First Tarsometatarsal Joint Fusion in Foot—A Biomechanical Human Anatomical Specimen Analysis with Use of Low-Profile Nitinol Staples Acting as Continuous Compression Implants
Abstract
:1. Introduction
2. Materials and Methods
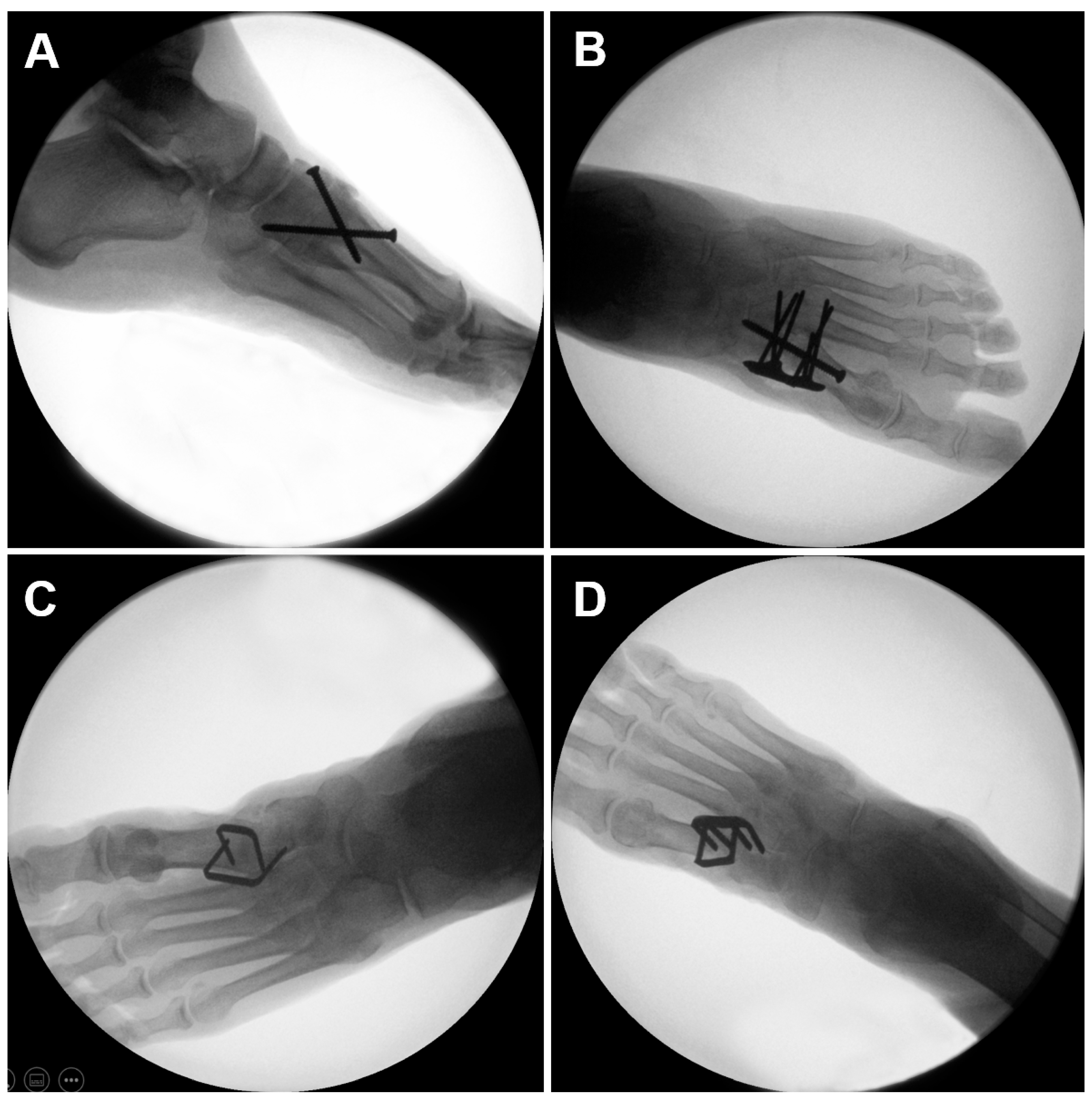
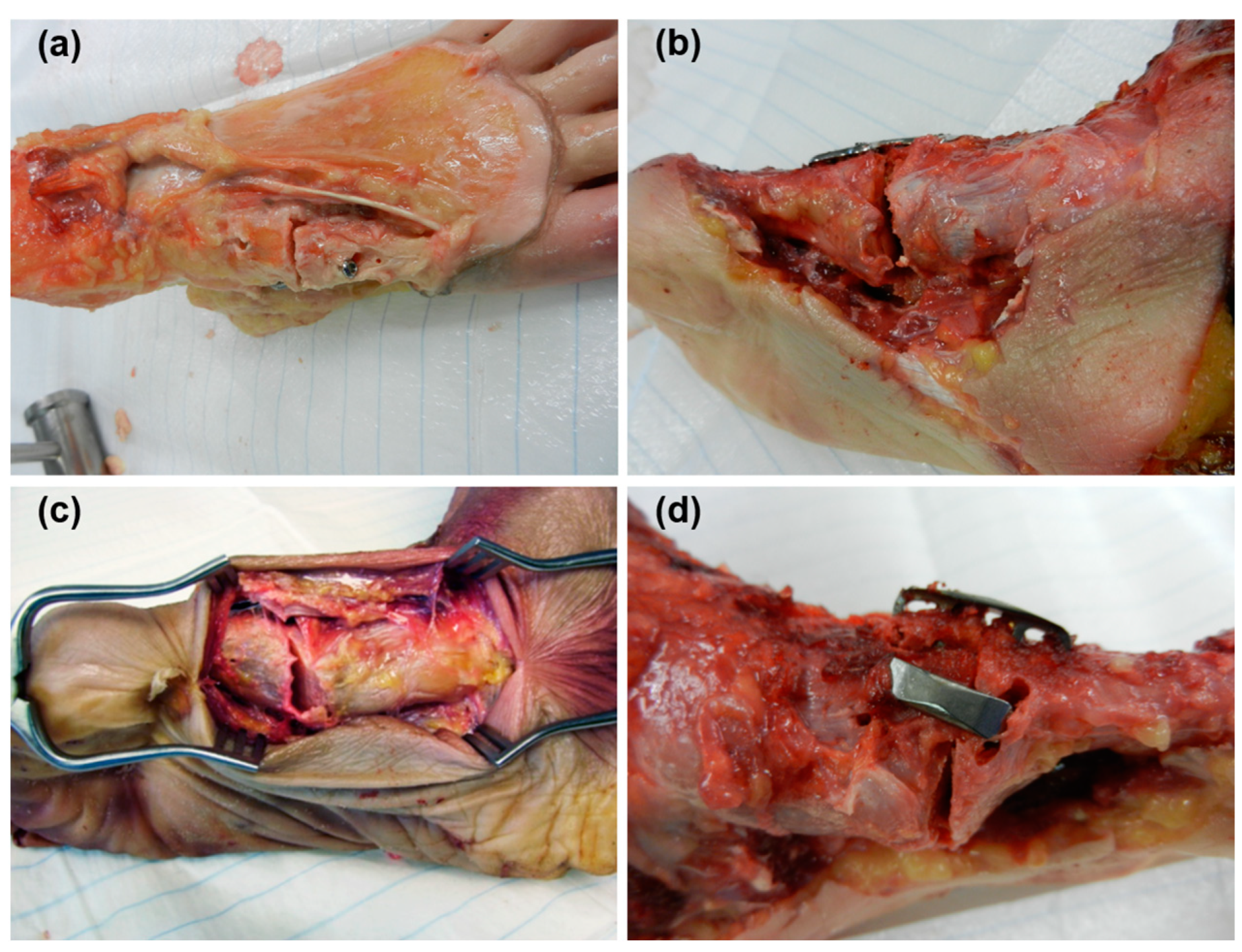
2.1. Specimens and Preparation
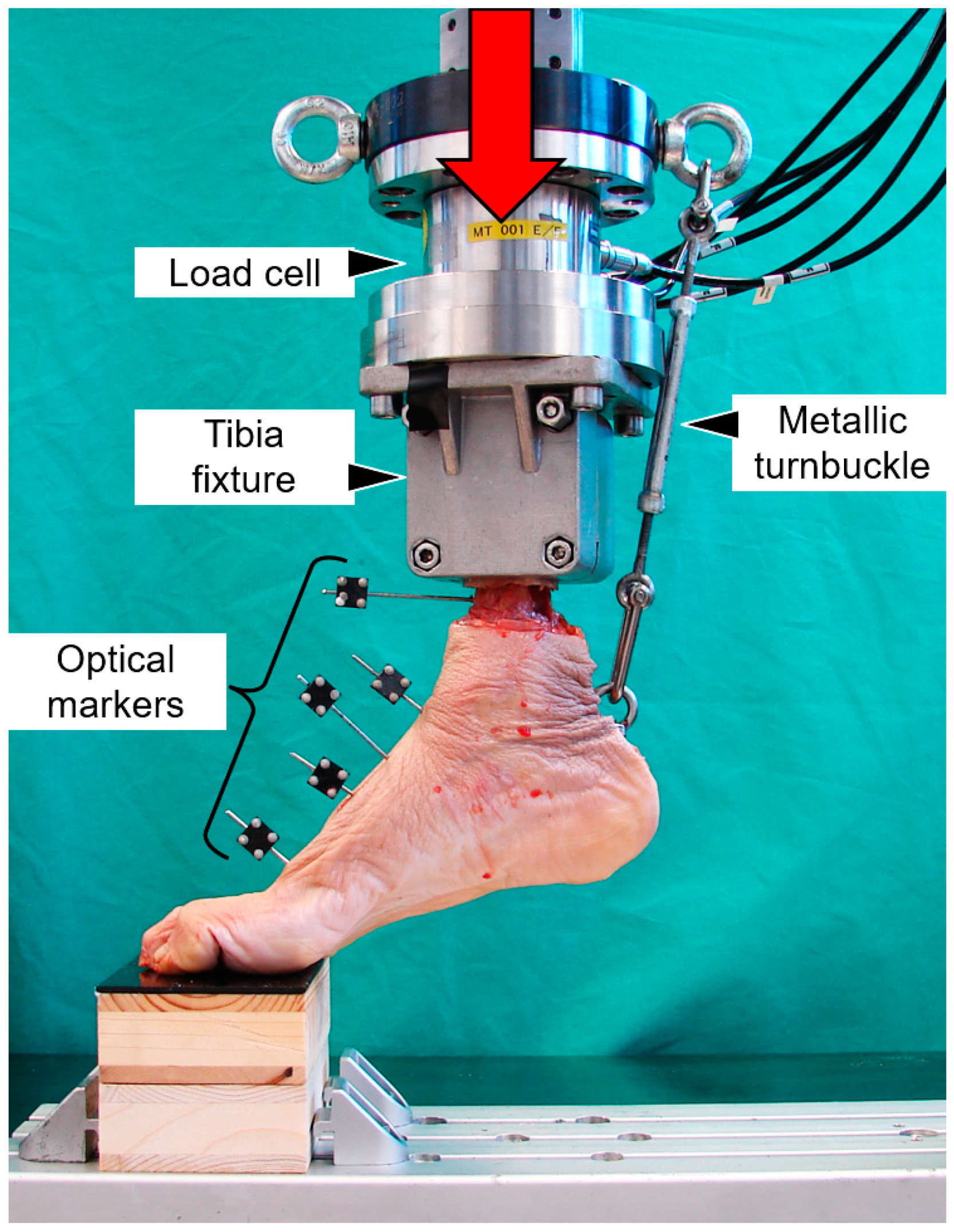
2.2. Biomechanical Testing
2.3. Data Acquisition and Evaluation
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fuhrmann, R.A. Arthrodesis of the first tarsometatarsal joint for correction of the advanced splayfoot accompanied by a hallux valgus. Oper Orthop. Traumatol. 2005, 17, 195–210. [Google Scholar] [CrossRef] [PubMed]
- Wanivenhaus, A.; Pretterklieber, M. First tarsometatarsal joint: Anatomical biomechanical study. Foot Ankle 1989, 9, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Baravarian, B.; Briskin, G.B.; Burns, P. Lapidus bunionectomy: Arthrodesis of the first metatarsocunieform joint. Clin. Podiatr. Med. Surg. 2004, 21, 97–111. [Google Scholar] [CrossRef]
- Faber, F.W.; Mulder, P.G.; Verhaar, J.A. Role of first ray hypermobility in the outcome of the Hohmann and the Lapidus procedure. A prospective, randomized trial involving one hundred and one feet. J. Bone Joint Surg. Am. 2004, 86, 486–495. [Google Scholar] [CrossRef]
- Bednarz, P.A.; Manoli, A. Modified lapidus procedure for the treatment of hypermobile hallux valgus. Foot Ankle Int. 2000, 21, 816–821. [Google Scholar] [CrossRef]
- Gruber, F.; Sinkov, V.S.; Bae, S.Y.; Parks, B.G.; Schon, L.C. Crossed screws versus dorsomedial locking plate with compression screw for first metatarsocuneiform arthrodesis: A cadaver study. Foot Ankle Int. 2008, 29, 927–930. [Google Scholar] [CrossRef]
