Short-Term Effects of Manual Therapy plus Capacitive and Resistive Electric Transfer Therapy in Individuals with Chronic Non-Specific Low Back Pain: A Randomized Clinical Trial Study
Abstract
:1. Introduction
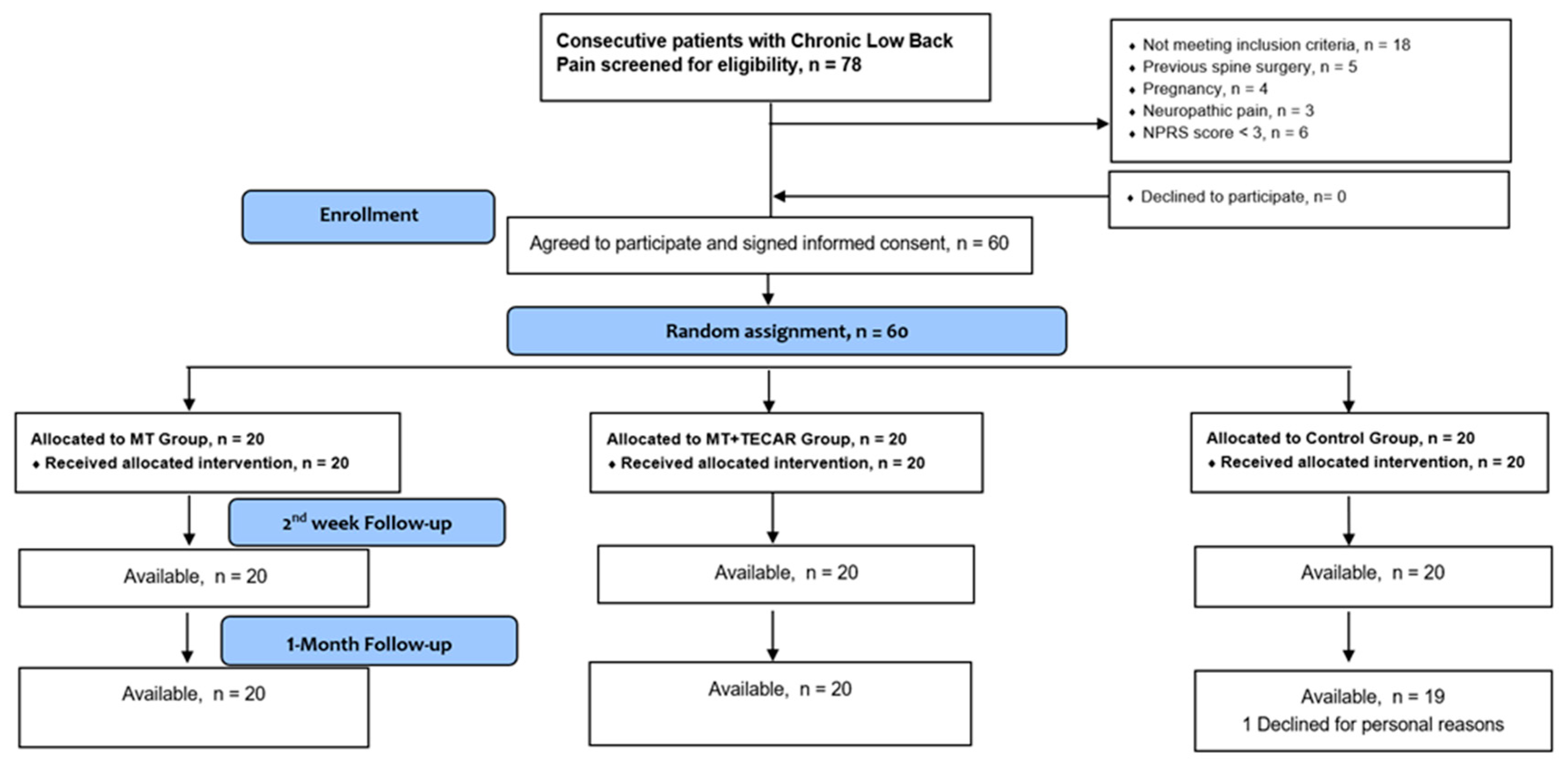
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Outcome Measures
2.3.1. Pain with the Numeric Pain Rating Scale (NPRS)
2.3.2. Functional Ability with the Roland–Morris Disability Questionnaire (RMDQ)
2.3.3. Pressure Pain Threshold (PPT) with Pressure Algometry
2.3.4. Lumbo-Pelvic Region Mobility with the Fingertip-to-Floor (FTF) Test
2.4. Experimental Protocols
2.4.1. Manual Therapy (MT) Protocol
2.4.2. Manual Therapy with TECAR (MT + TECAR) Protocol
2.4.3. Control
2.5. Sample Size Determination
2.6. Statistical Analysis
3. Results
3.1. NPRS Score
3.2. RMDQ Score
3.3. PPT of L4-L5 Paraspinal Intervertebral Space
3.4. Sacroiliac Joint PPT
3.5. Quadratus Lumborum Muscle PPT
3.6. FTF Test Score
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hutchinson, A.J.; Ball, S.; Andrews, J.C.; Jones, G.G. The effectiveness of acupuncture in treating chronic non-specific low back pain: A systematic review of the literature. J. Orthop. Surg. Res. 2012, 7, 36. [Google Scholar] [CrossRef] [Green Version]
- Balagué, F.; Mannion, A.F.; Pellisé, F.; Cedraschi, C. Non-specific Low Back Pain. Lancet 2012, 379, 482–491. [Google Scholar] [CrossRef] [Green Version]
- Hayden, J.A.; Ellis, J.; Ogilvie, R.; Stewart, S.A.; Bagg, M.K.; Stanojevic, S.; Yamato, T.P.; Saragiotto, B.T. Some Types of Exercise are more Effective than Others in People with Chronic Low Back Pain: A Network Meta-analysis. J. Physiother. 2021, 67, 252–262. [Google Scholar] [CrossRef] [PubMed]
- GBD Collaborators. Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 354 Diseases and Injuries for 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef] [Green Version]
- Hartvigsen, J.; Hancock, M.J.; Kongsted, A.; Louw, Q.; Ferreira, M.L.; Genevay, S.; Hoy, D.; Karppinen, J.; Pransky, G.; Sieper, J.; et al. What Low Back Pain is and Why we Need to Pay Attention. Lancet 2018, 391, 2356–2367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coulter, I.D.; Crawford, C.; Hurwitz, E.L.; Vernon, H.; Khorsa, R.; Suttorp Booth, M.; Herman, P.M. Manipulation and Mobilization for Treating Chronic Low Back Pain: A Systematic Review and Meta-analysis. Spine J. 2018, 18, 866–879. [Google Scholar] [CrossRef] [Green Version]
