1. Introduction
Since December 2019, the world has observed the appearance and spread of SARS-CoV-19, a novel coronavirus that causes a severe respiratory syndrome (COVID-19) and its disastrous impact on global health [
1,
2]. COVID-19 was declared as a pandemic on March 11, 2020 by the World Health Organization (WHO), and since then millions of people worldwide have been affected. As of May 2023, more than six million deaths have been confirmed.
Although in the majority of cases, the disease manifests with respiratory symptoms such as cough, fever and dyspnea, its extra-pulmonary manifestations are being increasingly acknowledged [
3] Specifically, the incidence of GI manifestations such as abdominal pain, nausea or diarrhea has ranged from 12% to as much as 61% in patients with COVID-19. Moreover, some patients might present only with GI symptoms, hindering diagnosis. Furthermore, it has been noted that patients presenting with GI symptoms tend to progress more to a severe form of disease with poor outcomes [
4,
5,
6]. As it is well documented that SARS-CoV-19 uses ACE-2 receptors to enter human cells, this receptor is found to be expressed not only in pulmonary epithelial cells but also in gastrointestinal and hepatobiliary cells, explaining the GI involvement [
7,
8]. Despite the broad recognition of respiratory imaging findings, until now few studies have been published about abdominal radiological presentation of patients with COVID-19 [
9,
10].
Taking into consideration the high possibility of GI tract involvement, it is crucial for radiologists to be aware of the variety of abdominal imaging findings in patients with COVID-19, as early recognition can aid the diagnosis in patients with nonspecific, atypical symptoms.
2. Materials and Methods
The aim of this study is to share our experience and present the imaging findings of GI manifestations that can be noted in patients with COVID 19.
The authors executed a retrospective study from our hospital archives starting from February 2020, when the first COVID-19 patient was admitted in our hospital, until 31 December of 2022. Six of the authors searched the institutional databases of COVID-19 patients who underwent abdominal CT. Confirmed COVID-19 patients who presented with abdominal signs and symptoms were included in the study. A dedicated 16-slice SIEMENS EMOTION CT scanner was used for confirmed COVID-19 cases. The final study population was 84 patients. Simultaneously, a search of the published literature was conducted on PubMed using suitable key words (e.g., COVID-19, abdominal manifestations, CT, imaging, and gastrointestinal).
2.1. Colon Manifestations
The bowel is the most commonly involved abdominal organ in COVID-19 patients. Frequent abdominal imaging features include intestinal imaging findings (24%), including colorectal (5%) and small bowel thickening (12%), intestinal distension (18%), pneumatosis and intestinal perforation [
11]. Bhayana R. et al. observed [
9] similar bowel-wall abnormalities in 31% of CT images in patients in the Intensive Care Unit (ICU). Common indications for CT evaluation include abdominal pain or distention, hematochezia and nausea [
3]. It is important to note that the presence of bowel abnormalities predict worse prognosis and increased clinical severity.
ACE 2 receptors are located in gastrointestinal cells (enterocytes and vascular cells) and can interpret the bowel manifestations of the virus, specifically the inflammatory bowel manifestations and ischemic bowel alterations. This is supported by the detection of SARS-CoV-19 virus in feces samples [
4,
12]. Infection of the GI tract epithelial cells is followed by an inflammatory response and a cytokine release.
A large bowel infection usually appears with diffuse circumferential and enhancing wall thickening (hyperenhancement) that can involve one or more segments of the colon (
Figure 1 and
Figure 2). Pericolic fluid or perintestinal fat stranding is common while pericolic lymphadenopathy is not (
Figure 3 and
Figure 4). If we suspect COVID-19-related colitis, clinical correlation is needed, and the detection of the virus in stools can establish the diagnosis.
It is very important to suspect and diagnose patients presenting acute mesenteric ischemia (AMI) or perforation of the bowel [
13]. It is well documented that critically ill COVID-19 patients are at risk for thrombosis or bleeding [
14].
AMI, is associated with severe symptoms, a worsening systemic status and high morbidity and mortality. Imaging has a crucial role in its detection and is the cornerstone of diagnosis [
15]. An ultrasound is nonspecific with low sensitivity but may reveal decreased peristalsis and intraluminal content that indicate stasis.
CT angiography remains the pillar for detection of signs of ischemia [
16]. Filling defects that represent emboli or thrombi within the lumen of the abdominal aorta and its branches are major findings along with hypoenhancement of the mesenteric vascular arcade and decreased contrast enhancement of the bowel wall that indicates hypoperfusion. In some cases, a target appearance of the bowel wall, representing mucosal hyperemia with adjacent mural edema, can be depicted in ischemic colitis. The affected segment appears with thickened bowel walls, and it may be pictured as fluid-filled due to disruption of peristalsis [
17]. Additional findings are porto-mesenteric venous gas, pneumatosis intestinalis and pneumoperitoneum [
18,
19]. In a later phase, the bowel wall is thinned due to loss of normal tone.
2.2. Small Intestine Manifestations
When COVID-19 affects the small intestine, it can cause a range of symptoms, including diarrhea, abdominal pain and vomiting, in both adults and children [
20].
One study published in the Journal of Medical Virology examined the fecal samples of COVID-19 patients and found that the virus was present in the samples of 23 out of 29 patients, indicating that SARS-CoV-19 can be transmitted via the fecal–oral route. Additionally, the virus was present in higher amounts in patients with gastrointestinal symptoms, such as diarrhea and nausea [
21].
Another study found that nearly half of COVID-19 patients had gastrointestinal symptoms, such as diarrhea, nausea and vomiting. Those patients experienced a longer duration of illness compared to those without digestive symptoms [
22].
