/PlGF ratio at the time of initial evaluation is associated with subsequent adverse maternal and perinatal outcomes in women with twin pregnancy and suspected preeclampsia |
| Boucoiran et al., 2013 [18] | Prospective Cohort | PlGF level was a good predictor of subsequent PE as early as 12 to 18 weeks in multiple-gestation pregnancies but was not clinically useful enough to be used as a single marker |
| Droge et al., 2015 [17] | Multicenter case–control study | In twin pregnancies with PE, sFlt-1 levels and the sFlt-1/PlGF ratio were increased and PlGF levels were decreased as compared to twin gestations with an uneventful pregnancy outcome sFlt-1/PlGF ratio did not differ between twin pregnancies with PE and singleton pregnancies with PE. In twin pregnancies with an uneventful outcome, sFlt-1 levels and sFlt-1/PlGF ratio were increased, but no differences in PlGF concentration were found when compared with that of singleton controls. Reference ranges of sFlt-1, PlGF and their ratio in singleton pregnancies are therefore not transferable to twin pregnancies |
| Faupel-Badger et al., 2015 [22] | Data analysis from two studies | sFlt-1 concentrations and the sFlt-1/PlGF ratio were higher in twins than singletons across pregnancy and at delivery, with the greatest differences at week 35. PlGF concentrations were lower in twin than singleton pregnancies at week 35 only. Placental weight appeared to be inversely correlated with maternal sFlt-1/PlGF ratio at the end of pregnancy in both twins and singletons |
| Saleh et al., 2018 [16] | Secondary analysis of a prospective multicenter cohort study | Serum sFlt-1levels are considerably higher in twin than in singleton control gestations. sFlt-1/PlGF ratio of ≤38 to predict short-term absence of PE is not applicable to twin pregnancies in predicting either the absence of PE or the absence of adverse pregnancy outcomes |
| Binder et al., 2020 [15] | Retrospective analysis | sFlt-1/PlGF ratio lower than 38 was able to rule-out delivery within 1 and 2 weeks with a negative predictive value of 98.8% and 96.4% for delivery because of preeclampsia within 1 and 2 weeks, respectively. A cutoff of 38 is applicable for ruling out delivery because of preeclampsia in twin pregnancies |
| Calle et al., 2021 [13] | Reference range analysis | Up to 28 weeks + 6 days’ gestation, median, 5th, and 95th percentile values for the sFlt-1/PlGF ratio in twin pregnancies were similar to singleton pregnancies. From 29 weeks of gestation onward, median, 5th, and 95th percentile values for the sFlt-1/PlGF ratio appear to be higher in twin pregnancies suggesting that ratio could be useful in those pregnancies |
| Karge et al., 2021 [14] | Retrospective cohort study | sFlt-1/PlGF ratio in twin pregnancies with suspected PE/HELLP may be useful for the prediction of adverse perinatal outcome, especially to identify cases of s-FGR. MTUD was significantly shortened in women with an elevated sFlt-1/PIGF ratio, an intensified clinical monitoring is required, mainly in women with early onset PE and a ratio > 85. However, a normal ratio may not rule out adverse perinatal outcome. |
| Kozłowski et al., 2021 [21] | Prospective observational study | sFlt-1 level was related to twin gestation chorionicity (significantly higher concentration of sFlt-1 in dichorionic in comparison to monochorionic pregnancies in both the first and third trimesters) |
| Shinohara et al., 2021 [11] | Retrospective observational cohort | A cutoff value of 22.2 for the sFlt-1/ PlGF ratio at 28–30 weeks of gestation may be useful to exclude the development of PE within 4 weeks in twin pregnancies |
| Martínez-Varea et al., 2022 [12] | Prospective study | sFlt-1/PlGF ratio ≥17 at 24 weeks in twin pregnancies is associated with a significant increase in the frequency of preeclampsia and FGR. sFlt-1/PlGF ratio at 24 weeks in twin pregnancies, combined with the mean PI UtA and maternal characteristics, could select patients at risk for placental dysfunction, such as preeclampsia or FGR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sapantzoglou, I.; Rouvali, A.; Koutras, A.; Chatziioannou, M.I.; Prokopakis, I.; Fasoulakis, Z.; Zachariou, E.; Douligeris, A.; Mortaki, A.; Perros, P.; et al. sFLT1, PlGF, the sFLT1/PlGF Ratio and Their Association with Pre-Eclampsia in Twin Pregnancies—A Review of the Literature. Medicina 2023, 59, 1232. https://doi.org/10.3390/medicina59071232
Sapantzoglou I, Rouvali A, Koutras A, Chatziioannou MI, Prokopakis I, Fasoulakis Z, Zachariou E, Douligeris A, Mortaki A, Perros P, et al. sFLT1, PlGF, the sFLT1/PlGF Ratio and Their Association with Pre-Eclampsia in Twin Pregnancies—A Review of the Literature. Medicina. 2023; 59(7):1232. https://doi.org/10.3390/medicina59071232
Chicago/Turabian StyleSapantzoglou, Ioakeim, Angeliki Rouvali, Antonios Koutras, Maria Ioanna Chatziioannou, Ioannis Prokopakis, Zacharias Fasoulakis, Eleftherios Zachariou, Athanasios Douligeris, Anastasia Mortaki, Paraskevas Perros, and et al. 2023. "sFLT1, PlGF, the sFLT1/PlGF Ratio and Their Association with Pre-Eclampsia in Twin Pregnancies—A Review of the Literature" Medicina 59, no. 7: 1232. https://doi.org/10.3390/medicina59071232
APA StyleSapantzoglou, I., Rouvali, A., Koutras, A., Chatziioannou, M. I., Prokopakis, I., Fasoulakis, Z., Zachariou, E., Douligeris, A., Mortaki, A., Perros, P., Ntounis, T., Pergialiotis, V., Domali, E., Athanasiou, S., Daskalakis, G., Rodolakis, A., Panagopoulos, P., & Pappa, K. I. (2023). sFLT1, PlGF, the sFLT1/PlGF Ratio and Their Association with Pre-Eclampsia in Twin Pregnancies—A Review of the Literature. Medicina, 59(7), 1232. https://doi.org/10.3390/medicina59071232











