1. Introduction
With the recent increase in the intensity and frequency of sports participation in the general population, the incidence of low back pain (LBP) has continuously increased in modern society [
1]. Under the complex influence of physical and psychological factors such as lifestyle, anxiety, stress, and depression, LBP increases in duration and repeating intervals of pain as it develops into chronic or recurrent pain [
2]. Recently, the International Association for the Study of Pain has reported that various types of pain, including LBP, are caused by the complexity of five different mechanisms (nociplastic, neuropathic, nociceptive, motor control, and psychological) [
3]. Taekwondo practice includes both physical and mental aspects. The physical part is challenged to increase muscle strength, flexibility, speed, and skill through various training exercises and techniques. Taekwondo players continuously practice formality (patterns of movement) and sparring. Through this, Taekwondo athletes experience back pain as frequently as the general population [
4,
5]. LBP has been reported as a common pain in Taekwondo [
6]. Studies have shown that the back is the third most injured body part in elite adult Canadian Taekwondo athletes [
7]. Although there are known data that LBP frequently occurs in judo and karate athletes, studies focusing on frequent LBP in Taekwondo athletes are lacking [
8].
Recurrent LBP (RLBP) frequently occurs among athletes performing martial arts sports, including Taekwondo, who predominantly perform high-intensity and repetitive training involving sparring with partners, an activity that induces various injuries. The time spent on recovery is short, and repeated overloading is applied to the lumbar spine [
9]. As such, LBP affects athletic performance [
10]. Athletes with RLBP often show the following symptoms: synesthesia in the area of pain, reduced range of motion (ROM), nerve signs, and radiating pain [
11], as well as compensatory responses to avoid pain, which can cause movement control disabilities [
12].
Athletes commonly spend considerable time and money on surgery and rehabilitation therapies to control the symptoms of RLBP [
13]. Previous studies have reported that athletes with LBP may undergo a general physical therapy intervention that has long been used for pain control or that they may need to take time off from training to facilitate recovery. Other applied methods include ultrasound, kinesio-taping, transcutaneous electrical nerve stimulation, and manual therapy [
14]. However, recent studies on RLBP rehabilitation have shown that, despite the short-term positive effects, general physical therapy interventions have negligible effects on long-term management [
15]. A previous study indicated that the lack of long-term effects of general therapeutic interventions were because they were based on a single rather than on several pain mechanisms that could occur simultaneously in athletes [