- Dayanlr, I.O.; Birinci, T.; Kaya Mutlu, E.; Akcetin, M.A.; Akdemir, A.O. Comparison of Three Manual Therapy Techniques as Trigger Point Therapy for Chronic Nonspecific Low Back Pain: A Randomized Controlled Pilot Trial. J. Altern. Complement. Med. 2020, 26, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Ulger, O.; Demirel, A.; Oz, M.; Tamer, S. The Effect of Manual Therapy and Exercise in Patients with Chronic Low Back Pain: Double Blind Randomized Controlled Trial. J. Back. Musculoskelet. Rehabil. 2017, 30, 1303–1309. [Google Scholar] [CrossRef]
- Chitale, N.; Patil, D.S.; Phansopkar, P.; Joshi, A. A Review on Treatment Approaches for Chronic Low Back Pain via Mulligans Movement with Mobilization and Physical Therapy. Cureus 2022, 18, e28127. [Google Scholar] [CrossRef]
- Szabo, D.A.; Neagu, N.; Teodorescu, S.; Predescu, C.; Sopa, I.S.; Panait, L. TECAR Therapy Associated with High-Intensity Laser Therapy (Hilt) and Manual Therapy in the Treatment of Muscle Disorders: A Literature Review on the Theorised Effects Supporting Their Use. J. Clin. Med. 2022, 11, 6149. [Google Scholar] [CrossRef]
- Kim, Y.J.; Joo-Hee, P.; Kim, J.; Moon, G.A.; Jeon, H. Effect of High-frequency Diathermy on Hamstring Tightness. Phys. Ther. Korea 2021, 28, 65–71. [Google Scholar] [CrossRef]
- Barassi, G.; Mariani, C.; Supplizi, M.; Prosperi, L.; Di Simone, E.; Marinucci, C.; Pellegrino, R.; Guglielmi, V.; Younes, A.; Di Iorio, A. Capacitive and Resistive Electric Transfer Therapy: A Comparison of Operating Methods in Non-specific Chronic Low Back Pain. Adv. Exp. Med. Biol. 2022, 1375, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Notarnicola, A.; Maccagnano, G.; Gallone, M.F.; Covelli, I.; Tafurp, S.; Moretti, B. Short Term Efficacy of Capacitive-resistive Diathermy Therapy in Patients with Low Back Pain: A Prospective Randomized Controlled Trial. J. Biol. Regul. Homeost. Agents 2017, 31, 509–515. [Google Scholar] [PubMed]
- Tashiro, Y.; Suzuki, Y.; Nakayama, Y.; Sonoda, T.; Yokota, Y.; Kawagoe, M.; Tsuboyama, T.; Aoyama, T. The Effect of Capacitive and Resistive Electric Transfer on Non-specific Chronic Low Back Pain. Electromagn. Biol. Med. 2020, 39, 437–444. [Google Scholar] [CrossRef]
- Urbaniak, G.C.; Plous, S. Research Randomizer (Version 4.0). 2013. Available online: https://www.randomizer.org/ (accessed on 22 December 2022).
