Flexural and Cell Adhesion Characteristic of Phakic Implantable Lenses
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
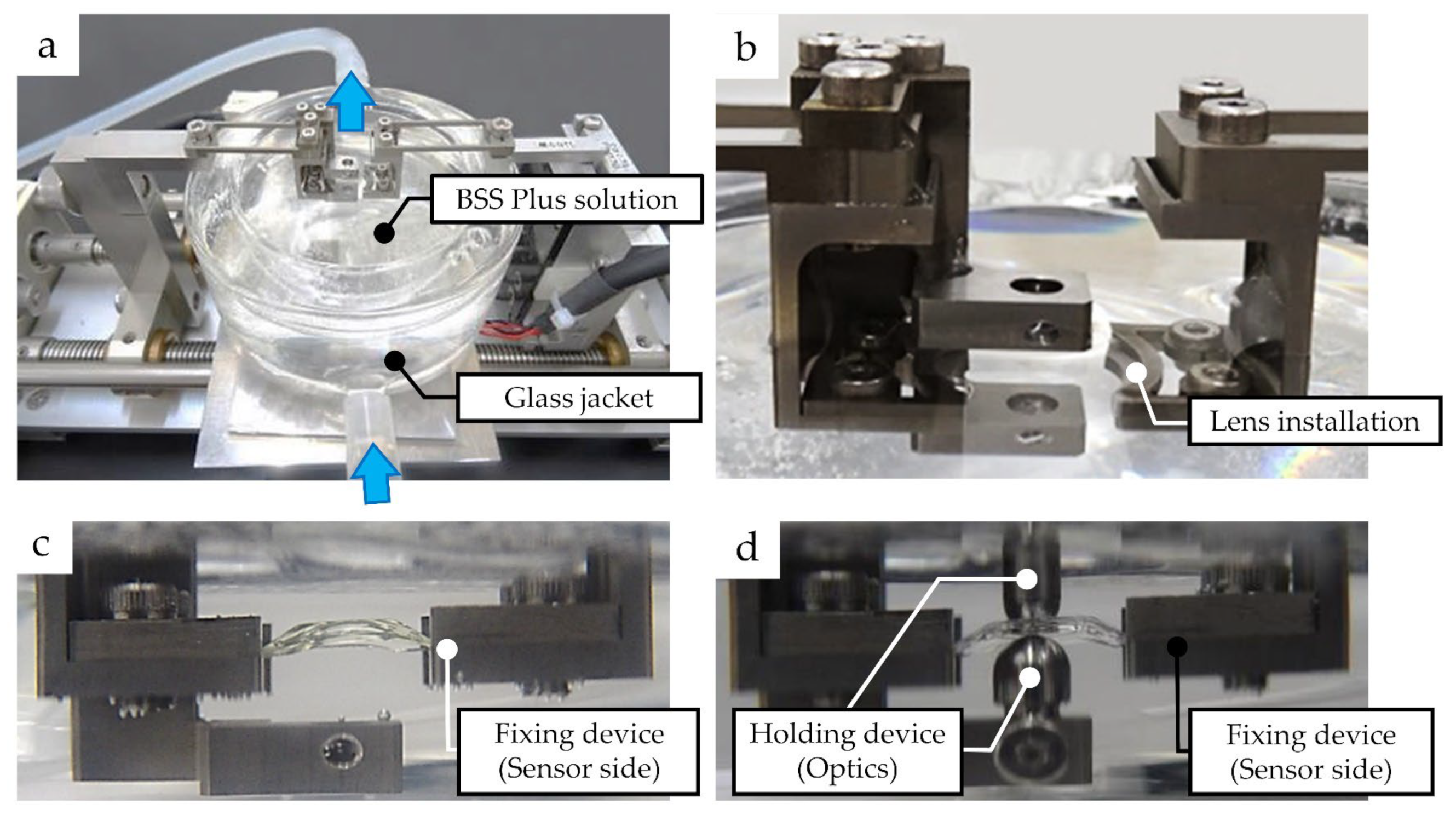
2.2. Measurement of Compressive Load
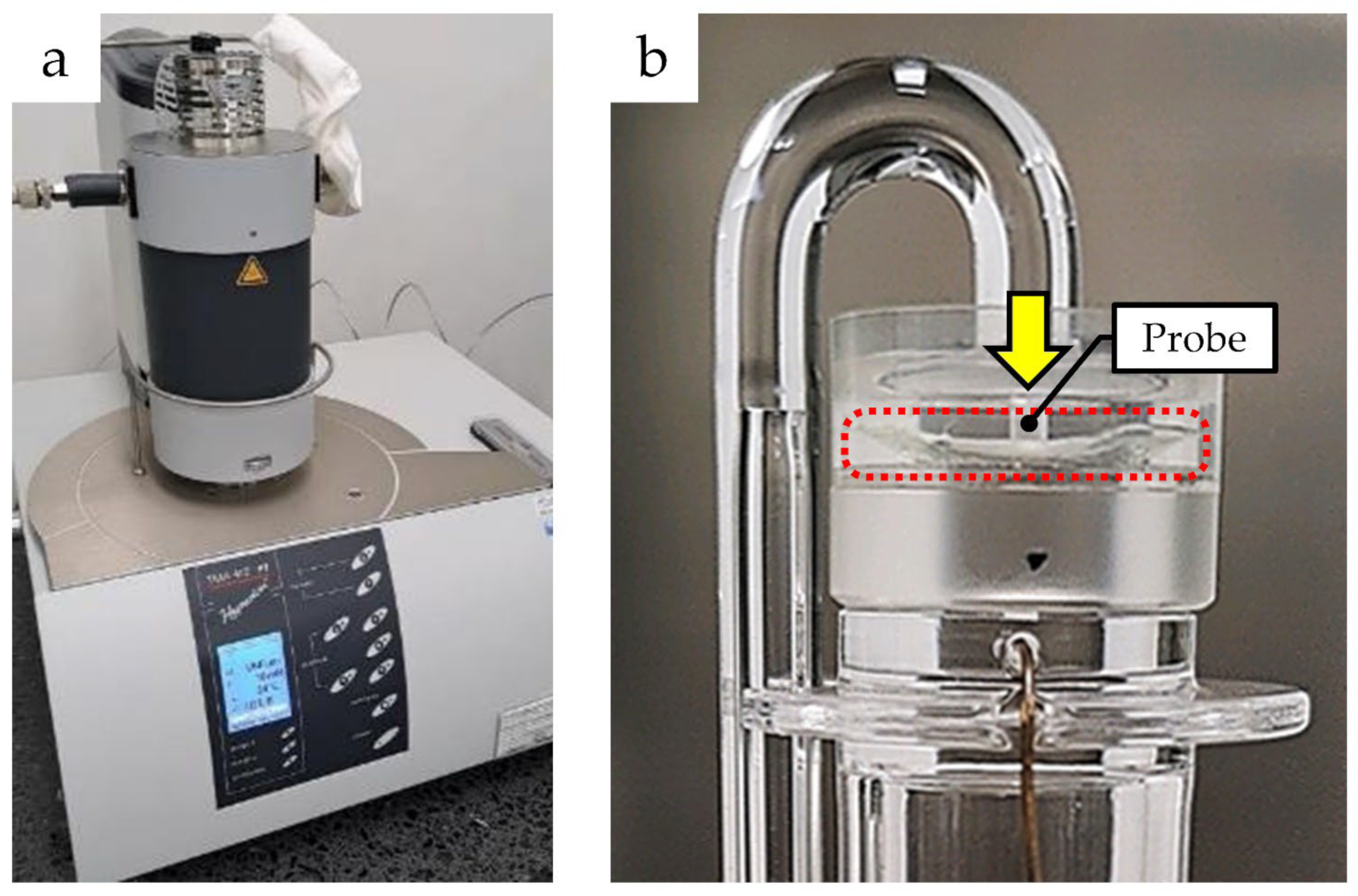
2.3. Measuring Static Young’s Modulus
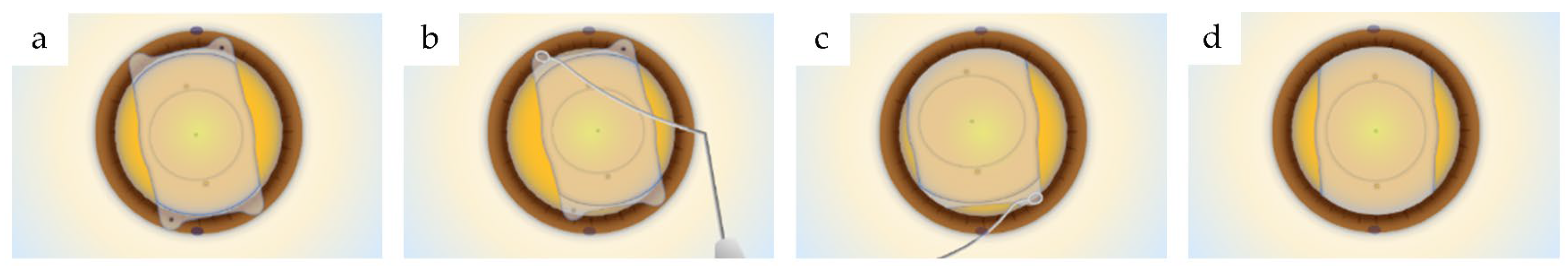
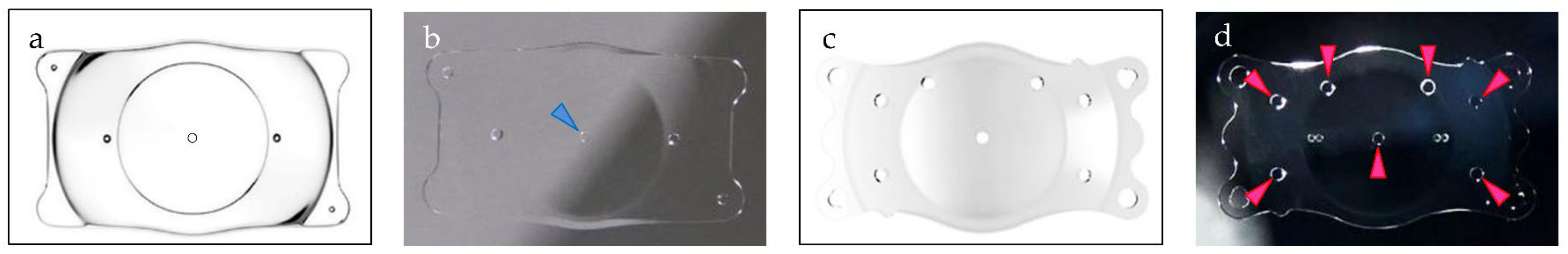
2.4. Cell Adhesion Experiments
2.5. Statistical Analysis
3. Results
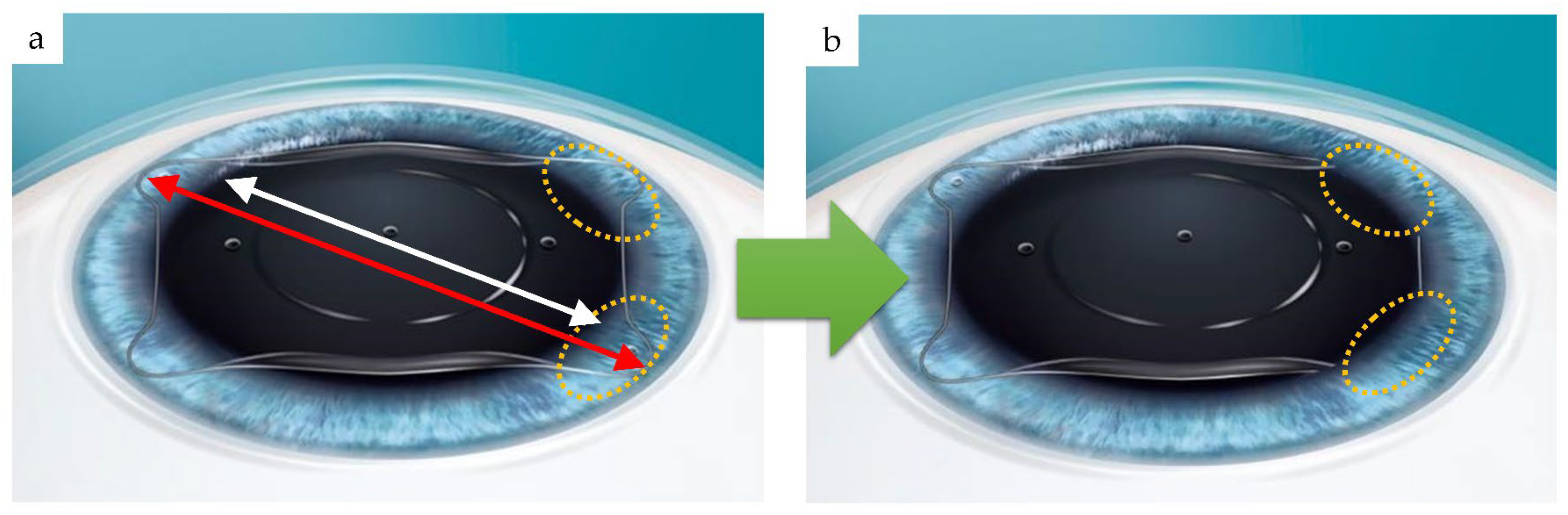
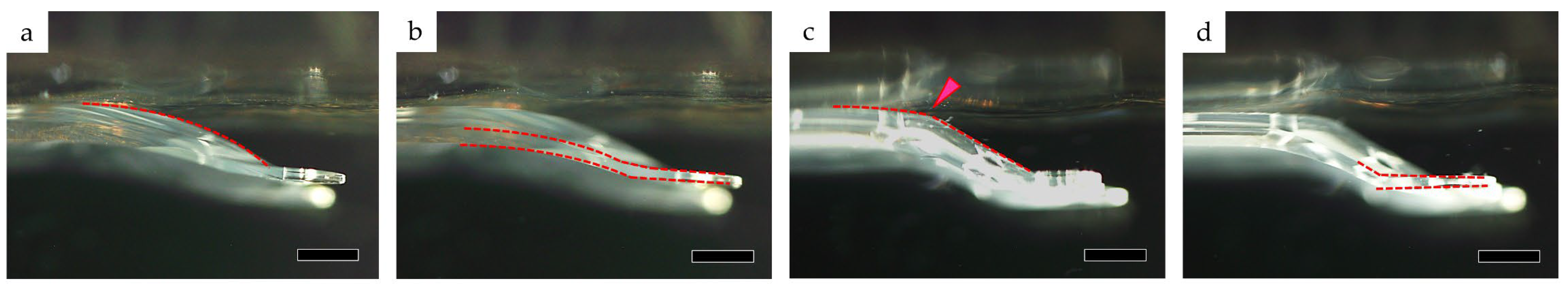
3.1. Compression Load and Comparisons among Lenses
3.1.1. Optic Section Unrestrained
3.1.2. Optic Section Restrained
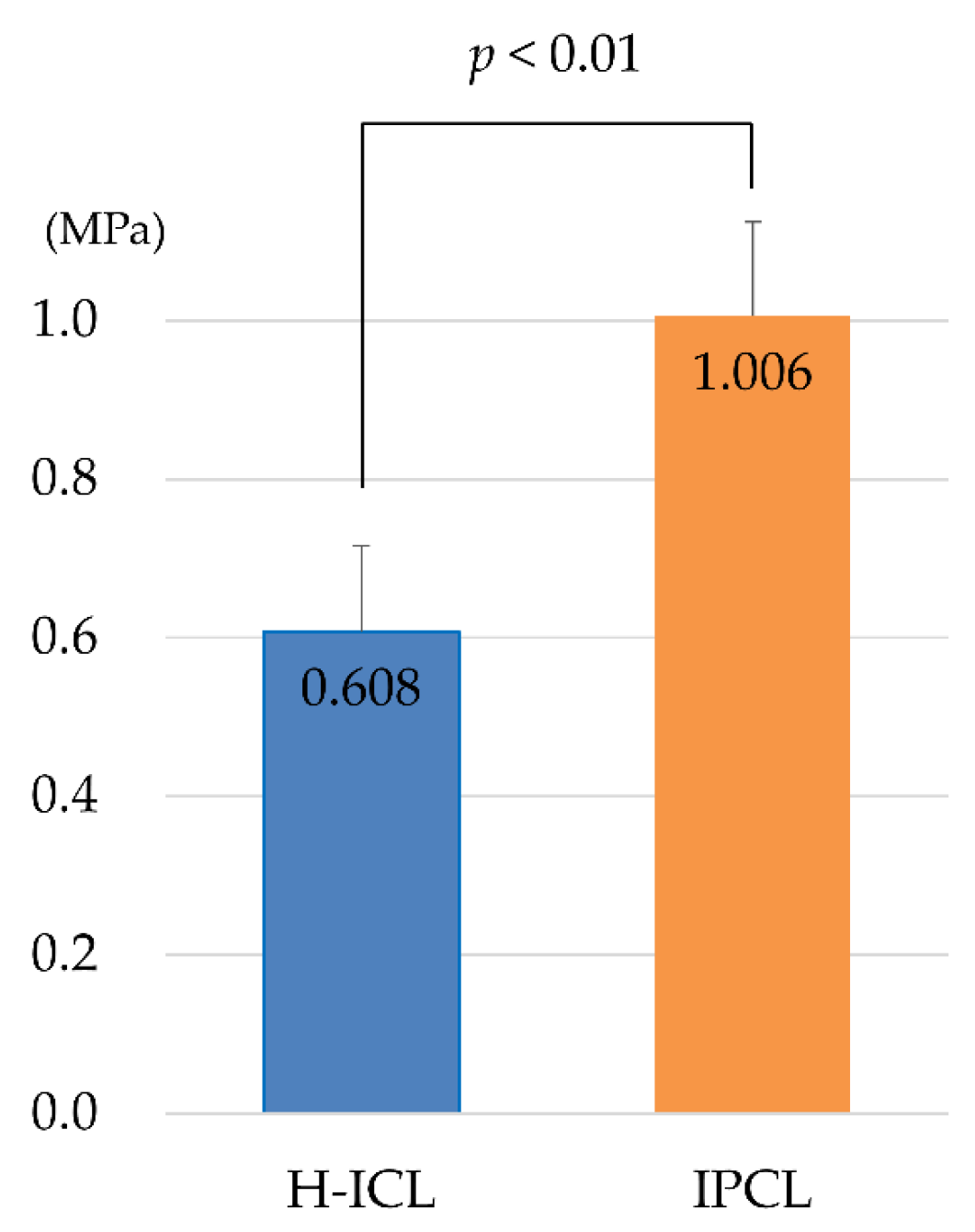
3.2. Comparison of Static Young’s Modulus
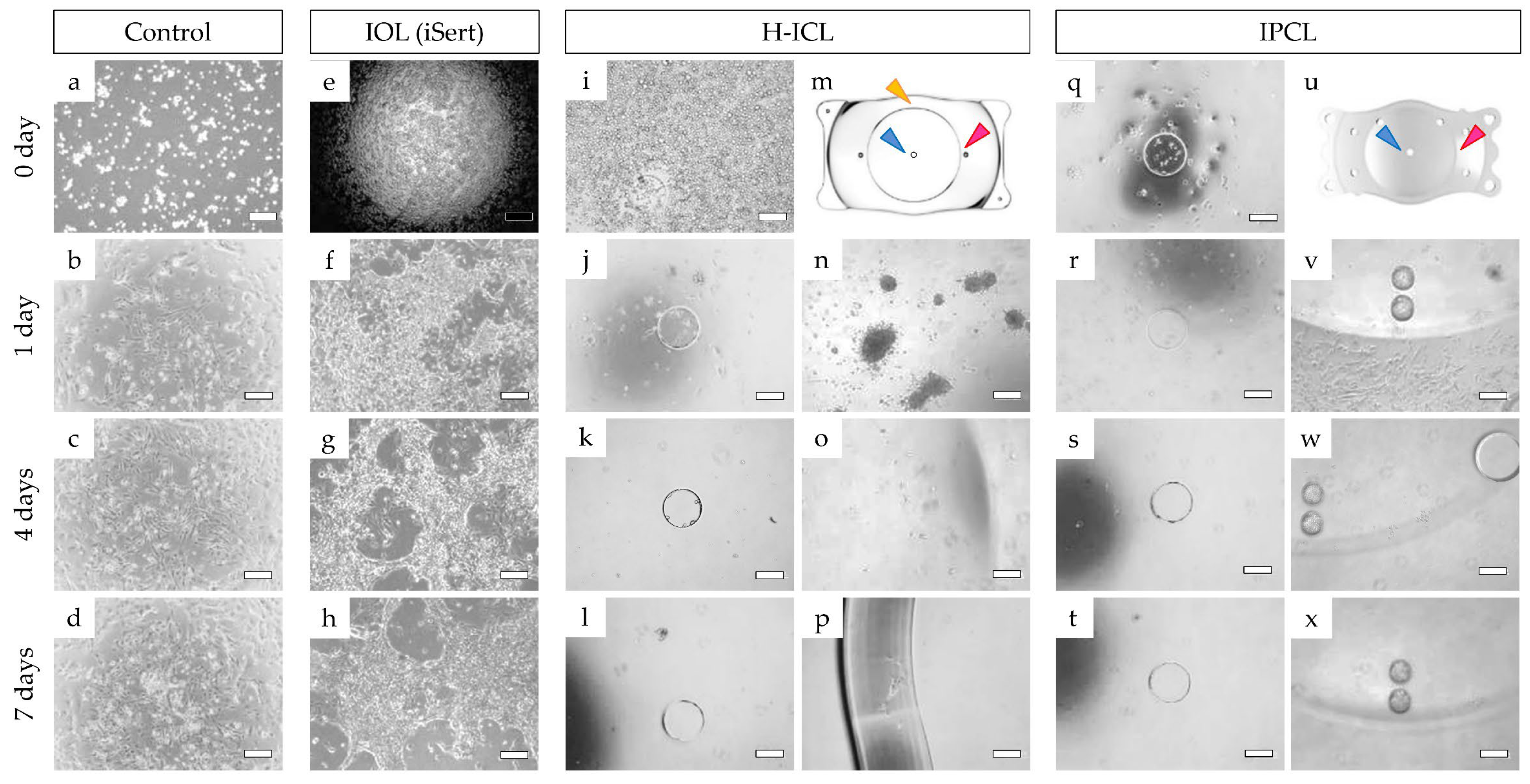
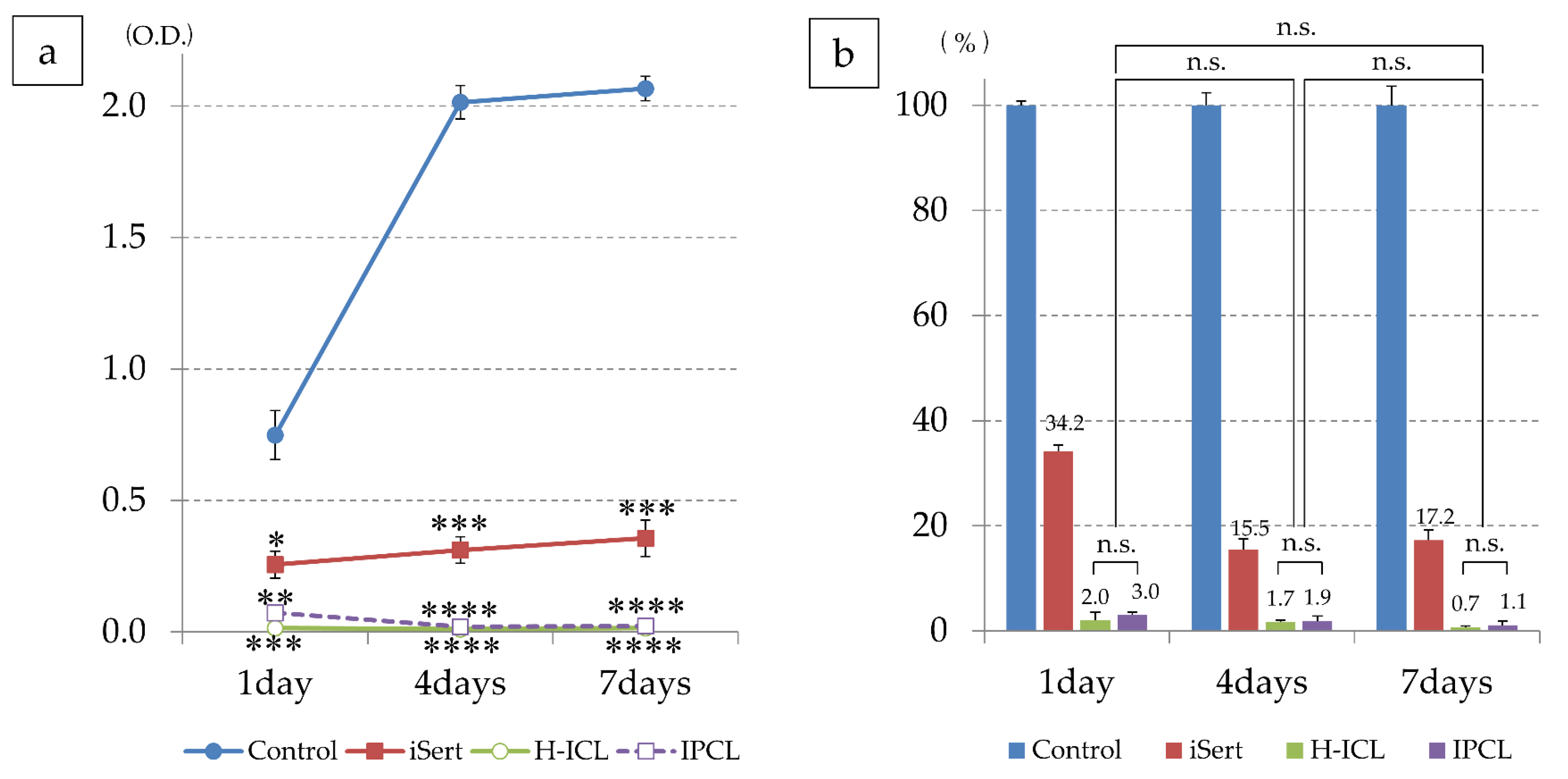
3.3. Cell Adhesion to Lens
3.3.1. iHLEC-NY2 Cell Adhesion Status
3.3.2. iHLEC-NY2 Proliferation Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torricelli, A.A.M.; Bechara, S.J.; Wilson, S.E. Screening of Refractive Surgery Candidates for LASIK and PRK. Cornea 2014, 33, 1051–1055. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, A.H. Past and present of corneal refractive surgery: A retrospective study of long-term results after photorefractive keratectomy and a prospective study of refractive lenticule extraction. Acta Ophthalmol. 