16]. This has led to recent claims that the effectiveness of the intervention may be maximized through a pain-mechanism-based therapy approach, reflecting several simultaneous pain mechanisms in athletes with RLBP [
17,
18,
19,
20]. Medical therapies commonly apply such mechanism-based approaches [
21,
22], which have several advantages, including a widened scope of therapeutic intervention for physical therapists, and the possibility of reflecting the results of the latest studies in various fields [
3]. Nevertheless, relatively few studies have investigated mechanism-based therapies. This study therefore aimed to verify the effects of complex pain control programs incorporating mechanism-based therapeutic approaches in Taekwondo athletes with LBP.
4. Discussion
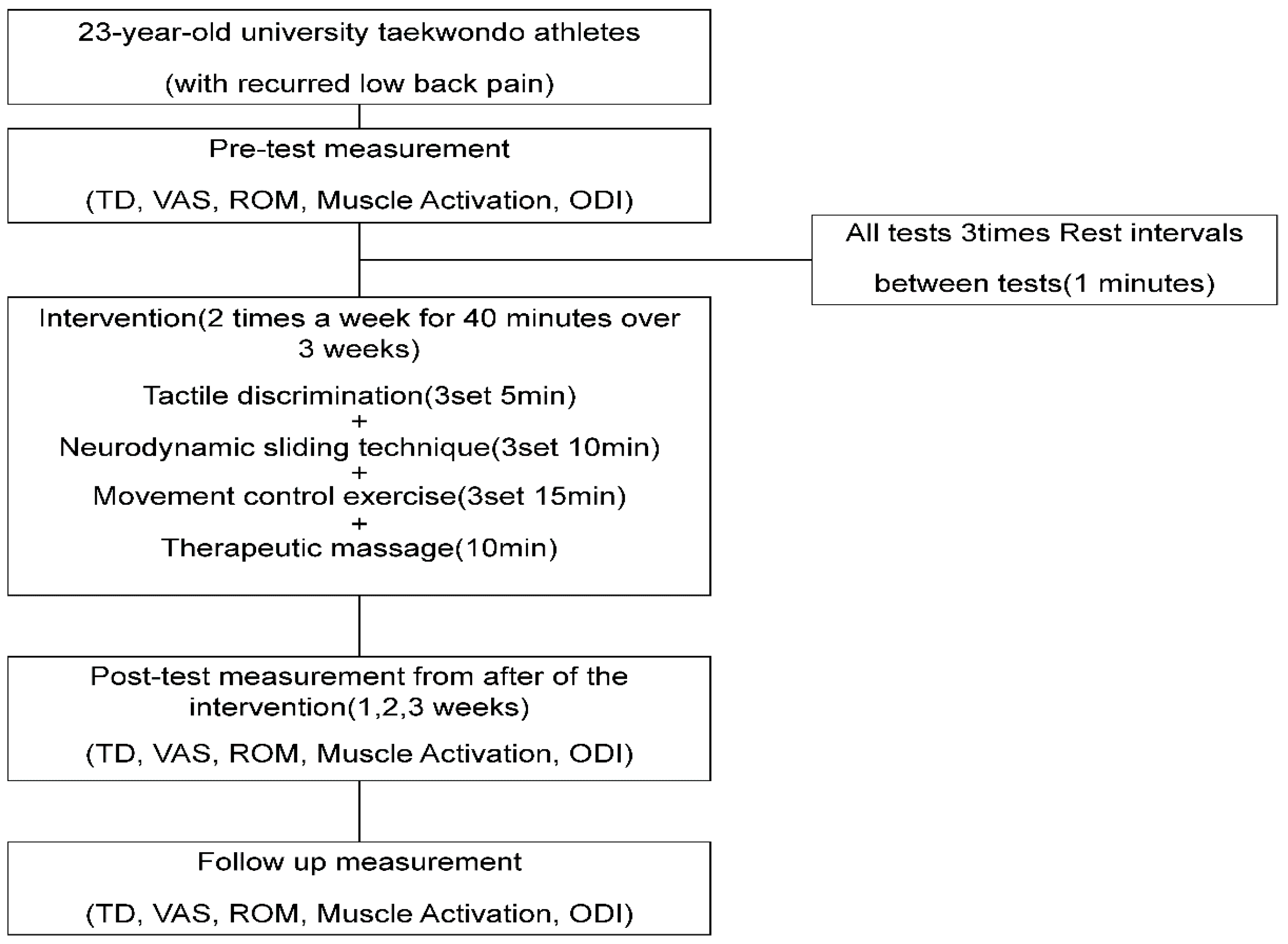
This study aimed to determine the effects of complex pain control programs involving the treatment of four of five pain mechanisms on TD, tissue mechanosensitivity, and lumbar functions in Taekwondo athletes with recurrent LBP.
The inclusion criteria for patients with RLBP in previous studies were individuals who complained of pain at least twice in the past 6 months, with a level of pain estimated as a VAS score ≥ 5 and an ODI score of ≥5, while the exclusion criteria were individuals with acute lumbar pain, spinal fracture, and a history of surgery, spondylolisthesis, or spinal tuberculosis [
11]. The same criteria were used to determine the eligibility of the present patient.
Advances in neuroscience and brain imaging research have allowed further research in the human brain, which have reported that the brain undergoes functional changes when a part of the body experiences pain [
32]. Pain-induced functional changes in the brain prevent normal cortical processing, causing complex cognitive, sensory, and motor control [
33]. In a previous study, an intervention combining TD and motor control training had a positive effect in individuals with RLBP [
16]. Similarly, in this study, the methods in the previous study were modified to apply a nine-point TD training during the 3-week intervention; the TDP scores were increased from 11-point pre-intervention to 16.5-point post-intervention, the latter of which was maintained during the 2-week follow-up period.
Previous studies have shown that motor control training leads to the control of compensation movement via education of the order of muscle recruitment, thereby reducing the load on the joint caused by the compensation movement [
34,
35]. Motor control training combined with TD training has been shown to induce changes in the cerebral cortex governing the lumbar pain area to correct paresthesia in the pain area, causing misinterpretation of normal stimuli from peripheral nerves [
36]. These claims lend support to the trend of an increase in TDP following the intervention combining TD training and motor control training in this study.