- Childs, J.D.; Piva, S.R.; Fritz, J.M. Responsiveness of the Numeric Pain Rating Scale in Patients with Low Back Pain. Spine 2005, 30, 1331–1334. [Google Scholar] [CrossRef]
- Jenks, A.; Hoekstra, T.; van Tulder, M.; Ostelo, R.W.; Rubinstein, S.M.; Chiarotto, A. Roland-Morris Disability Questionnaire, Oswestry Disability Index, and Quebec Back Pain Disability Scale: Which Has Superior Measurement Properties in Older Adults with Low Back Pain? J. Orthop. Sports Phys. Ther. 2022, 52, 457–469. [Google Scholar] [CrossRef]
- Roland, M.; Fairbank, J. The Roland-Morris Disability Questionnaire and the Oswestry Disability Questionnaire. Spine 2000, 25, 3115–3124. [Google Scholar] [CrossRef] [Green Version]
- Boscainos, P.J.; Sapkas, G.; Stilianessi, E.; Prouskas, K.; Papadakis, S.A. Greek Versions of the Oswestry and Roland-Morris Disability Questionnaires. Clin. Orthop. Relat. Res. 2003, 411, 40–53. [Google Scholar] [CrossRef]
- Imamura, M.; Alfieri, F.M.; Filippo, T.R.M.; Battistella, L.R. Pressure Pain Thresholds in Patients with Chronic Non-specific Low Back Pain. J. Back. Musculoskelet. Rehabil. 2016, 29, 327–336. [Google Scholar] [CrossRef]
- Imamura, M.; Chen, J.; Matsubayashi, S.R.; Targino, R.A.; Alfieri, F.M.; Bueno, D.K.; Hsing, W.T. Changes in Pressure Pain Threshold in Patients with Chronic Nonspecific Low Back Pain. Spine 2013, 38, 2098–2107. [Google Scholar] [CrossRef]
- Balaguier, R.; Madeleine, P.; Vuillerme, N. Intra-session Absolute and Relative Reliability of Pressure Pain Thresholds in the Low Back Region of Vine-workers: Effect of the Number of Trials. BMC Musculoskelet. Disord. 2016, 17, 350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perret, C.; Poiraudeau, S.; Fermanian, J.; Colau, M.M.; Benhamou, M.A.; Revel, M. Validity, Reliability, and Responsiveness of the Fingertip-to-floor Test. Arch. Phys. Med. Rehabil. 2001, 82, 1566–1570. [Google Scholar] [CrossRef] [PubMed]
- Ekedahl, K.H.; Jönsson, B.; Frobell, R.B. Validity of the Fingertip-to-floor Test and Straight Leg Raising Test in Patients with Acute and Subacute Low Back Pain: A Comparison by Sex and Radicular Pain. Arch. Phys. Med. Rehabil. 2010, 91, 1243–1247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaltenborn, F.M. The Spine, Orthopedic Physical Therapy Products. In Manual Mobilization of the Joints, Joint Examination and Basic Treatment; Norli: Oslo, Norway, 2018; Volume 2. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates Publishers: New York, NY, USA, 1988; Volume 4. [Google Scholar]