- Myerson, M.; Allon, S.; McGarvey, W. Metatarsocuneiform arthrodesis for management of hallux valgus and metatarsus primus varus. Foot Ankle 1992, 13, 107–115. [Google Scholar] [CrossRef]
- Sangeorzan, B.J.; Hansen, S.T., Jr. Modified Lapidus procedure for hallux valgus. Foot Ankle 1989, 9, 262–266. [Google Scholar] [CrossRef]
- Swords, M.; Tomlinson, M. Midfoot arthrodesis for adult acquired flatfoot. Fuß Sprunggelenk 2020, 18, 30–36. [Google Scholar] [CrossRef]
- Symeonidis, P.D.; Anderson, J.G. Original and Modified Lapidus Procedures: Proposals for a New Terminology. J. Bone Joint Surg. Am. 2021, 103, e15. [Google Scholar] [CrossRef] [PubMed]
- Coughlin, M.J.; Mann, R.A. Halux Valgus. In Surgery of the Foot and Ankle, 8th ed.; Coughlin, M.J., Mann, R.A., Saltzman, C.L., Eds.; Elsevier: Philadelphia, PA, USA, 2007; pp. 181–363. [Google Scholar]
- Thompson, I.M.; Bohay, D.R.; Anderson, J.G. Fusion rate of first tarsometatarsal arthrodesis in the modified Lapidus procedure and flatfoot reconstruction. Foot Ankle Int. 2005, 26, 698–703. [Google Scholar] [CrossRef] [PubMed]
- Horton, G.A.; Olney, B.W. Deformity correction and arthrodesis of the midfoot with a medial plate. Foot Ankle 1993, 14, 493–939. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.A.; Parks, B.G.; Schon, L.C. Screw fixation compared to H-locking plate fixation for first metatarsocuneiform arthrodesis: A biomechanical study. Foot Ankle Int. 2005, 26, 984–989. [Google Scholar] [CrossRef]
- Scranton, P.E.; Coetzee, J.C.; Carreira, D. Arthrodesis of the first metatarsocuneiform joint: A comparative study of fixation methods. Foot Ankle Int. 2009, 30, 341–345. [Google Scholar] [CrossRef]
