Remimazolam Induction in a Patient with Super-Super Obesity and Obstructive Sleep Apnea: A Case Report
Abstract
1. Introduction
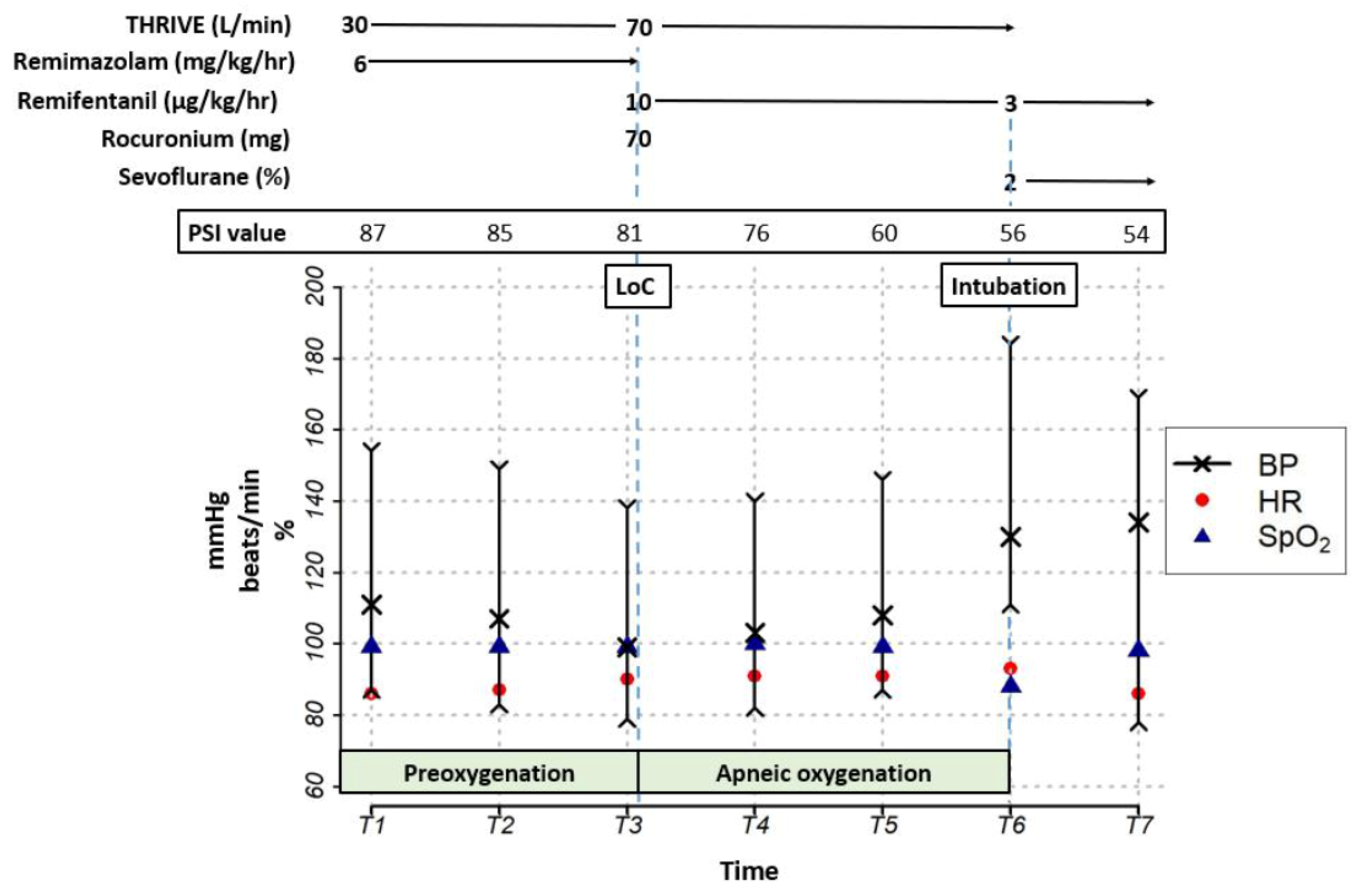
2. Detailed Case Description
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, S.; Arora, L. Anesthesia for the morbidly obese patient. Anesthesiol. Clin. 2020, 38, 197–212. [Google Scholar] [CrossRef]
- Grassi, L.; Kacmarek, R.; Berra, L. Ventilatory mechanics in the patient with obesity. Anesthesiology 2020, 132, 1246–1256. [Google Scholar] [CrossRef]
- Steier, J.; Lunt, A.; Hart, N.; Polkey, M.I.; Moxham, J. Observational study of the effect of obesity on lung volumes. Thorax 2014, 69, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Jehan, S.; Zizi, F.; Pandi-Perumal, S.R.; Wall, S.; Auguste, E.; Myers, A.K.; Jean-Louis, G.; McFarlane, S.I. Obstructive sleep apnea and obesity: Implications for public health. Sleep. Med. Disord. 2017, 1, 00019. [Google Scholar]
- Tsui, B.C.; Murtha, L.; Lemmens, H.J. Practical dosing of propofol in morbidly obese patients. Can. J. Anaesth. 2017, 64, 449–455. [Google Scholar] [CrossRef] [PubMed]
- van Kralingen, S.; Diepstraten, J.; van de Garde, E.M.; van der Lely, A.J.; van Dongen, E.P.; van Ramshorst, B.; Knibbe, C.A. Comparative evaluation of propofol 350 and 200 mg for induction of anaesthesia in morbidly obese patients: A randomized double-blind pilot study. Eur. J. Anaesthesiol. 2010, 27, 572–574. [Google Scholar] [CrossRef] [PubMed]
- Keam, S.J. Remimazolam: First approval. Drugs 2020, 80, 625–633. [Google Scholar] [CrossRef]
- Seyni-Boureima, R.; Zhang, Z.; Antoine, M.M.L.K.; Antoine-Frank, C.D. A review on the anesthetic management of obese patients undergoing surgery. BMC Anesthesiol. 2022, 22, 98. [Google Scholar] [CrossRef]
- Wiltshire, H.R.; Kilpatrick, G.J.; Tilbrook, G.S.; Borkett, K.M. A placebo- and midazolam-controlled phase I single ascending-dose study evaluating the safety, pharmacokinetics, and pharmacodynamics of remimazolam (CNS 7056): Part II. Population pharmacokinetic and pharmacodynamic modeling and simulation. Anesth. Analg. 2012, 115, 284–296. [Google Scholar] [CrossRef]