Mechanosensitivity indicates the level of nerve tissue activity in response to a mechanical force [
37]. A previous study reported that an increase in pain in patients with RLBP caused an increase in inflammation of the nerve and surrounding tissues, while decreasing nerve mobility and increasing pressure caused by edema around the nerve itself and circulatory disability [
38]. This phenomenon could also increase the pain in patients to induce involuntary contraction of the muscles in the vicinity, thereby causing pain [
38]. An increase in mechanosensitivity is indicative of a state in which even a small or normal stimulus is sensed as risk or pain [
39]. A previous study verified the effects of a neurodynamic technique applied to peripheral nerves to reduce pain, to increase hip joint ROM, and to decrease the activity of lower limb muscles in performing specific movements [
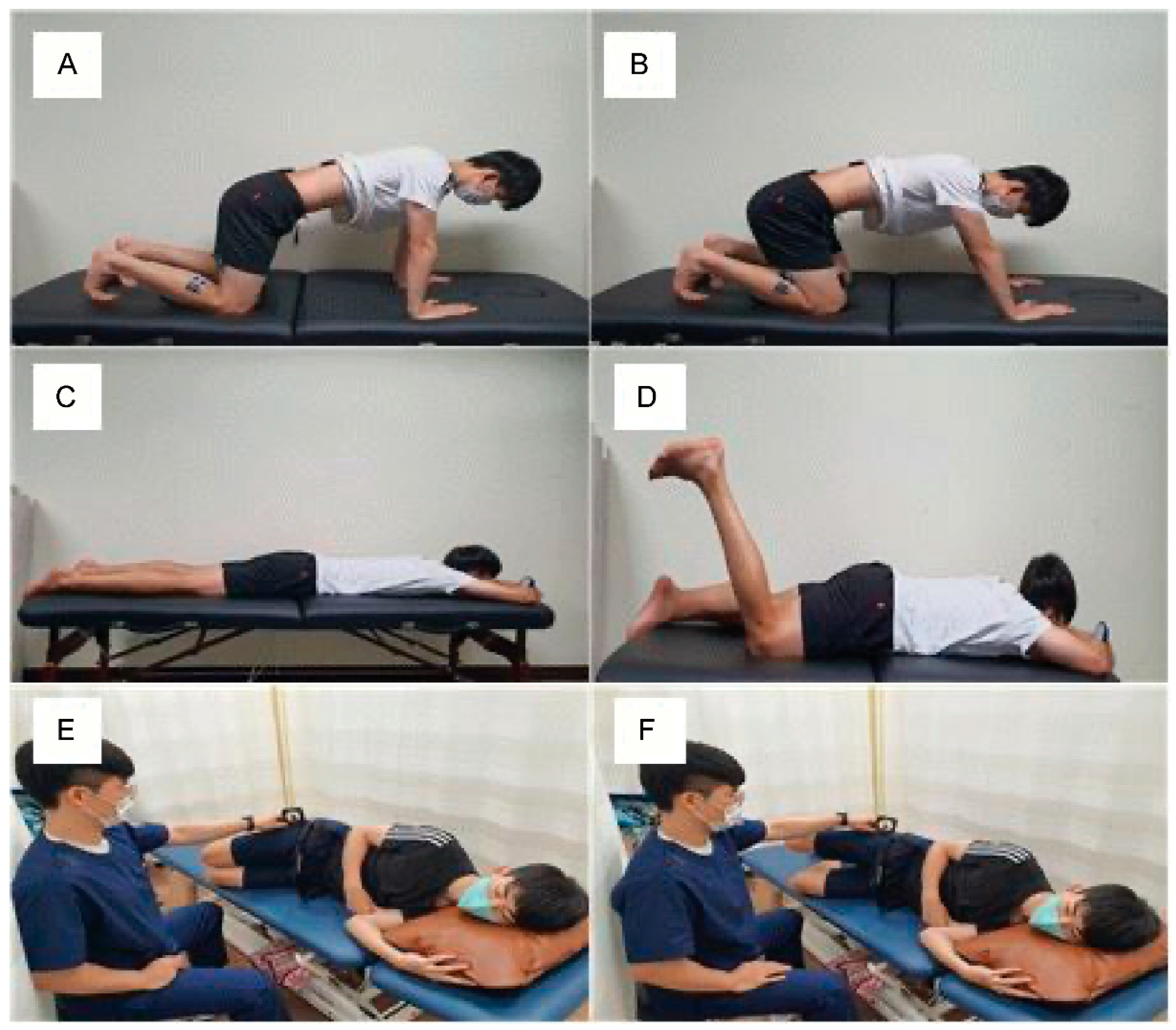
23]. Based on this study, the present investigation applied ASLR to test the reduction in mechanosensitivity and measure the subsequent pain, hamstring muscle activity, and hip joint ROM of the patient. The detailed methods used to measure these variables in this study were as follows: The point of onset of pain (P1) and point of maximum pain (P2) were set, and the scores were measured and compared pre-intervention, post-intervention after 3 weeks, and at follow-up after 2 weeks. To facilitate an accurate comparison of the pain levels at P1 and P2 at pre-intervention, post-intervention, and follow-up, the P1 and P2 scales at post-intervention and follow-up were compared based on the angles of P1 and P2 at pre-intervention. Meanwhile, the change in ROM was examined by recording the newly sensed angles of P1 and P2 after the 3-week intervention, using a motion analysis device. Simultaneously, the MA of the biceps femoris and semimembranosus muscles were measured to verify the reduction in mechanosensitivity in the lower limb, in parallel with the decrease in hamstring MA and increase in hip joint ROM.