- Russell, S.M. Design considerations for nitinol bone staples. J. Mater. Eng. Perform. 2009, 18, 831–835. [Google Scholar] [CrossRef]
- Sun, F.; Sask, K.N.; Brash, J.L.; Zhitomirsky, I. Surface modifications of Nitinol for biomedical applications. Colloids Surf. B Biointerfaces 2008, 67, 132–139. [Google Scholar] [CrossRef] [PubMed]
- McNaney, J.M.; Imbeni, V.; Jung, Y.; Papadopoulos, P.; Ritchie, R. An experimental study of the superelastic effect in a shape-memory Nitinol alloy under biaxial loading. Mech. Mater. 2003, 35, 969–986. [Google Scholar] [CrossRef]
- Shibuya, N.; Manning, S.N.; Meszaros, A.; Budny, A.M.; Malay, D.S.; Yu, G.V. A compression force comparison study among three staple fixation systems. J. Foot Ankle Surg. 2007, 46, 7–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rethnam, U.; Kuiper, J.; Makwana, N. Biomechanical characteristics of three staples commonly used in foot surgery. J. Foot Ankle Res. 2009, 2, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aiyer, A.; Russell, N.A.; Pelletier, M.H.; Myerson, M.; Walsh, W.R. The Impact of Nitinol Staples on the Compressive Forces, Contact Area, and Mechanical Properties in Comparison to a Claw Plate and Crossed Screws for the First Tarsometatarsal Arthrodesis. Foot Ankle Spec. 2016, 9, 232–240. [Google Scholar] [CrossRef]
- Hoon, Q.J.; Pelletier, M.H.; Christou, C.; Johnson, K.A.; Walsh, W.R. Biomechanical evaluation of shape-memory alloy staples for internal fixation-an in vitro study. J. Exp. Orthop. 2016, 3, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russell, N.A.; Regazzola, G.; Aiyer, A.; Nomura, T.; Pelletier, M.H.; Myerson, M.; Walsh, W.R. Evaluation of Nitinol Staples for the Lapidus Arthrodesis in a Reproducible Biomechanical Model. Front. Surg. 2015, 2, 65. [Google Scholar] [CrossRef] [Green Version]