- Wachi, M.; Jiroumaru, T.; Satonaka, A.; Ikeya, M.; Noguchi, S.; Suzuki, M.; Hyodo, Y.; Oka, Y.; Fujikawa, T. Effects of Capacitive and Resistive Electric Transfer Therapy on Pain and Lumbar Muscle Stiffness and Activity in Patients with Chronic Low Back Pain. J. Phys. Ther. Sci. 2022, 34, 400–403. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Q.; Jiang, A.Y.; Gao, Q. Effect of Manual Soft Tissue Therapy on the Pain in Patients with Chronic Neck Pain: A systematic Review and Meta-analysis. Complement. Ther. Clin. Pract. 2022, 49, 101619. [Google Scholar] [CrossRef]
- Bishop, M.D.; Torres-Cueco, R.; Gay, C.W.; Lluch-Girbés, E.; Beneciuk, J.M.; Bialosky, J.E. What effect can manual therapy have on a patient’s pain experience? Pain. Manag. 2015, 5, 455–464. [Google Scholar] [CrossRef] [Green Version]
- Ostelo, R.W.; de Vet, H.C. Clinically Important Outcomes in Low Back Pain. Best. Pract. Res. Clin. Rheumatol. 2005, 19, 593–607. [Google Scholar] [CrossRef]
- Clijsen, R.; Leoni, D.; Schneebeli, A.; Cescon, C.; Soldini, E.; Li, L.; Barbero, M. Does the Application of Tecar Therapy Affect Temperature and Perfusion of Skin and Muscle Microcirculation? A Pilot Feasibility Study on Healthy Subjects. J. Altern. Complement. Med. 2020, 26, 147–153. [Google Scholar] [CrossRef]

| Demographics | Group 1 (MT) | Group 2 (MT + TECAR)) | Group 3 (Control) |
|---|---|---|---|
| Age (years) | 37.85 (2.62) | 39.20 (2.63) | 38.10 (2.36) |
| Sex (Men/Women) | 35% (n = 7) Men | 30% (n = 6) Men | 35% (n = 7) Men |
| 65% (n = 13) Woman | 70% (n = 14) Woman | 65% (n = 13) Woman | |
| Affected side (Right/Left/Both) | 60% (n = 12) Right | 70% (n = 14) Right | 75% (n = 15) Right |
| 25% (n = 5) Left | 20% (n = 4) Left | 20% (n = 4) Left | |
| 15% (n = 3) Both | 10% (n = 2) Both | 5% (n = 1) Both | |
| Symptoms Duration (months) (3–6/6–12/More than 12) | 30% (n = 6) 3–6 | 35% (n = 7) 3–6 | 40% (n = 8) 3–6 |
| 50% (n = 10) 6–12 | 40% (n = 8) 6–12 | 40% (n = 8) 6–12 | |
| 20% (n = 4) More than 12 | 25% (n = 5) More than 12 | 20% (n = 4) More than 12 | |
| Previous Physiotherapy (Yes/No) | 80% (n = 16) Yes | 85% (n = 17) Yes | 85% (n = 17) Yes |
| 20% (n = 4) No | 15% (n = 3) No | 15% (n = 3) No |
| Baseline | Week 2 | 1-Month Follow-Up | |
|---|---|---|---|