The exact mechanisms by which COVID-19 affects the small intestine are still not fully understood, but it is thought that the virus may directly infect the intestinal cells or that it may cause an inflammatory response that affects the gut. Additionally, some patients may experience a dysregulated immune response, which can also contribute to small bowel manifestations, including inflammation, mucosal injury and microthrombi formation [
23].
Several studies have investigated the CT findings associated with small intestine involvement. In the CT scans of 149 COVID-19 patients, nearly 20% were indicative of small intestine involvement, with wall thickening, luminal dilatation and mucosal enhancement, especially in severe cases associated with worse outcomes (
Figure 5) [
22].
Bhayana et al. found that 29% of CTs showed bowel wall thickening involving the colon or small bowel, such as findings of ischemia with pneumatosis or portal venous gas and bowel perforation and a fluid-filled colon in 43% of patients, suggestive of diarrhea. In addition to small bowel abnormalities, (
Figure 6) CT imaging has also shown evidence of mesenteric lymphadenopathy, with mesenteric lymph node enlargement and increased enhancement in 22% of patients [
9].
However, as these findings are not specific, CT findings must be correlated with clinical and laboratory data to confirm a COVID-19 diagnosis.
2.3. Hepato-Biliary Involvement
ACE2 receptors are found in many organs including the liver and biliary system, thus giving the opportunity for a local inflammation of these organs [
24].
The hepatobiliary involvement in patients with COVID-19 infection and the abdominal imaging findings in those patients represent an interesting topic for further investigation.
One of the most common imaging findings in CT examinations of COVID-19 patients with hepatobiliary dysfunction (both in the literature and in our series) is hepatomegaly with or without a diffuse decrease in liver/spleen attenuation ratio. This can be due to liver injury due to the virus, pre-existing comorbidities (obesity, nonalcoholic fatty liver, and hepatitis) or as a result of hospitalization (parenteral nutrition and hepatotoxic drugs) [
25,
26].
In severe cases of COVID-19 infection, mostly in patients in ICU, a heterogenicity and mosaic pattern of the liver parenchyma is seen, combined with periportal edema and a contrast reflux in dilated hepatic veins indicating liver congestion due to hypoxia and cardiac failure [
25].
Biliary imaging findings are also encountered in patients with COVID-19 infection. In our institution, many patients presented with right quadrant pain and nausea/vomiting after a meal. In ultrasound and CT examinations, the findings included gallbladder distention, wall thickening and mural edema, pericholecystic fluid and inflammatory fat stranding with calculi and/or sludge (
Figure 7 and
Figure 8).
Less frequently, acalculous cholecystitis with sludge is seen in patients in the ICU, indicating cholestasis due to parenteral nutrition and/ or systemic inflammation [
27,
28].
2.4. Pancreatic Involvement
The data on pancreatic involvement during SARS-CoV-19 infection are limited. The frequency and severity of pancreatic damage and acute pancreatitis (AP) and its pathophysiology are still being studied.
AP is usually caused by increased alcohol consumption and gallstones. However, in 10%–20% of cases, an etiological factor cannot be identified [
29]. A number of infectious agents, such as Coxsackie B virus and hepatitis A virus, infect the pancreas [
30]. Radiology and imaging findings play a vital role mainly in the detection and follow up of complications of AP [
30].
According to the revised Atlanta classification, the diagnosis of AP requires two of the following: (a) typical abdominal pain, (b) a serum lipase level (or amylase) at least three times greater than the upper normal limit and (c) characteristic imaging findings on CT, MRI or ultrasonography [
31].
A study performed in pigeons with severe pancreatitis has managed to isolate COVID-19 or a Coronavirus-like virus, but no similar studies have been conducted in humans [
32]. SARS-COV-19 uses ACE-2 receptors to enter pancreatic ductal cells [
33], a fact that could explain the infection of the gland. Another possible way to induce the pancreatic injury is the caused cytokine storm, which produces pancreatic inflammation and an uncontrolled inflammatory immune systemic response caused by COVID-19. Finally, another important cause is the drug-induced pancreatic injury from antivirals, non-steroidal anti-inflammatory drugs (NSAIDs), tocilizumab and baricitinib, which belong to the approved treatment of COVID-19 [
4].
Amylase and lipase elevation has been reported in 8.5–17.3% of patients with COVID-19. However, those enzymes are also increased in other gastrointestinal diseases, such as gastritis and colitis that have also been reported in COVID-19 patients. A meta-analysis of patients with COVID-19 showed that 18% had gastrointestinal symptoms and raised pancreatic enzymes could not be directly associated with pancreatitis [
34]. Furthermore, kidneys play a major role in the clearance of both amylase and lipase, and their insufficiency could result in the elevation of these enzymes [
35]. It has been proved that COVID-19 can infect insulin-producing cells in the pancreas and change their function, potentially explaining the high privilege of diabetes in previously healthy individuals [
36].
The temporal relationship between the onset of COVID-19 infection and inflammation of the pancreas has not been clearly established. Some patients develop COVID-19 symptoms and abdominal pain when the infection begins, whereas others present with AP several days after COVID-19 diagnosis (
Figure 9) [
37].
A study of 52 patients with COVID-19 pneumonia showed that there was a 17% incidence of pancreatic injury. Kumar V. et al. studied patients with acute pancreatitis and COVID-19 infection and found that half of them developed AP after a median of 22.5 days from the onset of respiratory symptoms, while the rest of them were admitted for abdominal pain [
38] Another systematic review, including overall 37 patients, summarized that AP might be the first symptom of COVID-19 [
39]. In addition, COVID-19 may negatively influence the morbidity and mortality linked with AP [
40].