2014, 92, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Luger, M.H.; Ewering, T.; Arba-Mosquera, S. Myopia correction with transepithelial photorefractive keratectomy versus femtosecond−assisted laser in situ keratomileusis: One-year case-matched analysis. J. Cataract. Refract. Surg. 2016, 42, 1579–1587. [Google Scholar] [CrossRef]
- Ağca, A.; Demirok, A.; Yıldırım, Y.; Demircan, A.; Yasa, D.; Yeşilkaya, C.; Perente, I.; Taskapili, M. Refractive lenticule extraction (ReLEx) through a small incision (SMILE) for correction of myopia and myopic astigmatism: Current perspectives. Clin. Ophthalmol. 2016, 10, 1905–1912. [Google Scholar] [CrossRef] [PubMed]
- Gatinel, D.; Weyhausen, A.; Bischoff, M. The Percent Volume Altered in Correction of Myopia and Myopic Astigmatism with PRK, LASIK, and SMILE. J. Refract. Surg. 2020, 36, 844–850. [Google Scholar] [CrossRef]
- Zaldivar, R.; Gordillo, C.H.; Adamek, P. Visual Acuity Improvement in Low, Moderate and High Myopia After Posterior-Chamber Phakic Implantable Collamer Lens Surgery in a Large Patient Cohort. Clin. Ophthalmol. 2023, 17, 1179–1185. [Google Scholar] [CrossRef]
- Møller-Pedersen, T.; Cavanagh, H.; Petroll, W.; Jester, J.V. Stromal wound healing explains refractive instability and haze development after photorefractive keratectomy: A 1-year confocal microscopic study. Ophthalmology 2000, 107, 1235–1245. [Google Scholar] [CrossRef]