| NPRS score (SD) | |||
| Group 1 (MT) | 6.10 (1.33) | 4.10 (1.11) | 3.95 (0.99) |
| Group 2 (MT + TECAR) | 6.05 (1.39) | 3.20 (1.24) | 3.30 (1.08) |
| Group 3 (Control) | 5.95 (1.39) | 5.50 (1.14) | 6.25 (1.02) |
| p-Value (Interaction) | <0.001 | ||
| 0.02 | |||
| 0.00 | |||
| p-Value (between groups) | p a,b,c > 0.05 | p a,b,c.* | p b,c. * |
| RMDQ score (SD) | |||
| Group 1 (MT) | 11.40 (1.42) | 7.25 (2.69) | 8.80 (2.96) |
| Group 2 (MT + TECAR) | 11.75 (1.94) | 5.65 (2.90) | 6.10 (2.90) |
| Group 3 (Control) | 11.80 (1.67) | 10.95 (1.82) | 12.65 (1.81) |
| p-Value (Interaction) | <0.001 | ||
| p-Value (between groups) | p a,b,c > 0.05 | p b,c. * | p a,b,c. * |
| L4-L5 paraspinal intervertebral space PPT, kg/cm2 Right | |||
| Group 1 (MT) | 4.10 (1.57) | 5.64 (1.76) | 5.14 (1.57) |
| Group 2 (MT + TECAR) | 3.99 (1.24) | 6.03 (1.31) | 6.07 (1.28) |
| Group 3 (Control) | 4.06 (1.27) | 4.38 (1.31) | 4.33 (1.27) |
| p-Value (Interaction) | <0.001 | ||
| 0.00 | |||
| 0.00 | |||
| p-Value (between groups) | p a,b,c > 0.05 | p b,c.* | p c * |
| L4-L5 paraspinal intervertebral space PPT, kg/cm2 Left | |||
| Group 1 (MT) | 4.50 (1.45) | 5.34 (1.51) | 5.10 (1.53) |
| Group 2 (MT + TECAR) | 4.74 (0.86) | 6.38 (0.69) | 6.28 (0.69) |
| Group 3 (Control) | 4.48 (1.23) | 4.72 (1.21) | 4.25 (0.98) |
| p-Value (Interaction) | <0.001 | ||
| p-Value (between groups) | p a,b,c > 0.05 | p a,c.* | p a,c.* |
| Sacroiliac joint PPT, kg/cm2 Right | |||
| Group 1 (MT) | 3.83 (1.05) | 4.82 (1.07) | 4.57 (1.07) |
| Group 2 (MT + TECAR) | 3.93 (1.11) | 5.46 (1.10) | 5.36 (1.13) |
| Group 3 (Control) | 3.98 (0.89) | 4.10 (0.90) | 4.00 (0.91) |
| p-Value (Interaction) | <0.001 | ||
| p-Value (between groups) | p a,b,c > 0.05 | p c. * | p c. * |
| Sacroiliac joint PPT, kg/cm2 Left | |||
| Sacroiliac joint PPT, kg/cm2 Right | |||
| Group 1 (MT) | 4.23 (1.31) | 5.19 (1.26) | 4.94 (1.17) |
| Group 2 (MT + TECAR) | 4.31 (1.10) | 5.63 (1.18) | 5.44 (1.19) |
| Group 3 (Control) | 4.23 (1.01) | 4.41 (1.06) | 4.35 (1.01) |
| p-Value (Interaction) | <0.001 | ||
| p-Value (Interaction) | |||
| <0.001 | |||
| p-Value (between groups) | p a,b,c > 0.05 | p c. * | p c. * |
| Quadratus lumborum muscle PPT, kg/cm2 Right | |||
| Group 1 (MT) | 4.05 (0.89) | 4.86 (0.98) | 4.76 (0.94) |
| Group 2 (MT + TECAR) | 3.83 (1.21) | 5.36 (1.16) | 5.29 (1.13) |
| Group 3 (Control) | 3.76 (1.00) | 4.06 (0.86) | 3.97 (0.85) |