Finally, it should be noted that after the COVID-19 pandemic, pancreatic cancer and metastases rates have been dramatically raised, as there was a temporary cessation of screening during the pandemic. Less than 25% of patients had regular availability of diagnostic and staging tools, while 20% were unable to perform surgery [
41].
2.5. Thromboembolic Complications
Systemic coagulopathy is common in COVID-19 patients with severe pneumonia [
42].
Despite the limited available data, many thrombotic manifestations regarding the abdominal vessels have been documented in cases of COVID-19 inpatients. The CT depiction frequency is highly dependent on the performed protocol, which is usually unenhanced or contains only a portal venous phase. Only in a few cases the image acquisition is accomplished through an abdominal multiphase CT angiogram. Consequently, thromboembolic events regarding the arterial abdominal branches are scarcely reported, opposed to venous thrombi, possibly due to underdiagnosis [
43].
In many cases we can only depict indirect findings due to the small vessel thrombotic nature of the disease, a phenomenon that could also be related to hospitalization or comorbidities [
11]. When it comes to microvasculature, both arteries and veins are affected [
44]. An interesting fact is that in an accountable percentage of the arterial thrombi, the affected vessels did not have any atherosclerotic alterations, suggesting that COVID-19 was the generating factor of the thrombus [
45].
Solid abdominal organs infarcts have been documented during imaging protocols performed for pulmonary embolism detection, especially in patients with elevation of the D-dimers. Apart from those cases, renal infarcts were reported in scans performed for vague abdominal pain or due to acute kidney failure [
45].
Fewer reports about splenic infracts in COVID-19 patients also come from thoracic scans with abdominal extension [
46].
A single-center small retrospective study reported in COVID-19 patients 15 cases of acute aortic thrombosis, splenic artery thrombosis (associated with splenic infraction), superior mesenteric and renal artery thrombosis such as a celiac and an internal iliac thrombosis. An interesting part was a patient with infrarenal aortic wall inflammation and focal dissection, while many venous thromboses have been described (affecting the portal vein, inferior, superior mesenteric, renal, ovarian vein and inferior vena cava). Reports of indirect findings of the splanchnic branch venous occlusion described bowel wall severe edema, hyperenhancement or severe hypoenhancement, associated mesenteric and portal intravenous gas, bowel pneumatosis and pneumoperitoneum (
Figure 10) [
47]. Bari Dane et al. published a case of a simultaneous nonocclusive aortic, celiac and superior mesenteric artery thrombus combined with a complete common hepatic artery thrombus [
48].
Those findings are in accordance with reports of bowel pneumatosis as a thrombotic event outcome [
49], while many case reports and large case series demonstrate major abdominal—both arterial and venous—thrombosis in COVID-19 patients. In fact, many patients suffer from thrombotic occlusion despite prophylaxis or even the full-dose anticoagulation therapy supporting evidence of COVID-19 direct endothelial injury [
50].
2.6. Bleeding Manifestations
Bleeding in patients with COVID-19 can be the result of pre-existing risks factors, antithrombotic drugs and a massive immune response to the virus, [
51]. A common bleeding complication is abdominal hematomas, usually of the Iliopsoas and rectus abdominis muscle [
51,
52]. The role of a CT scan is major in both the diagnosis and treatment of these entities. They are usually seen as muscle enlargement with increased densities, blood-fluid level and possibly extravasation of contrast. When it comes to the GI tract, upper GI is the most common site of bleeding followed by the lower GI [
53,
54].
A potential pathogenic route is through the binding of the virus with Angiotensin Converting Enzyme-2 expressed in gastrointestinal epithelial cells [
55].
Underlying mucosal lesions in the GI (ulcers and vascular abnormalities) and prophylactic/therapeutic anticoagulant therapy should be considered and further investigated.
GI hemorrhage is less commonly encountered in abdominal imaging, and CT findings include active intraluminal extravasation of contrast and indirect signs such as luminal distention with blood clots (
Figure 11) [
56].
Imaging is additionally significant to the treatment plan by identifying the exact site and extent of the bleeding as well as offering a precise and minimally invasive treatment option. Digital Subtraction Angiography can confirm the active bleeding seen as a “contrast blush”, detect the responsible branch and provide occlusion via selective embolization.
3. Results
In our institution, 84 COVID-19 patients underwent abdominal CT imaging studies displaying positive GI imaging findings. The study population included 43 males and 41 females. The oldest patient was 90 years old, and the youngest patient was 18 years old. Fourteen patients were admitted to the ICU, and in total 26 patients died.
The most frequent indications for abdominal CT were abdominal pain and abdominal distention. The majority of the patients presented elevated levels of liver enzymes and C reactive protein.
The most frequent comorbidities included hypertension, heart disease, dyslipidemia, diabetes mellitus, obesity and cancer. Also, a patient with kidney transplant was included.
The most common imaging findings concerned the small intestine and colon. Bowel wall thickening was noted in 25 patients (22 of them in colorectal area and the rest in small bowel) accompanied with pericolic fluid (6 patients), submucosal edema (6 patients) or intestinal perforation (2 patients). Furthermore, thrombosis of SMA was noted in one patient.
Hepatobiliary manifestations were also common with 22 patients presenting liver steatosis, 3 patients suffering from acute cholecystitis and 24 patients in total presenting biliary sludge and/or gallstones.
Seven patients experienced pancreatitis during their hospitalization.
In our institution, we are in concordance with the few publications about the abdominal manifestations of COVID-19. It was not our intention to present a statistical analysis of our findings but to expound upon the plethora of imaging findings in regard to the digestive system involvement.
In the future, a more extensive study and analysis should take place, in collaboration with other tertiary referral centers, using a bigger sample.