- Nettune, G.R.; Pflugfelder, S.C. Post-LASIK Tear Dysfunction and Dysesthesia. Ocul. Surf. 2010, 8, 135–145. [Google Scholar] [CrossRef]
- Garcia-Zalisnak, D.; Nash, D.; Yeu, E. Ocular surface diseases and corneal refractive surgery. Curr. Opin. Ophthalmol. 2014, 25, 264–269. [Google Scholar] [CrossRef]
- Roberts, C.J.; Dupps, W., Jr. Biomechanics of corneal ectasia and biomechanical treatments. J. Cataract. Refract. Surg. 2014, 40, 991–998. [Google Scholar] [CrossRef]
- Fatseas, G.; Stapleton, F.; Versace, P. Role of percent peripheral tissue ablated on refractive outcomes following hyperopic LASIK. PLoS ONE 2017, 12, e0170559. [Google Scholar] [CrossRef] [PubMed]
- Munnerlyn, C.R.; Koons, S.J.; Marshall, J. Photorefractive keratectomy: A technique for laser refractive surgery. J. Cataract. Refract. Surg. 1988, 14, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Pallikaris, L.G.; Papatzanaki, M.E.; Stathi, E.Z.; Frenschock, O.; Georgiadis, A. Laser in situ keratomileusis. Lasers Surg. Med. 1990, 10, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Baumeister, M.; Bühren, J.; Kohnen, T. Position of angle-supported, iris-fixated, and ciliary sulcus–implanted myopic phakic intraocular lenses evaluated by Scheimpflug photography. Am. J. Ophthalmol. 2004, 138, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Lovisolo, C.F.; Reinstein, D.Z. Phakic Intraocular Lenses. Surv. Ophthalmol. 2005, 50, 549–587. [Google Scholar] [CrossRef] [PubMed]