- Kim, S.H.; Fechner, J. Remimazolam—Current knowledge on a new intravenous benzodiazepine anesthetic agent. Korean J. Anesthesiol. 2022, 75, 307–315. [Google Scholar] [CrossRef]
- Riley, D.S.; Barber, M.S.; Kienle, G.S.; Aronson, J.K.; von Schoen-Angerer, T.; Tugwell, P.; Kiene, H.; Helfand, M.; Altman, D.G.; Sox, H.; et al. CARE guidelines for case reports: Explanation and elaboration document. J. Clin. Epidemiol. 2017, 89, 218–235. [Google Scholar] [CrossRef]
- Juel, B.E.; Romundstad, L.; Kolstad, F.; Storm, J.F.; Larsson, P.G. Distinguishing Anesthetized from Awake State in Patients: A New Approach Using One Second Segments of Raw EEG. Front. Hum. Neurosci. 2018, 12, 40. [Google Scholar] [CrossRef]
- Schüttler, J.; Eisenried, A.; Lerch, M.; Fechner, J.; Jeleazcov, C.; Ihmsen, H. Pharmacokinetics and pharmacodynamics of remimazolam (CNS 7056) after continuous infusion in healthy male volunteers: Part I. Pharmacokinetics and clinical pharmacodynamics. Anesthesiology 2020, 132, 636–651. [Google Scholar] [CrossRef]
- Sheng, X.Y.; Liang, Y.; Yang, X.Y.; Li, L.E.; Ye, X.; Zhao, X.; Cui, Y.M. Safety, pharmacokinetic and pharmacodynamic properties of single ascending dose and continuous infusion of remimazolam besylate in healthy Chinese volunteers. Eur. J. Clin. Pharmacol. 2020, 76, 383–391. [Google Scholar] [CrossRef]
- Doi, M.; Hirata, N.; Suzuki, T.; Morisaki, H.; Morimatsu, H.; Sakamoto, A. Safety and efficacy of remimazolam in induction and maintenance of general anesthesia in high-risk surgical patients (ASA Class III): Results of a multicenter, randomized, double-blind, parallel-group comparative trial. J. Anesth. 2020, 34, 491–501. [Google Scholar] [CrossRef]
- Hirata, N.; Hayamizu, K.; Yamakage, M. How to administer remimazolam for anesthesia induction. J. Anesth. 2020, 34, 962. [Google Scholar] [CrossRef]
- Dai, G.; Pei, L.; Duan, F.; Liao, M.; Zhang, Y.; Zhu, M.; Zhao, Z.; Zhang, X. Safety and efficacy of remimazolam compared with propofol in induction of general anesthesia. Minerva Anestesiol. 2021, 87, 1073–1079. [Google Scholar] [CrossRef]
- Kim, K.M.; Lee, H.; Bang, J.Y.; Choi, B.M.; Noh, G.J. Anaphylaxis following remimazolam administration during induction of anaesthesia. Br. J. Anaesth. 2022, 129, e122–e124. [Google Scholar] [CrossRef]