4. Conclusions
COVID-19 is a multi-organ disease, and GI manifestation might be noted at the time of diagnosis or later in the course of the disease. The presence of GI manifestations is associated with increased clinical severity and poor outcome. Therefore, it is pivotal for radiologists to be aware of related imaging findings to aid diagnosis and appropriate management.
Author Contributions
Conceptualization: X.M., E.P. and V.R. Methodology: X.M., A.P., N.P., E.S., M.A., M.S. and A.T. Software, data curation, formal analysis, investigation, writing—original draft preparation: A.P., N.P., E.S., M.A., M.S. and A.T. Validation: X.M. Resources: T.C. Supervision: P.P. Project administration X.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to the retrospective nature of the study including non-identifying images acquired for clinically indicated reasons.
Informed Consent Statement
Informed consent acquired by all patients presented.
Data Availability Statement
Not available due to confidentiality of imaging examinations.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, H.; Wei, R.; Rao, G.; Zhu, J.; Song, B. Characteristic CT Findings Distinguishing 2019 Novel Coronavirus Disease (COVID-19) from Influenza Pneumonia. Eur. Radiol. 2020, 30, 4910–4917. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Cao, Q.; Qin, L.; Wang, X.; Cheng, Z.; Pan, A.; Dai, J.; Sun, Q.; Zhao, F.; Qu, J.; et al. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19):A multi-center study in Wenzhou city, Zhejiang, China. J. Infect. 2020, 80, 388–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, A.; Madhavan, M.V.; Sehgal, K.; Nair, N.; Mahajan, S.; Sehrawat, T.S.; Bikdeli, B.; Ahluwalia, N.; Ausiello, J.C.; Wan, E.Y.; et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020, 26, 1017–1032. [Google Scholar] [CrossRef]
- Tian, Y.; Rong, L.; Nian, W.; He, Y. Review article: Gastrointestinal features in COVID-19 and the possibility of faecal transmission. Aliment. Pharmacol. Ther. 2020, 51, 843–851. [Google Scholar] [CrossRef]
- Redd, W.D.; Zhou, J.C.; Hathorn, K.E.; McCarty, T.R.; Bazarbashi, A.N.; Thompson, C.C.; Shen, L.; Chan, W.W. Prevalence and Characteristics of Gastrointestinal Symptoms in Patients With Severe Acute Respiratory Syndrome Coronavirus 2 Infection in the United States: A Multicenter Cohort Study. Gastroenterology 2020, 159, 765–767.e2. [Google Scholar] [CrossRef] [PubMed]
- Mao, R.; Qiu, Y.; He, J.S.; Tan, J.Y.; Li, X.H.; Liang, J.; Shen, J.; Zhu, L.-R.; Chen, Y.; Iacucci, M.; et al. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2020, 5, 667–678. [Google Scholar] [CrossRef]
- Li, G.; He, X.; Zhang, L.; Ran, Q.; Wang, J.; Xiong, A.; Wu, D.; Chen, F.; Sun, J.; Chang, C. Assessing ACE2 expression patterns in lung tissues in the pathogenesis of COVID-19. J. Autoimmun. 2020, 112, 102463. [Google Scholar] [CrossRef]
- Hamming, I.; Timens, W.; Bulthuis, M.L.C.; Lely, A.T.; Navis, G.J.; van Goor, H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004, 203, 631–637. [Google Scholar] [CrossRef]
- Bhayana, R.; Som, A.; Li, M.D.; Carey, D.E.; Anderson, M.A.; Blake, M.A.; Catalano, O.; Gee, M.S.; Hahn, P.F.; Harisinghani, M.; et al. Abdominal imaging findings in COVID-19: Preliminary observations. Radiology 2020, 297, E207–E215. [Google Scholar] [CrossRef]
- Guo, Y.; Hu, X.; Yu, F.; Chen, J.; Zheng, W.; Liu, J.; Zeng, P. Abdomen CT findings in a COVID-19 patient with intestinal symptoms and possibly false negative RT-PCR before initial discharge. Quant. Imaging Med. Surg. 2020, 10, 1158–1161. [Google Scholar] [CrossRef]
- Horvat, N.; Pinto, P.V.; Araujo-Filho, J.D.; Santos, J.M.; Dias, A.B.; Miranda, J.A.; de Oliveira, C.V.; Barbosa, C.S.; Morais, T.C.; Assuncao, A.N., Jr.; et al. Abdominal gastrointestinal imaging findings on computed tomography in patients with COVID-19 and correlation with clinical outcomes. Eur. J. Radiol. Open 2021, 8, 100326. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Tang, M.; Zheng, X.; Liu, Y.; Li, X.; Shan, H. Evidence for Gastrointestinal Infection of SARS-CoV-2. Gastroenterology 2020, 158, 1831–1833.e3. [Google Scholar] [CrossRef] [PubMed]