- Güell, J.L.; Morral, M.; Kook, D.; Kohnen, T. Phakic intraocular lenses part 1: Historical overview, current models, selection criteria, and surgical techniques. J. Cataract. Refract. Surg. 2010, 36, 1976–1993. [Google Scholar] [CrossRef] [PubMed]
- Sanders, D.R.; Doney, K.; Poco, M. ICL in Treatment of Myopia Study Group United States Food and Drug Administration clinical trial of the Implantable Collamer Lens (ICL) for moderate to high myopia: Three-year follow-up. Ophthalmology 2004, 111, 1683–1692. [Google Scholar] [CrossRef]
- Kojima, T.; Maeda, M.; Yoshida, Y.; Ito, M.; Nakamura, T.; Hara, S.; Ichikawa, K. Posterior Chamber Phakic Implantable Collamer Lens: Changes in Vault During 1 Year. J. Refract. Surg. 2010, 26, 327–332. [Google Scholar] [CrossRef]
- Fernandes, P.; González-Méijome, J.M.; Madrid-Costa, D.; Ferrer-Blasco, T.; Jorge, J.; Montés-Micó, R. Implantable Collamer Posterior Chamber Intraocular Lenses: A Review of Potential Complications. J. Refract. Surg. 2011, 27, 765–776. [Google Scholar] [CrossRef]
- Packer, M. The Implantable Collamer Lens with a central port: Review of the literature. Clin. Ophthalmol. 2018, 12, 2427–2438. [Google Scholar] [CrossRef]