- Choudhary, R.K.; Theruvil, B.; Taylor, G.R. First metatarsophalangeal joint arthrodesis: A new technique of internal fixation by using memory compression staples. J. Foot Ankle Surg. 2004, 43, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Malal, J.J.; Hegde, G.; Ferdinand, R.D. Tarsal joint fusion using memory compression staples—A study of 10 cases. J. Foot Ankle Surg. 2006, 45, 113–117. [Google Scholar] [CrossRef]
- Tang, R.G.; Dai, K.R.; Chen, Y.Q. Application of a NiTi staple in the metatarsal osteotomy. Biomed. Mater. Eng. 1996, 6, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Ronchetti, P.J.; Topper, S.M. Lunocapitate fusion using the OSStaple compression staple. Tech. Hand Up. Extrem. Surg. 2006, 10, 231–234. [Google Scholar] [CrossRef]
- Mereau, T.M.; Ford, T.C. Nitinol compression staples for bone fixation in foot surgery. J. Am. Podiatr. Med. Assoc. 2006, 96, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Mallette, J.P.; Glenn, C.L.; Glod, D.J. The incidence of nonunion after Lapidus arthrodesis using staple fixation. J. Foot Ankle Surg. 2014, 53, 303–306. [Google Scholar] [CrossRef]
- Coetzee, J.C.; Wickum, D. The Lapidus procedure: A prospective cohort outcome study. Foot Ankle Int. 2004, 25, 526–531. [Google Scholar] [CrossRef]
- Patel, S.; Ford, L.A.; Etcheverry, J.; Rush, S.M.; Hamilton, G.A. Modified lapidus arthrodesis: Rate of nonunion in 227 cases. J. Foot Ankle Surg. 2004, 43, 37–42. [Google Scholar] [CrossRef]