- Childers, B.C.; Cater, S.W.; Horton, K.M.; Fishman, E.K.; Johnson, P.T. CT evaluation of acute enteritis and colitis: Is it infectious, inflammatory, or ischemic? Radiographics 2015, 35, 1940–1941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, N.; Li, D.; Wang, X.; Sun, Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020, 18, 844–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parry, A.H.; Wani, A.H.; Yaseen, M. Acute Mesenteric Ischemia in Severe Coronavirus-19 (COVID-19): Possible Mechanisms and Diagnostic Pathway. Acad. Radiol. 2020, 27, 1190. [Google Scholar] [CrossRef]
- Furukawa, A.; Kanasaki, S.; Kono, N.; Wakamiya, M.; Tanaka, T.; Takahashi, M.; Murata, K. CT diagnosis of acute mesenteric ischemia from various causes. Am. J. Roentgenol. 2009, 192, 408–416. [Google Scholar] [CrossRef] [Green Version]
- Taourel, P.G.; Deneuville, M.; Pradel, J.; Régent, D.; Bruel, J.M. Acute Mesenteric Ischemia: Diagnosis with Contrast-enhanced CT’. Radiology 1996, 199, 632–636. [Google Scholar] [CrossRef]
- Corrêa Neto, I.J.F.; Viana, K.F.; Silva, M.B.S.d.; Silva, L.M.d.; Oliveira, G.d.; Cecchini, A.R.d.; Rolim, A.S.; Robles, L. Perforated acute abdomen in a patient with COVID-19: An atypical manifestation of the disease. J. Coloproctol. 2020, 40, 269–272. [Google Scholar] [CrossRef]
- De Nardi, P.; Parolini, D.C.; Ripa, M.; Racca, S.; Rosati, R. Bowel perforation in a Covid-19 patient: Case report. Int. J. Color. Dis. 2020, 35, 1797–1800. [Google Scholar] [CrossRef]
- Behzad, S.; Aghaghazvini, L.; Radmard, A.R.; Gholamrezanezhad, A. Extrapulmonary manifestations of COVID-19: Radiologic and clinical overview. Clin. Imaging 2020, 66, 35–41. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, L.; Deng, Q.; Zhang, G.; Wu, K.; Ni, L.; Yang, Y.; Liu, B.; Wang, W.; Wei, C.; et al. The presence of SARS-CoV-2 RNA in the feces of COVID-19 patients. J. Med. Virol. 2020, 92, 833–840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, L.; Mu, M.; Yang, P.; Sun, Y.; Wang, R.; Yan, J.; Li, P.; Hu, B.; Wang, J.; Hu, C.; et al. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: A descriptive, cross-sectional, multicenter study. Am. J. Gastroenterol. 2020, 115, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Kang, Z.; Gong, H.; Xu, D.; Wang, J.; Li, Z.; Cui, X.; Xiao, J.; Meng, T.; Zhou, W.; et al. The digestive system is a potential route of 2019-nCov infection: A bioinformatics 1 analysis based on single-cell transcriptomes 2. bioRxiv 2020. [Google Scholar] [CrossRef]
- Abdelmohsen, M.A.; Alkandari, B.M.; Gupta, V.K.; ElBeheiry, A.A. Diagnostic value of abdominal sonography in confirmed COVID-19 intensive care patients. Egypt. J. Radiol. Nucl. Med. 2020, 51, 198. [Google Scholar] [CrossRef]
- Sodeifian, F.; Seyedalhosseini, Z.S.; Kian, N.; Eftekhari, M.; Najari, S.; Mirsaeidi, M.; Farsi, Y.; Nasiri, M.J. Drug-Induced Liver Injury in COVID-19 Patients: A Systematic Review. Frontiers in Medicine. Front. Media 2021, 8, 731436. [Google Scholar] [CrossRef]
- Metawea, M.I.; Yousif, W.I.; Moheb, I. COVID 19 and liver: An A–Z literature review. Dig. Liver Dis. 2021, 53, 146–152. [Google Scholar] [CrossRef]
- Mattone, E.; Sofia, M.; Schembari, E.; Palumbo, V.; Bonaccorso, R.; Randazzo, V.; La Greca, G.; Iacobello, C.; Russello, D.; Latteri, S. Acute acalculous cholecystitis on a COVID-19 patient: A case report. Ann. Med. Surg. 2020, 58, 73–75. [Google Scholar] [CrossRef]
- Mossaab, G.; Ben Khlifa, M.; Karim, N.; Moez, B.; Oussama, J.; Hajer, N.; Habiba, B.S.A.; Zoukar, O.; Jemaa, Y. Acute acalculous cholecystitis in hospitalized patients in intensive care unit: Study of 5 cases. Heliyon 2022, 8, e11524. [Google Scholar] [CrossRef]
- Whitcomb, D.C. Acute Pancreatitis [Internet]. 2013. Available online: https://pubmed.ncbi.nlm.nih.gov/16707751/ (accessed on 14 May 2023).