- Antonik, L.J.; Goldwater, D.R.; Kilpatrick, G.J.; Tilbrook, G.S.; Borkett, K.M. A placebo- and midazolam-controlled phase I single ascending-dose study evaluating the safety, pharmacokinetics, and pharmacodynamics of remimazolam (CNS 7056): Part I. Safety, efficacy, and basic pharmacokinetics. Anesth. Analg. 2012, 115, 274–283. [Google Scholar] [CrossRef]
- Sunzel, M.; Paalzow, L.; Berggren, L.; Eriksson, I. Respiratory and cardiovascular effects in relation to plasma levels of midazolam and diazepam. Br. J. Clin. Pharmacol. 1988, 25, 561–569. [Google Scholar] [CrossRef]
- Sneyd, J.R.; Rigby-Jones, A.E. Remimazolam for anaesthesia or sedation. Curr. Opin. Anesthesiol. 2020, 33, 506–511. [Google Scholar] [CrossRef]
- Kim, T.K.; Obara, S.; Johnson, K. Basic principles of pharmacology. In Miller’s Anesthesia, 9th ed.; Gropper, M.A., Ed.; Elsevier: Philadelphia, PA, USA, 2019; pp. 471–472. [Google Scholar]
- Vullo, P.A.; Real Navacerrada, M.I.; Navarro Suay, R. Hemodynamic impact of increasing time between fentanyl and propofol administration during anesthesia induction: A randomised, clinical trial. Braz. J. Anesthesiol. 2021. [Google Scholar] [CrossRef]
- Davis, D.P.; Kimbro, T.A.; Vilke, G.M. The use of midazolam for prehospital rapid-sequence intubation may be associated with a dose-related increase in hypotension. Prehosp Emerg. Care 2001, 5, 163–168. [Google Scholar] [CrossRef]
- Huang, X.; Cao, H.; Zhang, C.; Lan, H.; Gong, X.; Li, R.; Lin, Y.; Xu, B.; Chen, H.; Guan, X. The difference in mean arterial pressure induced by remimazolam compared to etomidate in the presence of fentanyl at tracheal intubation: A randomized controlled trial. Front. Pharmacol. 2023, 14, 1143784. [Google Scholar] [CrossRef]
- Hasegawa, G.; Hirata, N.; Yoshikawa, Y.; Yamakage, M. Differential effects of remimazolam and propofol on heart rate variability during anesthesia induction. J. Anesth. 2022, 36, 239–245. [Google Scholar] [CrossRef]
- Masui, K. Remimazolam besilate, a benzodiazepine, has been approved for general anesthesia!! J. Anesth. 2020, 34, 479–482. [Google Scholar] [CrossRef]
- Chae, D.; Kim, H.C.; Song, Y.; Choi, Y.S.; Han, D.W. Pharmacodynamic analysis of intravenous bolus remimazolam for loss of consciousness in patients undergoing general anaesthesia: A randomised, prospective, double-blind study. Br. J. Anaesth. 2022, 129, 49–57. [Google Scholar] [CrossRef]