- Choi, J.H.; Lim, D.H.; Nam, S.W.; Yang, C.M.; Chung, E.S.; Chung, T.-Y. Ten-year clinical outcomes after implantation of a posterior chamber phakic intraocular lens for myopia. J. Cataract. Refract. Surg. 2019, 45, 1555–1561. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Wang, H.; Chen, X.; Xu, J.; Yin, H.; Yao, K. Recent Advances of Intraocular Lens Materials and Surface Modification in Cataract Surgery. Front. Bioeng. Biotechnol. 2022, 10, 913383. [Google Scholar] [CrossRef]
- Rateb, M.; Gad, A.A.; Tohamy, D.; Elmohamady, M.N. A Prospective Comparative Study between Implantable Phakic Intraocular Contact Lens and Implantable Collamer Lens in Treatment of Myopia in Adults. J. Ophthalmol. 2022, 2022, 9212253. [Google Scholar] [CrossRef]
- Khoramnia, R.; Yildirim, T.M.; Łabuz, G.; Mayer, C.S.; Auffarth, G.U. Eintrübung von Intraokularlinsen: Erkenntnisse aus dem Labor und der Klinik. Der Ophthalmol. 2020, 118, 633–642. [Google Scholar] [CrossRef]
- ISO 11979-3:2012; Ophthalmic Implants—Intraocular lenses—Part 3: Mechanical Properties and Test Methods, Third ed. American National Standards Institute (ANSI): Geneva, Switzerland, 2012.
- Kodera, S.; Hirata, A.; Miura, F.; Rashed, E.A.; Hatsusaka, N.; Yamamoto, N.; Kubo, E.; Sasaki, H. Model-based approach for analyzing prevalence of nuclear cataracts in elderly residents. Comput. Biol. Med. 2020, 126, 104009. [Google Scholar] [CrossRef]