The results of this study showed that an intervention combining neurodynamic, movement control, and TD training facilitated a reduction in pain at P1 and P2 when the patient performed SLR. The angles at P1 and P2 that were reassessed after the intervention both showed an increase, which was interpreted as an increase in ROM. In addition, MA was shown to decrease the angles of the hamstring and gastrocnemius muscles, and the altered level from the initial pre-intervention level was maintained for 2 weeks post-intervention. In a previous study, motor control exercises could discriminate between the normal alignment of the body and isolated motions at each segment to disperse the load on each segment [
40]. Previous studies applying the neurodynamic technique reported that it could lower the hypersensitivity of the senses and tissues by reducing the pressure on the nerves and surrounding tissues and improving blood flow to the muscles and nerves [
36,
38,
41,
42,
43]. As such, the intervention program applied in this study is presumed to have improved both the pain and function of the lumbar area, as the neutral spinal position was recognized through movement control training, while the nearby joints were controlled to prevent excessive motion such that mechanosensitivity and pain were reduced in the surrounding tissues and the nerve fibers could recover from inflammation and hypoxia. Additionally, the key factor in the intervention program in this study was the effect of TD training. Previous studies have reported that pain could cause the cerebral cortex to fail to accurately identify the normal information sent from the body segments and recommended TD training as a solution to improve such errors in the cerebral cortex [
22]. The TDP results in this study indicated that the increases in scores after the intervention were due to the TD training effect. TD training, in which the lumbar area was divided into nine blocks for repeated practice of accurate identification of specific blocks, is likely to improve cognitive abilities regarding segments.
In several previous studies, the ODI was used to objectively measure pain in patients with LBP and to examine the functional levels associated with functional and cognitive impairments [
44,
45,
46]. Previous studies have shown that the use of a complex intervention, including TD training, could induce positive changes in body functions in patients with lumbar pain [
16,
22]. Similarly to this study, the ODI scores increased after the intervention, while the increased levels were maintained during the 2 weeks following the intervention. Factors related to pain and daily activities increased among the ODI scores. This is presumed to be because the intervention program in this study ultimately improved the pain in the subjects, and the reduced pain could improve movements, which in turn improved physical function.
The findings of this study suggest that complex pain control programs are effective for the management of pain in Taekwondo athletes with LBP. Through the management and improvement of the four pain mechanisms, the pain-related reduction of mechanosensitivity in the surrounding tissues and body’s functions and abilities to discriminate the segments could be improved. Based on these results, it is predicted that the use of a complex intervention based on pain mechanisms rather than a single perspective, even in the early days of rehabilitation, would be effective at promoting the functional recovery of training athletes with RLBP.
This study had some limitations. First, by applying for the intervention program, only secondary functional changes were confirmed without confirming changes in the activity of the back muscle. Second, the effects of a single intervention program could not be confirmed by confirming the impact of a complex intervention program. Third, an approach that reflects psychological and social factors related to chronic pain through repeated trauma and injury in Taekwondo players has not been investigated through various pain mechanisms.
Therefore, further studies are needed to determine the long-term effects of complex pain control programs based on pain mechanisms, including psychological and social factors.