- Almutairi, F.; Rabeie, N.; Awais, A.; Samannodi, M.; Aljehani, N.; Tayeb, S.; Elsayad, W. COVID-19 induced acute pancreatitis after resolution of the infection. J. Infect. Public Health 2022, 15, 282–284. [Google Scholar] [CrossRef]
- Kumaran, N.K.; Karmakar, B.K.; Taylor, O.M. Coronavirus disease-19 (COVID-19) associated with acute necrotising pancreatitis (ANP). BMJ Case Rep. CP 2020, 13, e237903. [Google Scholar] [CrossRef]
- Qian, D.H.; Zhu, G.J.; Wu, L.Z.; Hua, G.X. Isolation and characterization of a coronavirus from pigeons with pancreatitis. Am. J. Vet. Res. 2006, 67, 1575–1579. [Google Scholar] [CrossRef] [PubMed]
- Samanta, J.; Gupta, R.; Singh, M.P.; Patnaik, I.; Kumar, A.; Kochhar, R. Coronavirus disease 2019 and the pancreas. Pancreatology 2020, 20, 1567–1575. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.S.; Hung, I.F.N.; Chan, P.P.Y.; Lung, K.C.; Tso, E.; Liu, R.; Ng, Y.Y.; Chu, M.Y.; Chung, T.W.; Tam, A.R. Gastrointestinal Manifestations of SARS-CoV-2 Infection and Virus Load in Fecal Samples From a Hong Kong Cohort: Systematic Review and Meta-analysis. Gastroenterology 2020, 159, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Zippi, M.; Hong, W.; Traversa, G.; Maccioni, F.; De Biase, D.; Gallo, C.; Fiorino, S. Involvement of the exocrine pancreas during COVID-19 infection and possible pathogenetic hypothesis: A concise review Myc-mediated cell competition in cancer View project. Infez. Med. 2020, 28, 507–515. Available online: https://www.researchgate.net/publication/346609266 (accessed on 14 May 2023). [PubMed]
- Abramczyk, U.; Nowaczyński, M.; Słomczyński, A.; Wojnicz, P.; Zatyka, P.; Kuzan, A. Consequences of COVID-19 for the Pancreas. Int. J. Mol. Sci. 2022, 23, 864. [Google Scholar] [CrossRef] [PubMed]
- Sinagra, E.; Shahini, E.; Crispino, F.; Macaione, I.; Guarnotta, V.; Marasà, M.; Testai, S.; Pallio, S.; Albano, D.; Facciorusso, A.; et al. COVID-19 and the Pancreas: A Narrative Review. Life 2022, 12, 1292. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Barkoudah, E.; Souza, D.A.T.; Jin, D.X.; McNabb-Baltar, J. Clinical course and outcome among patients with acute pancreatitis and COVID-19. Eur. J. Gastroenterol. Hepatol. 2021, 33, 695–700. [Google Scholar] [CrossRef]
- Bircakova, B.; Bruha, R.; Lambert, L.; Grusova, G.; Michalek, P.; Burgetova, A. A bimodal pattern of the onset of COVID-19 related acute pancreatitis supports both the cytotoxic and immune-related pathogenesis—A systematic review. Scand. J. Gastroenterol. 2021, 56, 870–873. [Google Scholar] [CrossRef]
- Mutneja, H.R.; Bhurwal, A.; Arora, S.; Goel, A.; Vohra, I.; Attar, B.M. Acute pancreatitis in patients with COVID-19 is more severe and lethal: A systematic review and meta-analysis. Scand. J. Gastroenterol. 2021, 56, 1467–1472. [Google Scholar] [CrossRef]
- McKay, S.C.; Pathak, S.; Wilkin, R.J.W.; Kamarajah, S.K.; Wigmore, S.J.; Rees, J.; Dunne, D.F.; Garcea, G.; Ahmad, J.; de Liguori Carino, N.; et al. Impact of SARS-CoV-2 pandemic on pancreatic cancer services and treatment pathways: United Kingdom experience. HPB 2021, 23, 1656–1665. [Google Scholar] [CrossRef]
- Conti, C.B.; Henchi, S.; Coppeta, G.P.; Testa, S.; Grassia, R. Bleeding in COVID-19 severe pneumonia: The other side of abnormal coagulation pattern? Eur. J. Intern. Med. 2020, 77, 147. [Google Scholar] [CrossRef] [PubMed]
- De Roquetaillade, C.; Chousterman, B.G.; Tomasoni, D.; Zeitouni, M.; Houdart, E.; Guedon, A.; Reiner, P.; Bordier, R.; Gayat, E.; Montalescot, G.; et al. Unusual arterial thrombotic events in Covid-19 patients. Int. J. Cardiol. 2021, 323, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Js, A.; Sm, A.; Am, A.; Althobiani, M.; Rp, R.; Oyelade, T. Thoracic imaging outcomes in COVID-19 survivors. World J. Radiol. 2021, 13, 149–156. [Google Scholar]
- Han, H.; Yang, L.; Liu, R.; Liu, F.; Liu, F.; Wu, K.L.; Li, J.; Liu, X.-H.; Zhu, C.-L. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin. Chem. Lab. Med. 2020, 58, 1116–1120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pessoa, M.S.L.; Lima, C.F.C.; Pimentel, A.C.F.; José Carlos Godeiro Costa, J.; Holanda, J.L.B. Multisystemic Infarctions in COVID-19: Focus on the Spleen. Eur. J. Case Rep. Intern. Med. 2020, 7, 001747. [Google Scholar] [CrossRef]