- Yamamoto, N.; Takeda, S.; Hatsusaka, N.; Hiramatsu, N.; Nagai, N.; Deguchi, S.; Nakazawa, Y.; Takata, T.; Kodera, S.; Hirata, A.; et al. Effect of a Lens Protein in Low-Temperature Culture of Novel Immortalized Human Lens Epithelial Cells (iHLEC-NY2). Cells 2020, 9, 2670. [Google Scholar] [CrossRef] [PubMed]
- Gros-Otero, J.; Ketabi, S.; Cañones-Zafra, R.; Garcia-Gonzalez, M.; Villa-Collar, C.; Casado, S.; Teus, M.A. Atomic force microscopy comparative analysis of the surface roughness of two posterior chamber phakic intraocular lens models: ICL versus IPCL. BMC Ophthalmol. 2021, 21, 280. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Isogai, N.; Kojima, T.; Yoshida, Y.; Sugiyama, Y.; Tanaka, Y.; Ichikawa, K. Long-term In Vivo Stability of Posterior Chamber Phakic Intraocular Lens: Properties and Light Transmission Characteristics of Explants. Am. J. Ophthalmol. 2020, 219, 295–302. [Google Scholar] [CrossRef]
- Bhandari, V.; Karandikar, S.; Reddy, J.K.; Relekar, K. Implantable collamer lens V4b and V4c for correction of high myopia. J. Curr. Ophthalmol. 2015, 27, 76–81. [Google Scholar] [CrossRef]
- Alfonso, J.F.; Fernández-Vega-Cueto, L.; Alfonso-Bartolozzi, B.; Montés-Micó, R.; Fernández-Vega, L. Five-Year Follow-up of Correction of Myopia: Posterior Chamber Phakic Intraocular Lens With a Central Port Design. J. Refract. Surg. 2019, 35, 169–176. [Google Scholar] [CrossRef]