- Saxena, A.; Nguyen, A.; Nelsen, E. Lapidus Bunionectomy: Early Evaluation of Crossed Lag Screws versus Locking Plate with Plantar Lag Screw. J. Foot Ankle Surg. 2009, 48, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Simons, P.; Sommerer, T.; Zderic, I.; Wahl, D.; Lenz, M.; Skulev, H.; Knobe, M.; Gueorguiev, B.; Richards, R.G.; Klos, K. Biomechanical investigation of two plating systems for medial column fusion in foot. PLoS ONE 2017, 12, e0172563. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Morgan, E.F. Accuracy and precision of digital volume correlation in quantifying displacements and strains in trabecular bone. J. Biomech. 2007, 40, 3516–3520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, E.C.; Dear, K.A.; Hendow, C.; Miller, L.; Mehta, S.; Hast, M.W. Examining the novel use of continuous compression implants in clavicle reconstruction: A biomechanical study. Clin. Biomech. 2021, 88, 105437. [Google Scholar] [CrossRef] [PubMed]
- Appelt, A.; Schnabel, B.; Scharf, M.; Windolf, M.; Van der Pol, B.; Braunstein, V.; Schwieger, K. Alternative Versorgung von Patellafrakturen mit Memory-Metallklammern—Eine biomechanische Vergleichsstudie. In Proceedings of the Deutscher Kongress für Orthopädie und Unfallchirurgie, Berlin, Germany, 22–25 October 2008. [Google Scholar]
- Schnabel, B.; Scharf, M.; Schwieger, K.; Windolf, M.; van der Pol, B.; Braunstein, V.; Appelt, A. Biomechanical comparison of a new staple technique with tension band wiring for transverse patella fractures. Clin. Biomech. 2009, 24, 855–859. [Google Scholar] [CrossRef]
- Schipper, O.N.; Ellington, J.K. Nitinol compression staples in foot and ankle surgery. Orthop. Clin. 2019, 50, 391–399. [Google Scholar] [CrossRef]
- Bumpass, D.B.; Fuhrhop, S.K.; Schootman, M.; Smith, J.C.; Luhmann, S.J. Vertebral body stapling for moderate juvenile and early adolescent idiopathic scoliosis: Cautions and patient selection criteria. Spine 2015, 40, E1305–E1314. [Google Scholar] [CrossRef]