- Ghafoor, S.; Germann, M.; Jüngst, C.; Müllhaupt, B.; Reiner, C.S.; Stocker, D. Imaging features of COVID-19-associated secondary sclerosing cholangitis on magnetic resonance cholangiopancreatography: A retrospective analysis. Insights Imaging 2022, 13, 128. [Google Scholar] [CrossRef]
- Dane, B.; Smereka, P.; Wain, R.; Kim, D.; Katz, D.S. Hypercoagulability in Patients with Coronavirus Disease (COVID-19): Identification of Arterial and Venous Thromboembolism in the Abdomen, Pelvis, and Lower Extremities. AJR Am. J. Roentgenol. 2021, 216, 104–105. [Google Scholar] [CrossRef]
- Hellinger, J.C.; Sirous, R.; Hellinger, R.L.; Krauthamer, A. Abdominal Presentation of COVID-19. Appl. Radiol. 2020, 49, 24–26. Available online: https://appliedradiology.com/articles/abdominal-presentation-of-covid-19 (accessed on 14 May 2023). [CrossRef]
- Prasoppokakorn, T.; Kullavanijaya, P.; Pittayanon, R. Risk factors of active upper gastrointestinal bleeding in patients with COVID-19 infection and the effectiveness of PPI prophylaxis. BMC Gastroenterol. 2022, 22, 465. [Google Scholar] [CrossRef]
- Özer, M.; Terzioğlu, S.G.; KeskinkılıçYağız, B.; Gürer, A.; Dinç, T.; Coşkun, A. Does COVID-19 increase the incidence of spontaneous rectus sheath hematoma? Ulus. Travma Acil Cerrahi Derg. 2022, 28, 920–926. [Google Scholar] [CrossRef]
- Mackiewicz-Milewska, M.; Sakwińska, K.; Cisowska-Adamiak, M.; Szymkuć-Bukowska, I.; Ratuszek-Sadowska, D.; Mackiewicz-Nartowicz, H. Bleeding into the Abdominal and Ilio-Lumbar Muscles—A Rare Complication in the Course of COVID-19: Analysis of Four Cases and a Literature Review. J. Clin. Med. 2022, 11, 4712. [Google Scholar] [CrossRef] [PubMed]
- Ashktorab, H.; Russo, T.; Oskrochi, G.; Latella, G.; Massironi, S.; Luca, M.; Chirumamilla, L.G.; Laiyemo, A.O.; Brim, H. Clinical and Endoscopic Outcomes in COVID-19 Patients with Gastrointestinal Bleeding. Gastro Help Adv. 2022, 1, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.A.; Wan, D.W.; Hajifathalian, K.; Tewani, S.; Shah, S.L.; Mehta, A.; Kaplan, A.; Ghosh, G.; Choi, A.J.; Krisko, T.I.; et al. Gastrointestinal Bleeding in Patients With Coronavirus Disease 2019: A Matched Case-Control Study. Am. J. Gastroenterol. 2020, 115, 1609–1616. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.; Nassar, M.; Nso, N.; Alfishawy, M. Massive gastrointestinal bleeding in a patient with COVID-19. Arab. J. Gastroenterol. 2021, 22, 177. [Google Scholar] [CrossRef]
- Abdelmohsen, M.A.; Alkandari, B.M.; Gupta, V.K.; Elsebaie, N. Gastrointestinal tract imaging findings in confirmed COVID-19 patients: A non-comparative observational study. Egypt. J. Radiol. Nucl. Med. 2021, 52, 52. [Google Scholar] [CrossRef]
Figure 1.
COVID-19 (+) hospitalized 75-year-old male patient with abdominal pain and diarrhea. Abdominal Contrast Enhanced CT (CECT) depicted bowel wall thickening ((a), thick arrow) and mesenteric fat stranding ((b), thin arrow). The imaging findings are indicative of inflammatory colitis.
Figure 1.
COVID-19 (+) hospitalized 75-year-old male patient with abdominal pain and diarrhea. Abdominal Contrast Enhanced CT (CECT) depicted bowel wall thickening ((a), thick arrow) and mesenteric fat stranding ((b), thin arrow). The imaging findings are indicative of inflammatory colitis.
Figure 2.
COVID-19 (+) 68-year-old patient with a long hospital stay was subjected to an abdominal CECT for acute abdominal pain, which showed colonic wall thickening ((a), thick arrow) and adjacent fat stranding ((b), thin arrow).
Figure 2.
COVID-19 (+) 68-year-old patient with a long hospital stay was subjected to an abdominal CECT for acute abdominal pain, which showed colonic wall thickening ((a), thick arrow) and adjacent fat stranding ((b), thin arrow).
Figure 3.
55-year-old male patient with COVID-19 pneumonia with right lower quadrant pain. CT on admission demonstrating a thickened appendix and proximal fat stranding (yellow arrow), indicating acute appendicitis.
Figure 3.
55-year-old male patient with COVID-19 pneumonia with right lower quadrant pain. CT on admission demonstrating a thickened appendix and proximal fat stranding (yellow arrow), indicating acute appendicitis.