- Vargas, V.; Alió, J.L.; Barraquer, R.I.; Antin, J.C.D.; García, C.; Duch, F.; Balgos, J.; del Barrio, J.L.A. Safety and visual outcomes following posterior chamber phakic intraocular lens bilensectomy. Eye Vis. 2020, 7, 34. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, G.; Ramamurthy, D. Long-term safety of posterior chamber implantable phakic contact lens for the correction of myopia. Clin. Ophthalmol. 2019, 13, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Subudhi, P.; Patro, S.; Khan, Z.; Subudhi, B.N.R.; Silla, S. Refractive outcomes of implantation of an implantable phakic copolymer lens with peripheral holes in the intraocular posterior chamber in moderate to high myopia patients: A single-surgeon series. Clin. Ophthalmol. 2019, 13, 1887–1894. [Google Scholar] [CrossRef] [PubMed]
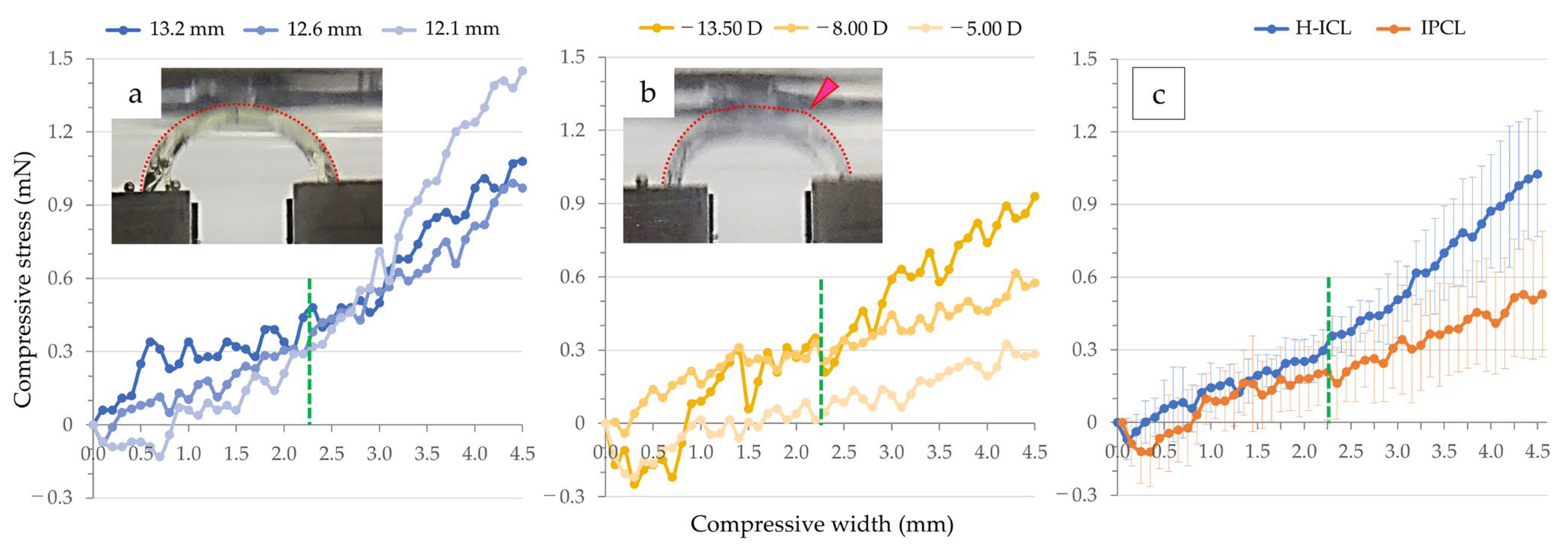

| Flexion (mm) | H-ICL (mN) | IPCL (mN) |
|---|---|---|
| 0.50 | 0.06 ± 0.12 | −0.04 ± 0.16 |
| 1.00 | 0.14 ± 0.10 | 0.09 ± 0.07 |
| 1.50 | 0.19 ± 0.08 | 0.11 ± 0.14 |
| 2.00 | 0.25 ± 0.09 | 0.18 ± 0.13 |
| 2.25 | 0.30 ± 0.09 | 0.21 ± 0.20 |
| 2.50 | 0.38 ± 0.10 | 0.24 ± 0.15 |
| 3.00 | 0.51 ± 0.16 | 0.34 ± 0.23 |
| 3.50 | 0.70 ± 0.20 | 0.38 ± 0.19 |
| 4.00 | 0.87 ± 0.24 | 0.41 ± 0.21 |
| 4.50 | 1.03 ± 0.26 | 0.53 ± 0.26 |
| Flexion (mm) | H-ICL (mN) | IPCL (mN) |
|---|---|---|