- Ibrahim, A.E.; Taraday, J.K.; Kharasch, E.D. Bispectral Index Monitoring during Sedation with Sevoflurane, Midazolam, and Propofol. Anesthesiology 2001, 95, 1151–1159. [Google Scholar] [CrossRef]
- Hu, B.; Zhang, M.; Wu, Z.; Zhang, X.; Zou, X.; Tan, L.; Song, T.; Li, X. Comparison of Remimazolam Tosilate and Etomidate on Hemodynamics in Cardiac Surgery: A Randomised Controlled Trial. Drug. Des. Devel Ther. 2023, 17, 381–388. [Google Scholar] [CrossRef]
- Malhotra, G.; Eckmann, D.M. Anesthesia for bariatric surgery. In Miller’s Anesthesia, 9th ed.; Michael, A.G., Roanld, D.M., Neal, H.C., Laris, I.E., Lee, A.F., Kate, L., Jeanine, P.W., Eds.; Elsevier: Philadelphia, PA, USA, 2019; pp. 1911–1928. [Google Scholar]
- Liu, H.; Ji, F.; Peng, K.; Applegate, R.L., 2nd; Fleming, N. Sedation After Cardiac Surgery: Is One Drug Better Than Another? Anesth. Analg. 2017, 124, 1061–1070. [Google Scholar] [CrossRef]
| Pre-Induction ABGA | 3 min Post-Intubation ABGA | |
|---|---|---|
| pH (mEq/L) | 7.384 | 7.371 |
| PaCO2 (mmHg) | 49 | 49.5 |
| PaO2 (mmHg) | 382 | 127 |
| HCO3− (mmol/L) | 29.3 | 28.7 |
| BE (mmol/L) | 3.3 | 2.6 |
| SatO2 (%) | 100 | 98.9 |
| Hb (g/dL) | 12.4 | 12.1 |
| Hct (%) | 38.1 | 37.2 |
| Lactic acid (mmol/L) | 1.6 | 1.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.H.; Han, H. Remimazolam Induction in a Patient with Super-Super Obesity and Obstructive Sleep Apnea: A Case Report. Medicina 2023, 59, 1247. https://doi.org/10.3390/medicina59071247
Lee SH, Han H. Remimazolam Induction in a Patient with Super-Super Obesity and Obstructive Sleep Apnea: A Case Report. Medicina. 2023; 59(7):1247. https://doi.org/10.3390/medicina59071247
Chicago/Turabian StyleLee, Sou Hyun, and Hyeji Han. 2023. "Remimazolam Induction in a Patient with Super-Super Obesity and Obstructive Sleep Apnea: A Case Report" Medicina 59, no. 7: 1247. https://doi.org/10.3390/medicina59071247
APA StyleLee, S. H., & Han, H. (2023). Remimazolam Induction in a Patient with Super-Super Obesity and Obstructive Sleep Apnea: A Case Report. Medicina, 59(7), 1247. https://doi.org/10.3390/medicina59071247