Figure 4.
A 48-year-old female patient with COVID-19 pneumonia and no other comorbidities. CT scan depicted a thickened cecum with proximal fat stranding (yellow arrow).
Figure 4.
A 48-year-old female patient with COVID-19 pneumonia and no other comorbidities. CT scan depicted a thickened cecum with proximal fat stranding (yellow arrow).
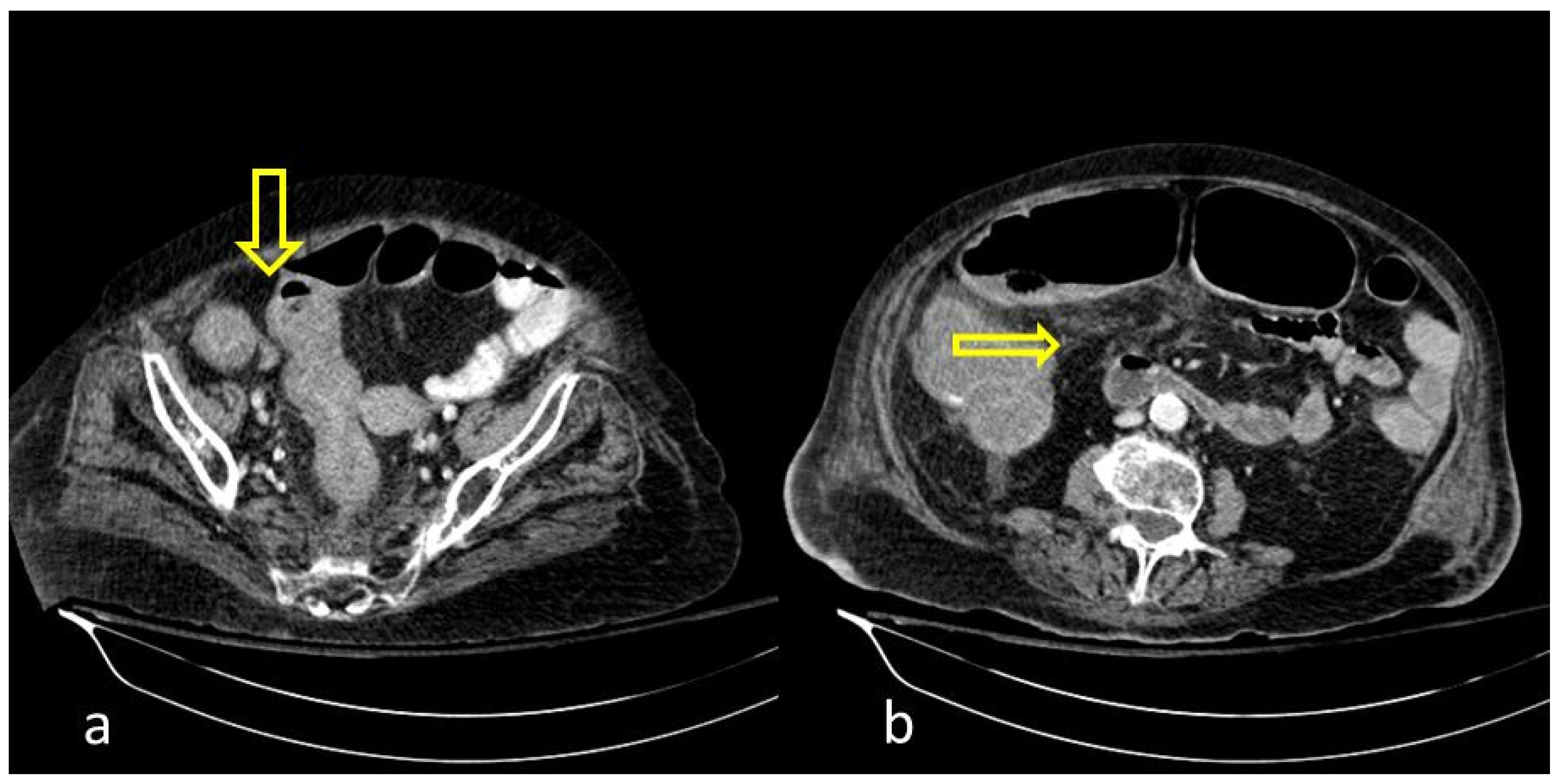
Figure 5.
59-year-old female patient with severe COVID-19 pneumonia hospitalized in the ICU. CT demonstrating distended large bowel with hyperenhanced walls and free abdominal fluid (shock bowel)—yellow arrow. Note the concomitant subcutaneous edema.
Figure 5.
59-year-old female patient with severe COVID-19 pneumonia hospitalized in the ICU. CT demonstrating distended large bowel with hyperenhanced walls and free abdominal fluid (shock bowel)—yellow arrow. Note the concomitant subcutaneous edema.
Figure 6.
85-year old-patient with small bowel ileus (fluid filled loops—yellow arrow) at the 4th day of hospitalization.
Figure 6.
85-year old-patient with small bowel ileus (fluid filled loops—yellow arrow) at the 4th day of hospitalization.
Figure 7.
A 69-year-old man with COVID-19 presented a positive Murphy sign. Ultrasound images showed gallbladder wall striation and increased thickness ((b)-arrowheads). The Color Doppler technique revealed hyperemia (a). No gallbladder stones were present indicating acalculous cholecystitis.
Figure 7.
A 69-year-old man with COVID-19 presented a positive Murphy sign. Ultrasound images showed gallbladder wall striation and increased thickness ((b)-arrowheads). The Color Doppler technique revealed hyperemia (a). No gallbladder stones were present indicating acalculous cholecystitis.
Figure 8.
A 90-year-old COVID-19 patient presented acute right quadrant abdominal pain and cholestasis. Computed tomography on the axial (a) and coronal (b) plane showed acute cholecystitis with diffuse thickening of the gallbladder wall and pericholecystic fluid (arrowheads). Disruption of the gallbladder wall (arrow) is also present with fluid collection around the liver indicating perforation.
Figure 8.
A 90-year-old COVID-19 patient presented acute right quadrant abdominal pain and cholestasis. Computed tomography on the axial (a) and coronal (b) plane showed acute cholecystitis with diffuse thickening of the gallbladder wall and pericholecystic fluid (arrowheads). Disruption of the gallbladder wall (arrow) is also present with fluid collection around the liver indicating perforation.
Figure 9.
81-year-old patient with COVID-19 pneumonia developed epigastric pain at the second day of hospitalization. CT demonstrating acute pancreatitis (yellow arrow).
Figure 9.
81-year-old patient with COVID-19 pneumonia developed epigastric pain at the second day of hospitalization. CT demonstrating acute pancreatitis (yellow arrow).
Figure 10.
56-year-old hospitalized male patient with COVID-19 pneumonia with raised level of d-dimers. CT depicting the thrombosed portal vein (yellow arrow). Note that in the same scan, there is extended small bowel thickening with mesenteric free fluid.
Figure 10.
56-year-old hospitalized male patient with COVID-19 pneumonia with raised level of d-dimers. CT depicting the thrombosed portal vein (yellow arrow). Note that in the same scan, there is extended small bowel thickening with mesenteric free fluid.
Figure 11.
A 75-year-old intubated patient with COVID-19 presented with hematemesis, hypotension and acute drop of hemoglobin level. Computed Tomography revealed active intraluminal extravasation of contrast into the 2nd part of duodenum (arrow) on arterial phase (a). Further pooling of the contrast (arrowhead) is shown on portal phase (b).
Figure 11.
A 75-year-old intubated patient with COVID-19 presented with hematemesis, hypotension and acute drop of hemoglobin level. Computed Tomography revealed active intraluminal extravasation of contrast into the 2nd part of duodenum (arrow) on arterial phase (a). Further pooling of the contrast (arrowhead) is shown on portal phase (b).
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).