| 0.50 | 1.24 ± 0.44 | 0.97 ± 0.71 |
| 1.00 | 2.05 ± 0.59 | 1.41 ± 0.90 |
| 1.50 | 1.79 ± 0.36 | 0.90 ± 0.81 |
| 2.00 | 3.84 ± 1.30 | 0.88 ± 1.29 |
| 2.25 | 4.91 ± 1.88 | 1.22 ± 1.81 |
| Culture Day | Control (O.D.) | IOL (O.D.) | H-ICL (O.D.) | IPCL (O.D.) |
|---|---|---|---|---|
| Day-1 | 0.748 ± 0.093 | 0.256 ± 0.051 | 0.015 ± 0.006 | 0.073 ± 0.015 |
| Day-4 | 2.014 ± 0.064 | 0.311 ± 0.051 | 0.011 ± 0.003 | 0.020 ± 0.009 |
| Day-7 | 2.066 ± 0.046 | 0.356 ± 0.069 | 0.015 ±0.002 | 0.023 ± 0.008 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ichikawa, K.; Ichikawa, K.; Yamamoto, N.; Horai, R. Flexural and Cell Adhesion Characteristic of Phakic Implantable Lenses. Medicina 2023, 59, 1282. https://doi.org/10.3390/medicina59071282
Ichikawa K, Ichikawa K, Yamamoto N, Horai R. Flexural and Cell Adhesion Characteristic of Phakic Implantable Lenses. Medicina. 2023; 59(7):1282. https://doi.org/10.3390/medicina59071282
Chicago/Turabian StyleIchikawa, Kazuo, Kei Ichikawa, Naoki Yamamoto, and Rie Horai. 2023. "Flexural and Cell Adhesion Characteristic of Phakic Implantable Lenses" Medicina 59, no. 7: 1282. https://doi.org/10.3390/medicina59071282
APA StyleIchikawa, K., Ichikawa, K., Yamamoto, N., & Horai, R. (2023). Flexural and Cell Adhesion Characteristic of Phakic Implantable Lenses. Medicina, 59(7), 1282. https://doi.org/10.3390/medicina59071282