| p-Value (Interaction) | < 0.001 | ||
| p-Value (Interaction) | |||
| < 0.001 | |||
| p-Value (between groups) | p a,b,c > 0.05 | p b,c.* | p b,c.* |
| Quadratus lumborum muscle PPT, kg/cm2 Left | |||
| Quadratus lumborum muscle PPT, kg/cm2 Right | |||
| Quadratus lumborum muscle PPT, kg/cm2 Right | |||
| Quadratus lumborum muscle PPT, kg/cm2 Right | |||
| Group 1 (MT) | 4.27 (1.04) | 5.12 (0.96) | 4.98 (0.97) |
| Group 2 (MT + TECAR) | 4.09 (0.92) | 5.67 (0.97) | 5.52 (0.93) |
| Group 3 (Control) | 4.00 (0.94) | 4.19 (0.89) | 4.04 (0.84) |
| p-Value (Interaction) | <0.001 | ||
| p-Value (Interaction) | |||
| p-Value (Interaction) | |||
| p-Value (between groups) | p a,b,c > 0.05 | p b,c.* | p b,c.* |
| FTF test score | |||
| Group 1 (MT) | 15.80 (4.39) | 11.50 (4.05) | 13.50 (4.09) |
| Group 2 (MT + TECAR) | 15.70 (2.13) | 6.45 (1.50) | 7.15 (1.89) |
| Group 3 (Control) | 15.50 (2.62)) | 14.50 (2.80) | 15.35 (4.00) |
| p-Value (Interaction) | >0.001 | ||
| p-Value (between groups) | p a,b,c > 0.05 | p a,b,c.* | p a,c. * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasimis, K.; Iakovidis, P.; Lytras, D.; Koutras, G.; Chatziprodromidou, I.P.; Fetlis, A.; Ntinou, S.R.; Keklikoglou, N.-M.; Samiotaki, A.; Chasapis, G.; et al. Short-Term Effects of Manual Therapy plus Capacitive and Resistive Electric Transfer Therapy in Individuals with Chronic Non-Specific Low Back Pain: A Randomized Clinical Trial Study. Medicina 2023, 59, 1275. https://doi.org/10.3390/medicina59071275
Kasimis K, Iakovidis P, Lytras D, Koutras G, Chatziprodromidou IP, Fetlis A, Ntinou SR, Keklikoglou N-M, Samiotaki A, Chasapis G, et al. Short-Term Effects of Manual Therapy plus Capacitive and Resistive Electric Transfer Therapy in Individuals with Chronic Non-Specific Low Back Pain: A Randomized Clinical Trial Study. Medicina. 2023; 59(7):1275. https://doi.org/10.3390/medicina59071275
Chicago/Turabian StyleKasimis, Konstantinos, Paris Iakovidis, Dimitrios Lytras, Georgios Koutras, Ioanna P. Chatziprodromidou, Antonis Fetlis, Stefania Rafailia Ntinou, Natalia-Maria Keklikoglou, Antigoni Samiotaki, Georgios Chasapis, and et al. 2023. "Short-Term Effects of Manual Therapy plus Capacitive and Resistive Electric Transfer Therapy in Individuals with Chronic Non-Specific Low Back Pain: A Randomized Clinical Trial Study" Medicina 59, no. 7: 1275. https://doi.org/10.3390/medicina59071275