- Singh, D.; Sinha, S.; Singh, H.; Jagetia, A.; Gupta, S.; Gangoo, P.; Tandon, M. Use of nitinol shape memory alloy staples (niti clips) after cervical discoidectomy: Minimally invasive instrumentation and long-term results. Min-Minim. Invasive Neurosurg. 2011, 54, 172–178. [Google Scholar] [CrossRef]
- Klos, K.; Gueorguiev, B.; Muckley, T.; Frober, R.; Hofmann, G.O.; Schwieger, K.; Windolf, M. Stability of medial locking plate and compression screw versus two crossed screws for lapidus arthrodesis. Foot Ankle Int. 2010, 31, 158–163. [Google Scholar] [CrossRef]

| Parameter | Groups | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| BMD (mgHA/cm3) | 123.5 (48.5) | 111.8 (41.3) | 119.7 (48.23) | 108.4 (49.9) | |
| Initial stiffness (N/mm) | 18.1 (4.0) | 19.0 (5.3) | 19.6 (7.7) | 20.2 (3.7) | |
| Cycles | |||||
| Gap angle unloaded (°) | 500 | 0.05 (0.10) | 0.00 (0.01) | 0.13 (0.17) | 0.19 (0.42) |
| 1000 | 0.06 (0.13) | 0.04 (0.07) | 0.41 (0.48) | 0.40 (0.61) | |
| 2500 | 0.16 (0.25) | 0.14 (0.24) | 0.99 (0.90) | 0.70 (0.84) | |
| Gap angle amplitude (°) | 500 | 0.56 (0.12) | 0.53 (0.09) | 1.00 (0.22) | 0.88 (0.46) |
| 1000 | 0.58 (0.14) | 0.61 (0.20) | 1.14 (0.21) | 0.92 (0.55) | |
| 2500 | 0.68 (0.19) | 0.66 (0.28) | 1.80 (0.98) | 1.31 (0.82) | |
| Displacement at dorsal aspect unloaded (mm) | 500 | 0.02 (0.07) | 0.01 (0.05) | 0.04 (0.05) | 0.11 (0.16) |
| 1000 | 0.04 (0.10) | 0.06 (0.10) | 0.08 (0.10) | 0.19 (0.29) | |
| 2500 | 0.09 (0.11) | 0.14 (0.23) | 0.19 (0.27) | 0.32 (0.34) | |
| Displacement amplitude at dorsal aspect (mm) | 500 | 0.32 (0.10) | 0.33 (0.08) | 0.41 (0.22) | 0.39 (0.20) |
| 1000 | 0.32 (0.11) | 0.36 (0.09) | 0.46 (0.29) | 0.45 (0.32) | |
| 2500 | 0.38 (0.12) | 0.37 (0.07) | 0.71 (0.69) | 0.48 (0.27) | |
| Displacement at plantar aspect unloaded (mm) | 500 | 0.02 (0.07) | 0.01 (0.05) | 0.07 (0.11) | 0.07 (0.10) |
| 1000 | 0.05 (0.10) | 0.10 (0.11) | 0.16 (0.19) | 0.14 (0.19) | |
| 2500 | 0.12 (0.10) | 0.13 (0.21) | 0.50 (0.67) | 0.29 (0.26) | |
| Displacement amplitude at plantar aspect (mm) | 500 | 0.47 (0.15) | 0.49 (0.10) | 0.77 (0.34) | 0.61 (0.31) |
| 1000 | 0.50 (0.19) | 0.86 (1.00) | 0.84 (0.51) | 0.75 (0.52) | |
| 2500 | 0.57 (0.22) | 0.67 (0.28) | 1.29 (1.14) | 0.76 (0.49) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sands, A.; Zderic, I.; Swords, M.; Gehweiler, D.; Ciric, D.; Roth, C.; Nötzli, C.; Gueorguiev, B. First Tarsometatarsal Joint Fusion in Foot—A Biomechanical Human Anatomical Specimen Analysis with Use of Low-Profile Nitinol Staples Acting as Continuous Compression Implants. Medicina 2023, 59, 1310. https://doi.org/10.3390/medicina59071310
Sands A, Zderic I, Swords M, Gehweiler D, Ciric D, Roth C, Nötzli C, Gueorguiev B. First Tarsometatarsal Joint Fusion in Foot—A Biomechanical Human Anatomical Specimen Analysis with Use of Low-Profile Nitinol Staples Acting as Continuous Compression Implants. Medicina. 2023; 59(7):1310. https://doi.org/10.3390/medicina59071310
Chicago/Turabian StyleSands, Andrew, Ivan Zderic, Michael Swords, Dominic Gehweiler, Daniel Ciric, Christoph Roth, Christoph Nötzli, and Boyko Gueorguiev. 2023. "First Tarsometatarsal Joint Fusion in Foot—A Biomechanical Human Anatomical Specimen Analysis with Use of Low-Profile Nitinol Staples Acting as Continuous Compression Implants" Medicina 59, no. 7: 1310. https://doi.org/10.3390/medicina59071310





